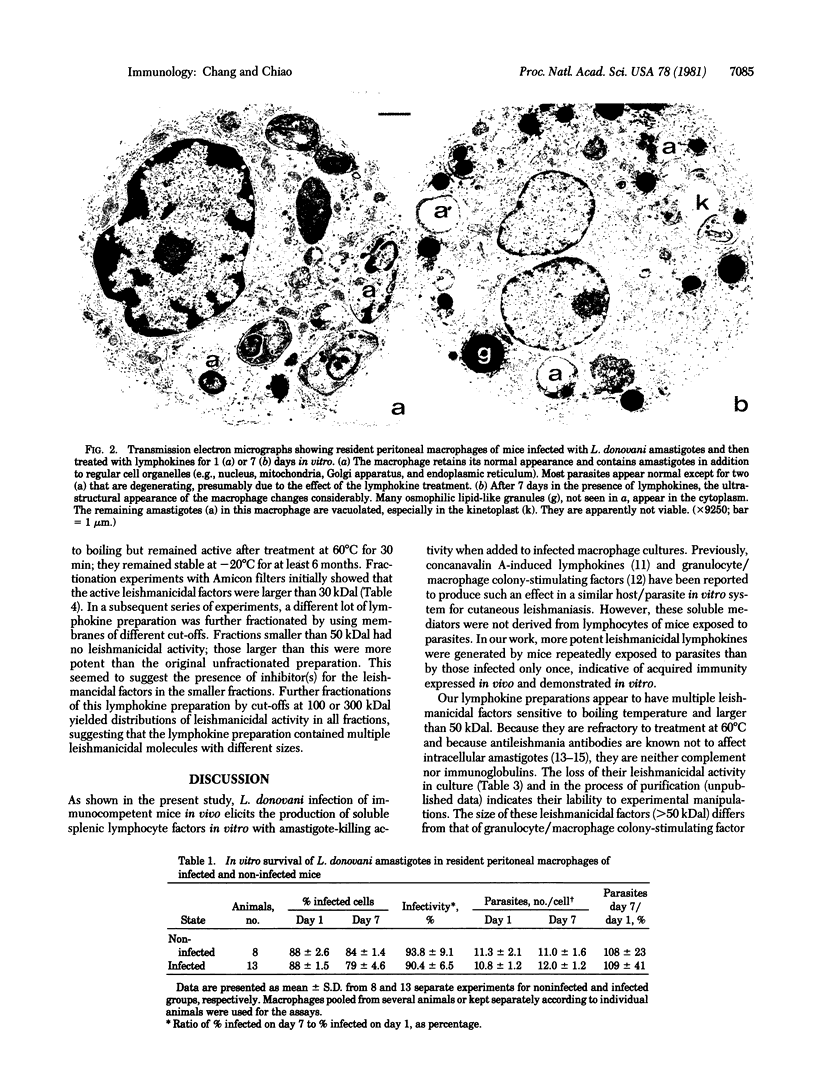
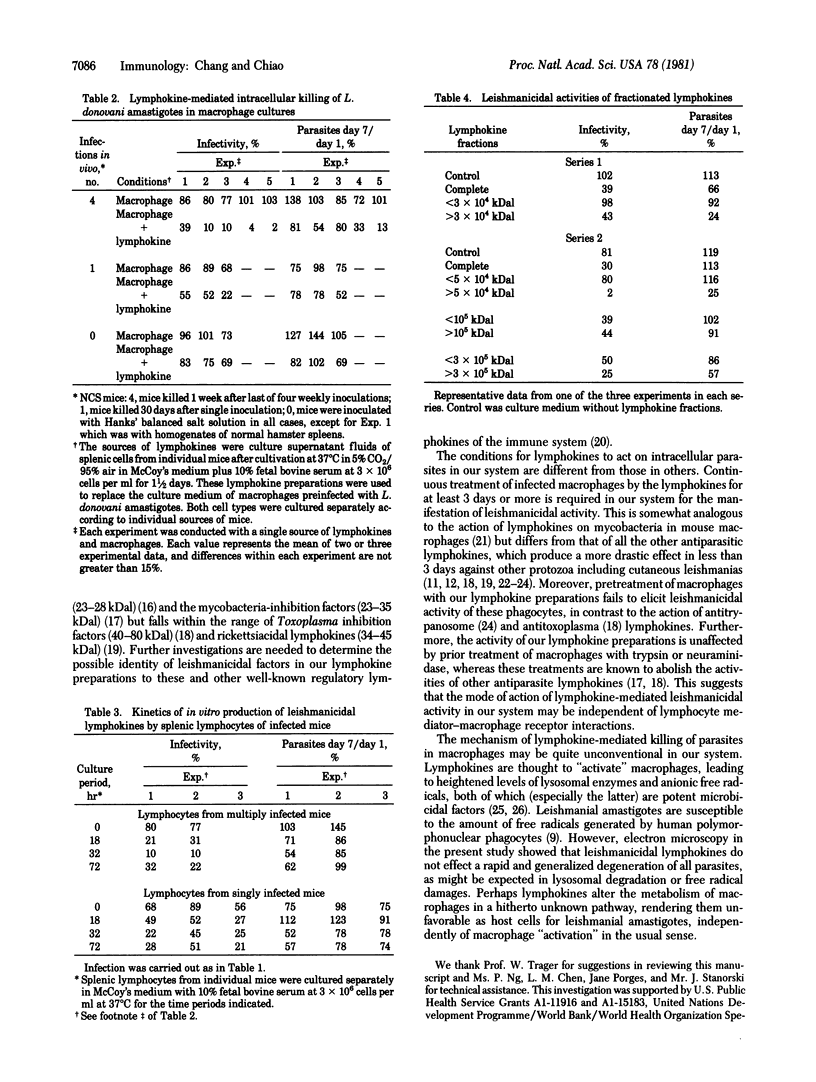
Abstract
Leishmania donovani, an intracellular protozoan, causes kala-azar by parasitizing the macrophages of its mammalian host. Outbred NCS and CD-1 mice develop immunity to this parasite. This immunity was demonstrable when supernatant fluids from cultured splenic lymphocytes were added to infected macrophages. Only the lymphokine preparations from infected mice showed significant leishmanicidal activity. Mice receiving multiple inocula were more potent producers of leishmanicidal lymphokines than were those receiving single inocula. The expression of leishmanicidal activity in our system required continuous presence of the lymphokine preparation and was independent of trypsin- or neuraminidase-sensitive receptors of the macrophages. Light and electron microscopy revealed that, in the presence of lymphokines, macrophages appeared to be "activated," and intracellular leishmanias developed specific subcellular lesions in the kinetoplast-mitochondria. A time-course study showed that cultivation of the lymphocytes for 1 1/2 days completed the release of their leishmanicidal lymphokines which were heat-labile molecules larger than 50,000 daltons.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyum A. Separation of blood leucocytes, granulocytes and lymphocytes. Tissue Antigens. 1974;4(4):269–274. [PubMed] [Google Scholar]
- Buchmüller Y., Mauel J. Studies on the mechanisms of macrophage activation. II. Parasite destruction in macrophages activated by supernates from concanavalin A-stimulated lymphocytes. J Exp Med. 1979 Aug 1;150(2):359–370. doi: 10.1084/jem.150.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess A. W., Camakaris J., Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem. 1977 Mar 25;252(6):1998–2003. [PubMed] [Google Scholar]
- Cahall D. L., Youmans G. P. Conditions for production, and some characteristics, of mycobacterial growth inhibitory factor produced by spleen cells from mice immunized with viable cells of the attenuated H37Ra strain of Mycobacterium tuberculosis. Infect Immun. 1975 Oct;12(4):833–840. doi: 10.1128/iai.12.4.833-840.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahall D. L., Youmans G. P. Macrophage migration inhibitory activity of mycobacterial growth inhibitory factor and the effect of a number of factors on mycobacterial growth inhibitory factor activity. Infect Immun. 1975 Oct;12(4):851–857. doi: 10.1128/iai.12.4.851-857.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahall D. L., Youmans G. P. Molecular weight and other characteristics of mycobacterial growth inhibitory factor produced by spleen cells obtained from mice immunized with viable attenuated mycobacterial cells. Infect Immun. 1975 Oct;12(4):841–850. doi: 10.1128/iai.12.4.841-850.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. P. Antibody-mediated inhibition of phagocytosis in Leishmania donovani-human phagocyte interactions in vitro. Am J Trop Med Hyg. 1981 Mar;30(2):334–339. doi: 10.4269/ajtmh.1981.30.334. [DOI] [PubMed] [Google Scholar]
- Chang K. P., Dwyer D. M. Leishmania donovani. Hamster macrophage interactions in vitro: cell entry, intracellular survival, and multiplication of amastigotes. J Exp Med. 1978 Feb 1;147(2):515–530. doi: 10.1084/jem.147.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. P. Leishmanicidal mechanisms of human polymorphonuclear phagocytes. Am J Trop Med Hyg. 1981 Mar;30(2):322–333. doi: 10.4269/ajtmh.1981.30.322. [DOI] [PubMed] [Google Scholar]
- Dumonde D. C., Wolstencroft R. A., Panayi G. S., Matthew M., Morley J., Howson W. T. "Lymphokines": non-antibody mediators of cellular immunity generated by lymphocyte activation. Nature. 1969 Oct 4;224(5214):38–42. doi: 10.1038/224038a0. [DOI] [PubMed] [Google Scholar]
- Handman E., Burgess A. W. Stimulation by granulocyte-macrophage colony-stimulating factor of Leishmania tropica killing by macrophages. J Immunol. 1979 Mar;122(3):1134–1137. [PubMed] [Google Scholar]
- Herman R. Cytophilic and opsonic antibodies in visceral leishmaniasis in mice. Infect Immun. 1980 May;28(2):585–593. doi: 10.1128/iai.28.2.585-593.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Masur H., Len L., Fu T. L. Lymphocyte-macrophage interaction during control of intracellular parasitism. Am J Trop Med Hyg. 1977 Nov;26(6 Pt 2):187–193. doi: 10.4269/ajtmh.1977.26.187. [DOI] [PubMed] [Google Scholar]
- Marsden P. D. Current concepts in parasitology. Leishmaniasis. N Engl J Med. 1979 Feb 15;300(7):350–352. doi: 10.1056/NEJM197902153000706. [DOI] [PubMed] [Google Scholar]
- Mauel J., Behin R. Cell-mediated and humoral immunity to protozoan infections. Transplant Rev. 1974;19(0):121–146. doi: 10.1111/j.1600-065x.1974.tb00130.x. [DOI] [PubMed] [Google Scholar]
- Miller H. C., Twohy D. W. Cellular immunity to Leishmania donovani in macrophages in cultures. J Parasitol. 1969 Feb;55(1):200–207. [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. I. Susceptibility of Toxoplasma gondii to oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):938–949. doi: 10.1084/jem.150.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacy C. A., Leonard E. J., Meltzer M. S. Macrophages in resistance to rickettsial infections: characterization of lymphokines that induce rickettsiacidal activity in macrophages. J Immunol. 1981 Jan;126(1):204–207. [PubMed] [Google Scholar]
- Nathan C., Nogueira N., Juangbhanich C., Ellis J., Cohn Z. Activation of macrophages in vivo and in vitro. Correlation between hydrogen peroxide release and killing of Trypanosoma cruzi. J Exp Med. 1979 May 1;149(5):1056–1068. doi: 10.1084/jem.149.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. A. Trypanosoma cruzi: in vitro induction of macrophage microbicidal activity. J Exp Med. 1978 Jul 1;148(1):288–300. doi: 10.1084/jem.148.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi K. K., Pelster B., Suzuki N., Piekarski G., Brandis H. Immunity to Toxoplasma gondii induced in vitro in non-immune mouse macrophages with specifically immune lymphocytes. J Immunol. 1975 Oct;115(4):1151–1158. [PubMed] [Google Scholar]
- Shirahata T., Shimizu K., Noda S., Suzuki N. Studies on production of biologically active substance which inhibits the intracellular multiplication of Toxoplasma within mouse macrophages. Z Parasitenkd. 1977 Aug 25;53(1):31–40. doi: 10.1007/BF00383112. [DOI] [PubMed] [Google Scholar]
- Skov C. B., Twohy D. W. Cellular immunity to Leishmania donovani. I. The effect of T cell depletion on resistance to L. donovani in mice. J Immunol. 1974 Dec;113(6):2004–2011. [PubMed] [Google Scholar]
- Skov C. B., Twohy D. W. Cellular immunity to Leishmania donovani. II. Evidence for synergy between thymocytes and lymph node cells in reconstitution of acquired resistance to L. donovani in mice. J Immunol. 1974 Dec;113(6):2012–2019. [PubMed] [Google Scholar]