Abstract
There are concerns that prenatal exposure to endocrine-disrupting chemicals increases children’s risk of obesity. African-American and Hispanic children born in the Bronx or Northern Manhattan, New York (1998–2006), whose mothers underwent personal air monitoring for polycyclic aromatic hydrocarbon (PAH) exposure during pregnancy, were followed up to ages 5 (n = 422) and 7 (n = 341) years. At age 5 years, 21% of the children were obese, as were 25% of those followed to age 7 years. After adjustment for child’s sex, age at measurement, ethnicity, and birth weight and maternal receipt of public assistance and prepregnancy obesity, higher prenatal PAH exposures were significantly associated with higher childhood body size. In adjusted analyses, compared with children of mothers in the lowest tertile of PAH exposure, children of mothers in the highest exposure tertile had a 0.39-unit higher body mass index z score (95% confidence interval (CI): 0.08, 0.70) and a relative risk of 1.79 (95% CI: 1.09, 2.96) for obesity at age 5 years, and they had a 0.30-unit higher body mass index z score (95% CI: 0.01, 0.59), a 1.93-unit higher percentage of body fat (95% CI: 0.33, 3.54), and a relative risk of 2.26 (95% CI: 1.28, 4.00) for obesity at age 7 years. The data indicate that prenatal exposure to PAHs is associated with obesity in childhood.
Keywords: cohort studies; environment; obesity; pediatrics; polycyclic hydrocarbons, aromatic
Childhood obesity clearly has multiple causes, with the literature identifying proximal, individual-level behavioral risk factors (e.g., high-fat diets, sedentary behavior) and more distal societal-level risk factors affecting diet and physical activity (e.g., suburbanization, automobile dependence, and agricultural policies) (1–3). However, a provocative emerging hypothesis is that exposure to environmental endocrine disruptors plays a role in the obesity epidemic by altering metabolic programming in early life (4–10). The polycyclic aromatic hydrocarbons (PAHs) are a family of chemicals created during incomplete combustion processes and are known human carcinogens that also have endocrine-disrupting effects (11–13). Hydroxy-PAHs, in particular, are structurally similar to estrogen and have been shown to have estrogenic activity in T47D breast adenoma cell lines (ER-CALUX assay; BioDetection Systems, Amsterdam, the Netherlands) (14). Likewise, PAH-containing particulate air pollution has been shown to be estrogenic in the T47D cell line and BG1Luc4E2 ovarian carcinoma cell line and in yeast (14–17). Recent murine and human adipocyte cell culture experiments showed that treatment with benzo[a]pyrene, a model PAH, inhibits lipolysis, and recent mouse studies found that benzo[a]pyrene exposure caused gains in fat mass (18).
In the Columbia Center for Children’s Environmental Health birth cohort study, the Mothers and Children Study in Northern Manhattan and the South Bronx, we investigated the effects of prenatal exposure to airborne PAHs on the child’s body size at ages 5 and 7 years and body composition at age 7 years. Because exposures to airborne PAHs were expected to be strongly influenced by individual activities, personal air monitoring was used for exposure assessment (19). Stationary ambient and indoor monitors cannot integrate exposures across times and locations experienced by an individual during a day in the way that personal monitoring can, and thus personal monitoring is thought to better characterize exposure. Prior analyses of these prenatal air monitoring data found that higher PAH exposures were associated with smaller birth size for gestational age among African Americans (20) and lower full-scale intelligence quotient and verbal intelligence quotient at age 5 years (21).
MATERIALS AND METHODS
Study participants were from a longitudinal birth cohort of mothers and children that has been described extensively elsewhere (22, 23). Nonsmoking pregnant women were recruited through prenatal clinics at New York-Presbyterian Hospital and Harlem Hospital Center between 1998 and 2006. The cohort was restricted to women aged 18–35 years who self-identified as either African-American or Dominican and had resided in Northern Manhattan or the South Bronx in New York City for at least 1 year prior to pregnancy. A questionnaire, administered to each woman in her home by a bilingual interviewer during the third trimester of pregnancy, collected information on demographic characteristics, history of active and passive smoking, educational and income levels, receipt of public assistance during pregnancy, maternal height, and maternal prepregnancy weight. Information on infant sex and birth weight was abstracted from the mothers’ and infants’ medical records following delivery. Women’s home addresses during pregnancy were geocoded, and the physical and sociodemographic characteristics of their neighborhoods, defined as a 1-km radial buffer around the home, were characterized as previously described (24).
As described in detail previously, during the third trimester of pregnancy the women wore a small backpack holding a personal ambient air monitor; the backpack was worn during the daytime for 2 consecutive days, and at night the women placed it near the bed (22, 24). A random subset of backpacks contained motion detectors, and detected motion was consistent with the verbal reports from the subjects that they were wearing the backpacks during daytime hours (21, 23). In ambient air, PAHs are found in vapors and aerosols and are bound to particle matter; particulate matter less than 2.5 μm in diameter (PM2.5) is of primary health importance, because fine particles of this size can penetrate deeply into the lungs (25). The personal air sampling pumps operated continuously at 4 L/minute, collecting PM2.5 on a precleaned quartz microfiber filter and collecting volatile and semivolatile vapors and aerosols on a polyurethane foam cartridge. Each personal monitoring result was assessed for accuracy in flow rate, time, and completeness of documentation. As described previously (23), particle bound and volatile and semivolatile PAHs were extracted from the filter and polyurethane foam via a Soxhlet Extractor (Corning, Inc., Corning, New York), and extracts were assayed by gas chromatography-mass spectrometry for 8 carcinogenic PAHs: benz[a]anthracene, chrysene, benzo[b]fluroanthene, benzo[k]fluroanthene, benzo[a]pyrene, indeno[1,2,3-cd]pyrene, dibenz[a,h]anthracene, and benzo[g,h,i]perylene. Comparisons between PAH levels measured in these air samples and levels measured in other cities have been reported previously (19). A substudy of 84 women was conducted to measure pollutants in residential indoor air during the last 6–8 weeks of pregnancy (26). Personal air samples were collected from the mothers over a period of 48 hours during the 32nd week of pregnancy, and then 2-week integrated indoor residential air samples were collected sequentially for the remaining 6–8 weeks of the pregnancy (26). Personal and indoor air samples were analyzed for PAHs using the same methods as in the full study (23).
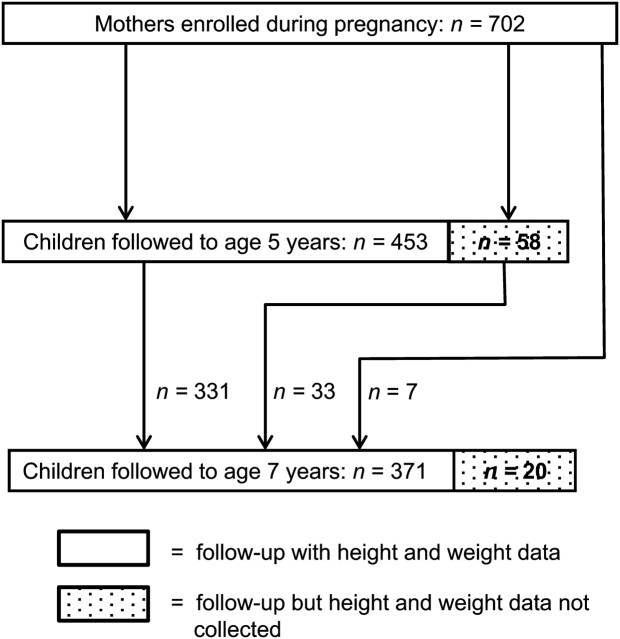
Personal air monitoring and measurement of PAHs was completed for 702 women; 511 of the children born to these women have been followed up to age 5 years, and thus far (as of 2011) 391 children have been followed up to age 7 years. Height and weight data were collected from 453 of the children at age 5 years and from 371 of the children at age 7 years, and body composition data were collected from 324 of these children. Figure 1 documents follow-up and attrition.
Figure 1.
Follow-up of children from a New York City birth cohort at ages 5 and 7 years and from whom height and weight data were collected, Mothers and Children Study in Northern Manhattan and the South Bronx, 1998–2011. Some children who were not followed up to age 5 years (n = 7) or from whom height and weight data were not collected at age 5 years (n = 33) were followed up at age 7 years and were measured for height and weight.
Weight at age 5 years was measured to the nearest 0.1 kg using a Detecto Cardinal 750 digital scale (Cardinal Scale Manufacturing Company, Webb City, Missouri) while the child was wearing light clothes and no shoes. At age 7 years, weight (to the nearest 0.1 kg) and body composition were measured using a Tanita scale (model BC-418; Tanita Corporation of America, Arlington Heights, Illinois). The Tanita scale reported percentage of body fat, fat mass, and lean mass using bioimpedance formulas validated in children as young as age 7 years. Height (to the nearest 0.1 cm) was measured using a SECA wall-mounted stadiometer (SECA, Hamburg, Germany).
Data analyses
Concentrations of the 8 PAHs measured in the air samples were summed and natural log-transformed (21, 23). Log-transformed personal air PAH levels were compared via t test across categories of obesity risk factors for the entire cohort and for children from whom anthropometric data were collected at ages 5 and 7 years. For the indoor air PAH exposure substudy, intraclass correlation coefficients were calculated to assess the variability of the integrated indoor air monitoring data during the last 6–8 weeks of pregnancy. Correlation coefficients were calculated comparing the mean residential indoor air PAH exposure calculated from the sequential 2-week indoor air monitoring with the 48-hour personal air monitoring data.
Children’s body mass index (BMI; weight (kg)/height (m)2) z scores and percentiles were calculated using the Centers for Disease Control and Prevention SAS macro (27); children were classified as obese if their BMI percentile was greater than or equal to the 95th percentile. Children were assigned to low, medium, and high maternal prenatal exposure categories based on tertile cutpoints of the distribution of prenatal PAH exposure levels. A series of linear regression analyses was used to determine whether children whose mothers were in the second (middle) or third (highest) tertile of personal air exposure to PAHs as compared with the first (lowest) tertile had higher BMI z scores at ages 5 and 7 years and a higher percentage of body fat, total fat mass, and fat-free mass at age 7 years. Relative risks of obesity at ages 5 and 7 years by exposure tertile were estimated using Poisson regression models (28, 29). All analyses controlled for the child’s sex, age at anthropometric measurement, ethnicity, and birth weight and maternal prepregnancy obesity. Measures of the mother’s socioeconomic status during pregnancy, including self-reported family income, mother’s educational attainment, and receipt of public assistance, were assessed as potential confounders. Receipt of public assistance was found to be the only predictor of the anthropometric outcomes and was included in all statistical models.
Additional sensitivity analyses were conducted assessing prenatal factors that might affect children’s growth trajectories and are also thought to be related to PAH exposure: season of monitoring, the mother’s living with a smoker, the mother’s living in a neighborhood with a high level of automobile traffic, and neighborhood socioeconomic status. Monitoring periods were classified as to whether or not they occurred between October 15 and April 31, the dates when owners of New York City apartment buildings are required to provide heat, predominantly via oil-fueled furnaces. Questionnaire data were used to assess whether a mother had lived with a smoker during her pregnancy. As a measure of exposure to automobile traffic, street density (linear distance of streets/area of neighborhood) within a 1-km radial buffer around the mother’s residence during pregnancy was measured. Neighborhood socioeconomic status was measured using 2000 US Census block group data on poverty and median household income aggregated to the 1-km radial neighborhood buffers using aerial weighting interpolation (24, 30). As described in prior work, for analyses that included neighborhood-level variables, linear regression generalized estimating equations with robust standard error calculations were used to account for clustering of subjects within New York City Community District neighborhoods (24, 31). In addition, analyses were conducted using inverse probability weights for successful follow-up and complete data collection to assess the effects of incomplete follow-up and missing data on effect estimates (32–34). These analyses are described in detail in the Web Appendix (http:/aje.oxfordjournals.org/).
All study procedures used at enrollment and at ages 5 and 7 years were approved by the Columbia University Institutional Review Board. Informed consent was obtained from all participating women, and assent was provided by the children at age 7 years.
RESULTS
Table 1 shows the geometric mean levels of PAH exposure overall and by categories of obesity risk factors for the entire cohort and for children at ages 5 and 7 years. For the cohort overall, mothers receiving public assistance had significantly higher exposure levels, and for children followed up to age 5 years, mothers of African-American children had higher exposure levels than mothers of Dominican children. In the indoor air monitoring substudy, the intraclass correlation coefficient for the consecutive 2-week indoor air sampling periods was 0.65 during the last 6–8 weeks of pregnancy. Estimates of prenatal PAH exposure based on individual 48-hour personal air monitoring and based on the mean of the consecutive 2-week integrated indoor air monitoring periods were significantly correlated (r = 0.58, P < 0.001).
Table 1.
Geometric Mean Levels (ng/m3) of Polycyclic Aromatic Hydrocarbons in Prenatal Personal Air Monitoring Samples in the Mothers and Children Study in Northern Manhattan and the South Bronx, 1998–2011
| Characteristic | Total Enrolled Cohort (n = 702) | Children With Anthropometric Measures at Age 5 Years (n = 453) | Children With Anthropometric Measures at Age 7 Years (n = 371) |
| Overall | 2.39 (2.12)a | 2.34 (2.05) | 2.53 (2.03) |
| Child’s sex | |||
| Girls | 2.34 (2.08) | 2.34 (1.99) | 2.53 (1.97) |
| Boys | 2.44 (2.18) | 2.32 (2.14) | 2.53 (2.10) |
| Child’s ethnicity | |||
| African-American | 2.53 (2.01) | 2.53 (1.90) | 2.69 (1.92) |
| Dominican | 2.29 (2.18) | 2.20 (2.16)* | 2.44 (2.10) |
| Maternal prepregnancy obesity | |||
| No | 2.39 (2.23) | 2.29 (2.14) | 2.53 (2.08) |
| Yes | 2.36 (1.93) | 2.56 (1.92) | 2.69 (1.93) |
| Maternal receipt of public assistance during pregnancy | |||
| No | 2.25 (2.14) | 2.25 (2.08) | 2.44 (2.03) |
| Yes | 2.53 (2.08)* | 2.46 (2.05) | 2.66 (2.03) |
* P < 0.05 (by t test).
Numbers in parentheses, standard deviation.
Complete data for outcomes and key covariates (sex, ethnicity, receipt of public assistance during pregnancy, birth weight, and maternal prepregnancy obesity) were available from 422 of the 453 children followed to age 5 years for anthropometric outcomes and from 341 of the 371 children followed to age 7 years. Data on body composition and key covariates were available for 297 children. The primary missing data element was maternal prepregnancy weight. Table 2 presents descriptive statistics for the sociodemographic characteristics and risk factors for all cohort children at baseline and for those followed up. At age 5 years, 21% of the children were obese, and at age 7 years, 25% were obese; the mean percentage of body fat at age 7 years was 24.1% (7.2 kg of fat mass). As expected, for the 331 children from whom anthropometric data were available at ages 5 and 7 years, obesity at age 5 was strongly predictive of obesity at age 7 (κ = 0.67, P < 0.001; relative risk = 8.39, 95% confidence interval (CI): 5.63, 12.50).
Table 2.
Sociodemographic and Anthropometric Characteristics of the Cohort at Baseline and During Follow-up, Mothers and Children Study in Northern Manhattan and the South Bronx, 1998–2011
| Characteristic | Total Enrolled Cohort (n = 702) | Children With Anthropometric Measures at Age 5 Years (n = 453) | Children With Anthropometric Measures at Age 7 Years (n = 371) | ||||||
| No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | |
| Child’s sex | |||||||||
| Female | 352 | 50 | 240 | 53 | 200 | 54 | |||
| Male | 335 | 48 | 213 | 47 | 171 | 46 | |||
| Unknown | 15 | 2 | 0 | 0 | |||||
| Child’s ethnicity | |||||||||
| African-American | 256 | 37 | 185 | 41 | 160 | 43 | |||
| Dominican | 446 | 63 | 268 | 59 | 211 | 57 | |||
| Child’s birth weight, g | 3,369 (477) | 3,380 (480) | 3,406 (486) | ||||||
| Maternal prepregnancy obesity | |||||||||
| No | 499 | 71 | 330 | 73 | 269 | 73 | |||
| Yes | 166 | 24 | 101 | 22 | 81 | 22 | |||
| Unknown | 37 | 5 | 22 | 5 | 21 | 6 | |||
| Maternal receipt of public assistance during pregnancy | |||||||||
| No | 402 | 57 | 255 | 56 | 211 | 57 | |||
| Yes | 294 | 42 | 194 | 43 | 156 | 42 | |||
| Unknown | 6 | 1 | 4 | 1 | 4 | 1 | |||
| Mother’s years of education at time of pregnancy | 12 (2) | 12 (2) | 12 (2) | ||||||
| % of residents living in poverty in the mother’s neighborhood | 36 (5) | 35 (5) | 35 (4) | ||||||
| Annual household income in the mother’s neighborhood, dollars | 22,661 (3,621) | 22,489 (3,430) | 22,517 (3,285) | ||||||
| Geometric mean PAH level in personal air monitoring samples, ng/m3 | 2.38 (2.13) | 2.34 (2.06) | 2.54 (2.03) | ||||||
| Body mass indexa z score | NA | 0.64 (1.37) | 0.82 (1.15) | ||||||
| Childhood obesity | |||||||||
| No | NA | 356 | 79 | 277 | 75 | ||||
| Yes | NA | 97 | 21 | 94 | 25 | ||||
| Mean % of body fat (n = 324) | NA | NA | 24 (6) | ||||||
Abbreviations: NA, not applicable; PAH, polycyclic aromatic hydrocarbon; SD, standard deviation.
Weight (kg)/height (m)2.
In unadjusted analyses, compared with children whose mothers were in the first tertile of prenatal PAH exposure, at age 5 years the BMI z score was 0.33 units higher (95% CI: 0.02, 0.65) for children of mothers in the second exposure tertile, and it was 0.43 units higher (95% CI: 0.12, 0.75) for children of mothers in the third exposure tertile. Similar effects were seen at age 7 years; compared with the first tertile, the second tertile of exposure was associated with a 0.17-unit higher BMI z score (95% CI: −0.14, 0.48), and the third tertile of exposure was associated with a 0.32-unit higher BMI z score (95% CI: 0.01, 0.62).
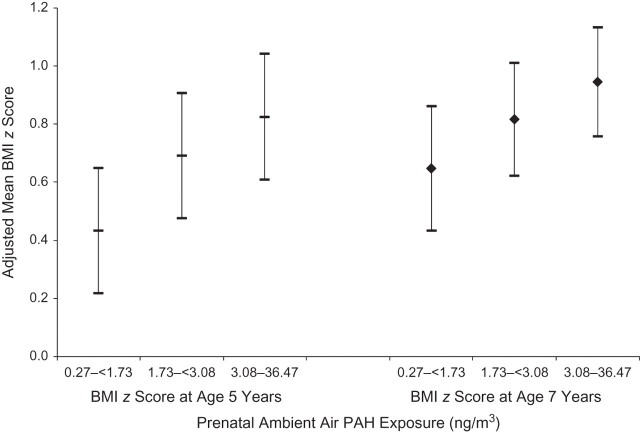
Table 3 shows the multivariate associations between model covariates and categories of maternal PAH exposure and BMI z score and body composition measures. After control for the model covariates, the highest tertile of prenatal PAH exposure was significantly associated with higher BMI z score at both age 5 years and age 7 years. In addition, at age 7 years, higher prenatal PAH exposure was significantly associated with increased percentage of body fat and fat mass but not with variation in lean mass (e.g., organs, bone, and muscle). Figure 2 shows the covariate-adjusted mean BMI z scores (and 95% confidence intervals) at ages 5 and 7 years by tertile of prenatal PAH exposure. To place the results in context, for a 5-year-old boy with a weight of 21 kg and a height of 115 cm (BMI z score = 0.37, BMI percentile = 64.5—median values for the study population), an increase of 0.39 z-score units equates to a 0.7-kg increase in weight and a 13-unit increase in BMI percentile. Similarly, for a 7-year-old boy with a weight of 27 kg and a height of 126 cm (BMI z score = 0.86, BMI percentile = 80.5), an increase of 0.30 z-score units equates to a 1.11-kg increase in weight and places the child at the 87.7th BMI percentile. Being in the third tertile of prenatal PAH exposure as compared with the first was associated with 1.1-kg higher fat mass. Table 4 shows that the second and third tertiles of maternal PAH exposure were associated with higher relative risks of obesity at ages 5 and 7 years.
Table 3.
Associations of Prenatal Exposure to Polycyclic Aromatic Hydrocarbons and Sociodemographic and Early-Life Characteristics With Body Size and Composition at Ages 5 and 7 Years Among Children in the Mothers and Children Study in Northern Manhattan and the South Bronx, 1998–2011a
| Risk Factor | BMIb z Score at Age 5 Years | BMI z Score at Age 7 Years | % Body Fat at Age 7 Years | Fat Mass at Age 7 Years, kg | Fat-Free Mass at Age 7 Years, kg | |||||
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | |
| Birth weight (per 100 g) | 0.05 | 0.02, 0.08 | 0.04 | 0.02, 0.07 | 0.14 | 0.00, 0.27 | 0.11 | 0.03, 0.20 | 0.18 | 0.10, 0.26 |
| Ethnicity | ||||||||||
| Dominican | 0 | Reference | 0 | Reference | 0 | Reference | 0 | Reference | 0 | Reference |
| African-American | −0.10 | −0.37, 0.17 | −0.11 | −0.35, 0.14 | −1.59 | −2.97, −0.21 | −0.60 | −1.46, 0.26 | 0.16 | −0.65, 0.99 |
| Maternal receipt of public assistance during pregnancy | ||||||||||
| No | 0 | Reference | 0 | Reference | 0 | Reference | 0 | Reference | 0 | Reference |
| Yes | −0.07 | −0.32, 0.19 | −0.20 | −0.43, 0.04 | −1.38 | −2.70, −0.05 | −1.05 | −1.88, −0.23 | −0.79 | −1.57, −0.01 |
| Child’s sex | ||||||||||
| Female | 0 | Reference | 0 | Reference | 0 | Reference | 0 | Reference | 0 | Reference |
| Male | 0.02 | −0.23, 0.27 | 0.19 | −0.05, 0.42 | −1.61 | −2.92, −0.30 | −0.23 | −1.05, 0.59 | 1.08 | 0.31, 1.85 |
| Maternal prepregnancy obesity | ||||||||||
| No | 0 | Reference | 0 | Reference | 0 | Reference | 0 | Reference | 0 | Reference |
| Yes | 0.38 | 0.07, 0.68 | 0.72 | 0.44, 1.00 | 3.85 | 2.27, 5.44 | 2.41 | 1.42, 3.40 | 1.91 | 0.98, 2.85 |
| Child’s age at measurement, months | 0.06 | 0.01, 0.11 | 0.01 | −0.04, 0.06 | 0.09 | −0.18, 0.36 | 0.15 | −0.02, 0.32 | 0.26 | 0.10, 0.42 |
| Tertile of prenatal PAH exposure | ||||||||||
| First (<1.73 ng/m3) | 0 | Reference | 0 | Reference | 0 | Reference | 0 | Reference | 0 | Reference |
| Second (1.73–3.07 ng/m3) | 0.26 | −0.05, 0.57 | 0.17 | −0.13, 0.46 | 1.91 | 0.27, 3.55 | 1.19 | 0.17, 2.22 | 0.45 | −0.52, 1.41 |
| Third (≥3.08 ng/m3) | 0.39 | 0.08, 0.70 | 0.30 | 0.01, 0.59 | 1.93 | 0.33, 3.54 | 1.11 | 0.10, 2.11 | 0.34 | −0.61, 1.29 |
Abbreviations: BMI, body mass index; CI, confidence interval; PAH, polycyclic aromatic hydrocarbon.
All linear regression β coefficients predicting the anthropometric outcomes were mutually adjusted for the other predictor variables in the table.
Weight (kg)/height (m)2.
Figure 2.
Covariate-adjusted mean body mass index (BMI; weight (kg)/height (m)2) z scores according to tertile of prenatal ambient air polycyclic aromatic hydrocarbon (PAH) exposure for children at ages 5 and 7 years, Mothers and Children Study in Northern Manhattan and the South Bronx, 1998–2011. Median PAH exposure levels for tertile categories: first tertile, 1.19 ng/m3; second tertile, 2.30 ng/m3; third tertile, 4.68 ng/m3. Bars, 95% confidence interval.
Table 4.
Risks of Obesity at Ages 5 and 7 Years According to Prenatal Exposure to Polycyclic Aromatic Hydrocarbons and Sociodemographic and Early-Life Characteristics Among Children in the Mothers and Children Study in Northern Manhattan and the South Bronx, 1998–2011
| Obesity at Age 5 Years | Obesity at Age 7 Years | |||
| RRa | 95% CI | RRa | 95% CI | |
| Birth weight (per 100 g) | 1.04 | 1.00, 1.08 | 1.02 | 0.98, 1.06 |
| Ethnicity | ||||
| Dominican | 1 | Reference | 1 | Reference |
| African-American | 0.68 | 0.45, 1.01 | 0.72 | 0.49, 1.06 |
| Maternal receipt of public assistance during pregnancy | ||||
| No | 1 | Reference | 1 | Reference |
| Yes | 0.94 | 0.65, 1.36 | 0.78 | 0.54, 1.13 |
| Child’s sex | ||||
| Female | 1 | Reference | 1 | Reference |
| Male | 1.01 | 0.70, 1.44 | 1.28 | 0.90, 1.82 |
| Maternal prepregnancy obesity | ||||
| No | 1 | Reference | 1 | Reference |
| Yes | 1.39 | 0.93, 2.08 | 2.01 | 1.39, 2.92 |
| Child’s age at measurement, months | 1.05 | 1.00, 1.10 | 1.04 | 0.97, 1.11 |
| Tertile of prenatal PAH exposure | ||||
| First (<1.73 ng/m3) | 1 | Reference | 1 | Reference |
| Second (1.73–3.07 ng/m3) | 1.79 | 1.08, 2.98 | 2.25 | 1.27, 4.01 |
| Third (≥3.08 ng/m3) | 1.79 | 1.09, 2.96 | 2.26 | 1.28, 4.00 |
Abbreviations: CI, confidence interval; PAH, polycyclic aromatic hydrocarbon; RR, relative risk.
All relative risks were mutually adjusted for other variables in the table.
We conducted further analyses to assess the sensitivity of the results to additional model specifications and adjustment for other covariates. Neighborhood socioeconomic status represents a potential confounding factor; however, further control for neighborhood poverty rate or median household income did not alter the results. In regards to factors hypothesized to be associated with higher ambient air PAH levels, PAH levels were not associated with the mother’s reporting that a smoker lived in the home or with neighborhood socioeconomic status. Air monitoring samples collected during heating months showed 47% higher PAH levels (95% CI: 29, 67) than samples collected during the nonheating months, and higher PAH levels were associated with higher street density within a 1-km radius of the home (2.94% higher per linear kilometer of street; 95% CI: 0.20, 5.65). However, season of sampling and neighborhood street density did not predict BMI z score at age 5 or 7 years or body composition at age 7 years, and adjustment for these variables did not alter the analytical results.
Weighting the data by the inverse probability of follow-up and complete data collection at age 5 years only slightly reduced the estimated effect of the third tertile of PAH exposure on BMI z score (weighted estimate = 0.34 BMI z-score units; 95% CI: 0.02, 0.66) and did not alter the effect estimate for the second tertile of exposure. However, inverse probability-weighted analyses for BMI z score at age 7 years suggested that the initial analyses were biased toward the null value. Compared with the first tertile of PAH exposure, the covariate-adjusted weighted β coefficient for the second tertile of exposure was 0.28 BMI z-score units (95% CI: −0.01, 0.58), while in the unweighted analyses the covariate-adjusted coefficient was 0.17 units (95% CI: −0.12, 0.46). For comparisons between the third and first tertiles of exposure, the covariate-adjusted weighted β coefficient was 0.40 BMI z-score units (95% CI: 0.11, 0.69), while the corresponding covariate-adjusted coefficient in the unweighted analyses was 0.30 BMI z-score units (95% CI: 0.01, 0.58).
DISCUSSION
Prenatal exposure to ambient PAHs was found to predict higher BMI z score and obesity at age 5 years and higher BMI z score, obesity, and fat mass at age 7 years. The observed effect of prenatal exposure to PAHs on children’s body size appears to be due to the accumulation of fat mass and not to differences in lean mass. In our analyses, we considered possible confounding by measures of socioeconomic status and by prenatal exposure to sources of PAHs and found that adjustment for these factors did not alter the results. Similar to a prior report using data from midway through cohort recruitment, the mother’s report of a smoker living in the home did not predict PAH levels in the prenatal air monitoring samples (19). Season of monitoring and neighborhood street density did predict PAH levels, but not body size at ages 5 and 7 years. These results provide the first evidence that childhood obesity can be influenced by prenatal exposure to PAHs.
These results are consistent with past laboratory experiments showing PAH and particulate air pollution effects on lipolysis and fat accumulation. Murine and human adipocyte experiments showed that treatment with benzo[a]pyrene, a model PAH, suppressed the normal release of free fatty acids by adipocytes upon exposures to β1, β2, and β3-adrenoreceptor-specific agonists (18). Similarly, C57B1/6J mouse experiments showed that benzo[a]pyrene treatment inhibited epinephrine-induced release of free fatty acids, and 15 days of chronic exposure caused weight gain due to accumulation of fat mass (18). A second study found that starting at week 3 of life, 10 weeks of exposure to a PM2.5 particle mixture representative of the northeastern region of the United States caused weight gain in C57Bl/6J mice due to increases in subcutaneous and visceral fat mass, although PM2.5 exposure concentrations were 10-fold higher than ambient levels (35, 36). Cigarette smoke is a source of PAHs, and maternal smoking during pregnancy has consistently been found to be associated with higher body weight in the child during childhood and adolescence and into adulthood (37, 38). While the literature on maternal smoking during pregnancy tends to ascribe smoking’s effects on children’s weight to nicotine exposure, it is possible that it is the PAH component of cigarette smoke that affects the children’s growth trajectory (37).
Recently, substantial attention has been focused on the hypothesis that prenatal and early-life exposure to environmental estrogens causes obesity (7–10, 39). Studies with CD-1 mice have shown that treatment during the neonatal period with diethylstilbestrol, a model estrogenic compound, causes increased weight gain (8). Although PAHs have been referred to as xenoestrogens, both estrogenic and antiestrogenic effects have been documented, with the primary endocrine effect relating to interference with estrogen signaling (11, 12). The effects of PAHs on estrogen receptor-mediated signaling have been shown to be compound-specific and cellular model-dependent (12). However, studies have shown that PAHs induce estrogen-dependent cell proliferation and estrogen receptor-mediated reporter gene expression and that they induce uterine growth in Wistar rats (12, 40, 41). Hydroxy-PAHs, in particular, have structural similarities to estrogen, and in vitro test systems have shown them to have estrogenic activity; in addition, PAH-containing particulate air pollution has been shown to have estrogenic effects in estrogen-responsive carcinoma cell lines and in yeast models (14–17). PAHs may also alter estrogen signaling through cross-talk between the estrogen receptor and the aryl hydrocarbon receptor, for which PAHs are a ligand (42). In human breast tissue, we have shown that levels of PAH-DNA adducts in breast tumors are significantly correlated with estrogen receptor expression (43). However, data are not available for testing whether weight gain associated with PAH exposure is mediated via an estrogen receptor agonist-based mechanism.
Several caveats must be considered when interpreting these results. Firstly, the study was restricted to nonsmoking women and so may not be generalizable to women who smoke during pregnancy. The personal air monitoring was conducted for only 48 hours in the third trimester, and PAH levels detected during this short duration of monitoring may not be representative of longer-term exposures incurred during pregnancy. However, the indoor air monitoring substudy showed that residential indoor PAH exposure levels were fairly stable during the last 6–8 weeks of pregnancy and that home indoor PAH levels were correlated with those in the personal air monitoring samples. Secondly, we were not able to adjust for ambient air PAH exposures in early childhood prior to age 5 years, which may be correlated with the mother’s exposures during pregnancy. Thus, it is not absolutely clear that pregnancy is the critical exposure window, and we may be indirectly observing the effects of early childhood exposures on subsequent growth trajectories. Body composition was measured using Tanita bioimpedance technology and a 2-compartment model of the body, which, while it has been validated (44–48), still represents a clinical standard rather than a gold-standard, 4-compartment measure of body composition. Additionally, the Tanita bioimpedance technology is not recommended for assessing body composition in children before age 7 years; thus, fat and lean mass data were not gathered at age 5 years. Lastly, follow-up was not complete, particularly to age 7 years, and it was associated with several predictors of body size. However, reanalysis of the data using inverse probability weighting for successful follow-up suggested that incomplete follow-up did not bias the age 5 results and that it caused a bias toward the null for the age 7 results (32, 33).
In conclusion, this study suggests that prenatal exposure to PAHs causes increased fat mass gain during childhood and a higher risk of childhood obesity. The strengths of this study include the use of personal air monitoring to measure exposure to PAHs during pregnancy, the prospective cohort design, and the use of body composition data to demonstrate specific effects on fat mass. These results provide further data on the negative health consequences of air pollution and suggest that prenatal exposure to endocrine-disrupting chemicals contributes to obesity risk.
Supplementary Material
Acknowledgments
Author affiliations: Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, New York (Andrew Rundle); Department of Environmental Health Sciences, Mailman School of Public Health, Columbia University, New York, New York (Lori Hoepner, Greg Freyer, Darrell Holmes, Marilyn Reyes, Frederica Perera, Robin Whyatt); Department of Pediatrics, New York-Presbyterian Hospital, Columbia University, New York, New York (Abeer Hassoun, Sharon Oberfield); Institute for Social and Economic Research and Policy, Columbia University, New York, New York (James Quinn); and Southwest Research Institute, San Antonio, Texas (David Camann).
This study was supported by the National Institute of Environmental Health Sciences (grants 5P01ES09600, 5R01ES08977, R01ES010165, and R01ES015282), the Environmental Protection Agency (grants R827027, 8260901, and RR00645), the Educational Foundation of America, the John and Wendy Neu Family Foundation, the New York Community Trust, and the Trustees of the Blanchette Hooker Rockefeller Fund.
Conflict of interest: none declared.
Glossary
Abbreviations
- BMI
body mass index
- CI
confidence interval
- PAH(s)
polycyclic aromatic hydrocarbon(s)
- PM2.5
particulate matter less than 2.5 μm in diameter
References
- 1.Sallis JF, Cervero RB, Ascher W, et al. An ecological approach to creating active living communities. Annu Rev Public Health. 2006;27:297–322. doi: 10.1146/annurev.publhealth.27.021405.102100. [DOI] [PubMed] [Google Scholar]
- 2.Sallis JF, Glanz K. The role of built environments in physical activity, eating, and obesity in childhood. Future Child. 2006;16(1):89–108. doi: 10.1353/foc.2006.0009. [DOI] [PubMed] [Google Scholar]
- 3.Sallis JF, Glanz K. Physical activity and food environments: solutions to the obesity epidemic. Milbank Q. 2009;87(1):123–154. doi: 10.1111/j.1468-0009.2009.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grün F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147(6 suppl):S50–S55. doi: 10.1210/en.2005-1129. [DOI] [PubMed] [Google Scholar]
- 5.Grün F, Blumberg B. Perturbed nuclear receptor signaling by environmental obesogens as emerging factors in the obesity crisis. Rev Endocr Metab Disord. 2007;8(2):161–171. doi: 10.1007/s11154-007-9049-x. [DOI] [PubMed] [Google Scholar]
- 6.Grün F, Blumberg B. Minireview: the case for obesogens. Mol Endocrinol. 2009;23(8):1127–1134. doi: 10.1210/me.2008-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newbold RR, Padilla-Banks E, Jefferson WN, et al. Effects of endocrine disruptors on obesity. Int J Androl. 2008;31(2):201–208. doi: 10.1111/j.1365-2605.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 8.Newbold RR, Padilla-Banks E, Snyder RJ, et al. Perinatal exposure to environmental estrogens and the development of obesity. Mol Nutr Food Res. 2007;51(7):912–917. doi: 10.1002/mnfr.200600259. [DOI] [PubMed] [Google Scholar]
- 9.Newbold RR, Padilla-Banks E, Snyder RJ, et al. Developmental exposure to endocrine disruptors and the obesity epidemic. Reprod Toxicol. 2007;23(3):290–296. doi: 10.1016/j.reprotox.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heindel JJ. Endocrine disruptors and the obesity epidemic. Toxicol Sci. 2003;76(2):247–249. doi: 10.1093/toxsci/kfg255. [DOI] [PubMed] [Google Scholar]
- 11.Davis DL, Bradlow HL, Wolff M, et al. Medical hypothesis: xenoestrogens as preventable causes of breast cancer. Environ Health Perspect. 1993;101(5):372–377. doi: 10.1289/ehp.93101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kummer V, Masková J, Zralý Z, et al. Estrogenic activity of environmental polycyclic aromatic hydrocarbons in uterus of immature Wistar rats. Toxicol Lett. 2008;180(3):212–221. doi: 10.1016/j.toxlet.2008.06.862. [DOI] [PubMed] [Google Scholar]
- 13.Santodonato J. Review of the estrogenic and antiestrogenic activity of polycyclic aromatic hydrocarbons: relationship to carcinogenicity. Chemosphere. 1997;34(4):835–848. doi: 10.1016/s0045-6535(97)00012-x. [DOI] [PubMed] [Google Scholar]
- 14.Wenger D, Gerecke AC, Heeb NV, et al. In vitro estrogenicity of ambient particulate matter: contribution of hydroxylated polycyclic aromatic hydrocarbons. J Appl Toxicol. 2009;29(3):223–232. doi: 10.1002/jat.1400. [DOI] [PubMed] [Google Scholar]
- 15.Klein GP, Hodge EM, Diamond ML, et al. Gas-phase ambient air contaminants exhibit significant dioxin-like and estrogen-like activity in vitro. Environ Health Perspect. 2006;114(5):697–703. doi: 10.1289/ehp.8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Wu W, Henkelman B, et al. Presence of estrogenic activity from emission of fossil fuel combustion as detected by a recombinant yeast bioassay. Atmos Environ. 2003;37(23):3225–3235. [Google Scholar]
- 17.Wang J, Xie P, Kettrup A, et al. Inhibition of progesterone receptor activity in recombinant yeast by soot from fossil fuel combustion emissions and air particulate materials. Sci Total Environ. 2005;349(1–3):120–128. doi: 10.1016/j.scitotenv.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Irigaray P, Ogier V, Jacquenet S, et al. Benzo[a]pyrene impairs beta-adrenergic stimulation of adipose tissue lipolysis and causes weight gain in mice. A novel molecular mechanism of toxicity for a common food pollutant. FEBS J. 2006;273(7):1362–1372. doi: 10.1111/j.1742-4658.2006.05159.x. [DOI] [PubMed] [Google Scholar]
- 19.Tonne CC, Whyatt RM, Camann DE, et al. Predictors of personal polycyclic aromatic hydrocarbon exposures among pregnant minority women in New York City. Environ Health Perspect. 2004;112(6):754–759. doi: 10.1289/ehp.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi H, Rauh V, Garfinkel R, et al. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and risk of intrauterine growth restriction. Environ Health Perspect. 2008;116(5):658–665. doi: 10.1289/ehp.10958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perera FP, Li Z, Whyatt R, et al. Prenatal airborne polycyclic aromatic hydrocarbon exposure and child IQ at age 5 years. Pediatrics. 2009;124(2):e195–e202. doi: 10.1542/peds.2008-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whyatt RM, Barr DB, Camann DE, et al. Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environ Health Perspect. 2003;111(5):749–756. doi: 10.1289/ehp.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perera FP, Rauh V, Tsai WY, et al. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect. 2003;111(2):201–205. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lovasi GS, Quinn JW, Rauh VA, et al. Chlorpyrifos exposure and urban residential environment characteristics as determinants of early childhood neurodevelopment. Am J Public Health. 2011;101(1):63–70. doi: 10.2105/AJPH.2009.168419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pope CA, III, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287(9):1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whyatt RM, Garfinkel R, Hoepner LA, et al. Within- and between-home variability in indoor-air insecticide levels during pregnancy among an inner-city cohort from New York City. Environ Health Perspect. 2007;115(3):383–389. doi: 10.1289/ehp.9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. A SAS Program for the CDC Growth Charts. Atlanta, GA: Centers for Disease Control and Prevention; 2004. [Google Scholar]
- 28.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 29.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 30.Flowerdew R, Green M. Data integration: statistical methods for transferring data between zonal systems. In: Masser I, Blakemore M, editors. Handling Geographical Information: Methodology and Potential Applications. New York, NY: Longman Publisher’s; 1991. pp. 38–54. [Google Scholar]
- 31.Hubbard AE, Ahern J, Fleischer NL, et al. To GEE or not to GEE: comparing population average and mixed models for estimating the associations between neighborhood risk factors and health. Epidemiology. 2010;21(4):467–474. doi: 10.1097/EDE.0b013e3181caeb90. [DOI] [PubMed] [Google Scholar]
- 32.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 33.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Curtis LH, Hammill BG, Eisenstein EL, et al. Using inverse probability-weighted estimators in comparative effectiveness analyses with observational databases. Med Care. 2007;45(10 suppl 2):S103–S107. doi: 10.1097/MLR.0b013e31806518ac. [DOI] [PubMed] [Google Scholar]
- 35.Xu X, Yavar Z, Verdin M, et al. Effect of early particulate air pollution exposure on obesity in mice: role of p47phox. Arterioscler Thromb Vasc Biol. 2010;30(12):2518–2527. doi: 10.1161/ATVBAHA.110.215350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun Q, Wang A, Jin X, et al. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA. 2005;294(23):3003–3010. doi: 10.1001/jama.294.23.3003. [DOI] [PubMed] [Google Scholar]
- 37.Oken E, Levitan EB, Gillman MW. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int J Obes (Lond) 2008;32(2):201–210. doi: 10.1038/sj.ijo.0803760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Power C, Jefferis BJ. Fetal environment and subsequent obesity: a study of maternal smoking. Int J Epidemiol. 2002;31(2):413–419. [PubMed] [Google Scholar]
- 39.Newbold RR, Padilla-Banks E, Jefferson WN. Environmental estrogens and obesity. Mol Cell Endocrinol. 2009;304(1-2):84–89. doi: 10.1016/j.mce.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdelrahim M, Ariazi E, Kim K, et al. 3-Methylcholanthrene and other aryl hydrocarbon receptor agonists directly activate estrogen receptor alpha. Cancer Res. 2006;66(4):2459–2467. doi: 10.1158/0008-5472.CAN-05-3132. [DOI] [PubMed] [Google Scholar]
- 41.Tsai KS, Yang RS, Liu SH. Benzo[a]pyrene regulates osteoblast proliferation through an estrogen receptor-related cyclooxygenase-2 pathway. Chem Res Toxicol. 2004;17(5):679–684. doi: 10.1021/tx0499517. [DOI] [PubMed] [Google Scholar]
- 42.Safe S, Wormke M. Inhibitory aryl hydrocarbon receptor-estrogen receptor alpha cross-talk and mechanisms of action. Chem Res Toxicol. 2003;16(7):807–816. doi: 10.1021/tx034036r. [DOI] [PubMed] [Google Scholar]
- 43.Rundle A, Tang D, Hibshoosh H, et al. The relationship between genetic damage from polycyclic aromatic hydrocarbons in breast tissue and breast cancer. Carcinogenesis. 2000;21(7):1281–1289. [PubMed] [Google Scholar]
- 44.Jebb SA, Cole TJ, Doman D, et al. Evaluation of the novel Tanita body-fat analyser to measure body composition by comparison with a four-compartment model. Br J Nutr. 2000;83(2):115–122. doi: 10.1017/s0007114500000155. [DOI] [PubMed] [Google Scholar]
- 45.Pietrobelli A, Rubiano F, St-Onge MP, et al. New bioimpedance analysis system: improved phenotyping with whole-body analysis. Eur J Clin Nutr. 2004;58(11):1479–1484. doi: 10.1038/sj.ejcn.1601993. [DOI] [PubMed] [Google Scholar]
- 46.Xie X, Kolthoff N, Bärenholt O, et al. Validation of a leg-to-leg bioimpedance analysis system in assessing body composition in postmenopausal women. Int J Obes Relat Metab Disord. 1999;23(10):1079–1084. doi: 10.1038/sj.ijo.0801034. [DOI] [PubMed] [Google Scholar]
- 47.Nuñez C, Gallagher D, Visser M, et al. Bioimpedance analysis: evaluation of leg-to-leg system based on pressure contact footpad electrodes. Med Sci Sports Exerc. 1997;29(4):524–531. doi: 10.1097/00005768-199704000-00015. [DOI] [PubMed] [Google Scholar]
- 48.Cable A, Nieman DC, Austin M, et al. Validity of leg-to-leg bioelectrical impedance measurement in males. J Sports Med Phys Fitness. 2001;41(3):411–444. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.