Abstract
The authors assessed the association between cognitive function and incidence and maintenance of overweight in preschool children. A population-based birth cohort was established in Menorca, Spain, between 1997 and 1999 (n = 482). Body mass index (weight (kg)/height (m)2) was measured at ages 4 years and 6 years (n = 421). At age 4 years, children were assessed for cognitive function (McCarthy Scales of Children's Abilities) (n = 395). After multivariable adjustment for a wide range of factors, including maternal education and body mass index, children with higher general cognition at age 4 years had a lower likelihood of being overweight (odds ratio = 0.47, 95% confidence interval (CI): 0.25, 0.88) at age 6 years. Children with higher general cognition at age 4 years had a lower likelihood of maintaining an unhealthy weight status (being at risk of overweight or overweight) between ages 4 years and 6 years, as well as worsening weight status over time, than children who maintained a healthy weight (odds ratios were 0.78 (95% CI: 0.54, 1.14) and 0.77 (95% CI: 0.51, 1.14), respectively). When specific dimensions of cognitive function were assessed, associations were mainly found for verbal and executive function areas. Children with higher cognitive function in early life might be at decreased risk of overweight later in childhood.
Keywords: body weight; child development; child, preschool; cognition; cognitive science; intelligence; obesity; overweight
The prevalence of childhood overweight has increased considerably within the last 20 years in nearly all developed countries (1). Overweight in childhood has been associated with the long-term risk of health problems such as metabolic syndrome, hypertension, diabetes, cardiovascular disease, and cancer (2). There is growing evidence of a possible association between overweight and poor cognitive function (3–10). Several mechanisms have been proposed to explain this relation. It has been suggested that poor control of neural centers related to impulsivity and/or addiction could foster impaired control of food intake, leading to overeating and subsequent overweight (11). Alternatively, physiologic brain changes caused by overweight—such as subclinical inflammatory changes, vascular changes, or dysmyelinization of white matter—could impair general cognitive function or performance in some cognitive areas (12, 13). Moreover, common genetic, environmental, or biologic factors could also play a role in the development of both phenotypes.
Previous studies have shown a relation between overweight and intelligence quotient or poor general cognitive performance, as well as relations with a variety of cognitive domains, including executive function, memory, verbal and motor abilities, and attention at different ages (3–10, 14–29). Most of the literature on this topic is based on cross-sectional studies, which limits our ability to assess the potential causality of this relation. In one longitudinal study, both rapid and slower growth during the first years of life were related to lower scores on cognitive tests in early childhood (6). In another study, men who were overweight earlier in adulthood had poorer cognitive performance during middle age and the elderly years (4). Conversely, investigators in several studies reported an association between lower childhood intelligence scores and higher adult body mass index (BMI) (15, 23), although these effects were attenuated after adjustment for educational attainment. In another study, Halkjaer et al. (20) found that intelligence assessed between ages 18 years and 24 years was negatively associated with the incidence of obesity some years later, with no association with the risk of remaining obese, after adjustment for educational level. Nevertheless, to our knowledge, no studies have yet assessed the possible influence of early cognitive function on the risk of subsequent overweight in early childhood.
In the present study, we examined the relation between early cognitive function and subsequent overweight, using data from a birth cohort study. We first assessed the longitudinal association between cognitive function at age 4 years and weight status at age 6 years, taking into account the possible influence of earlier weight status on this relation. Next, we assessed the association between cognitive function at age 4 years and changes in weight status between ages 4 years and 6 years.
MATERIALS AND METHODS
Study participants
This study was based on a population-based birth cohort from Menorca, a Balearic island in the northwestern Mediterranean Sea in Spain. The cohort was established within the Asthma Multicenter Infants Cohort Study (30). All women seeking antenatal care over a 12-month period starting in mid-1997 were recruited; they were then followed periodically until their children were 6 years of age. A total of 482 children were enrolled at birth, and 421 (87.3%) were followed through the age 6 visit. Cognitive function was assessed at age 4 years, and these data were available for 395 children (93.8%).
A flood in 2001 damaged some records, including weight and height measurements taken at age 4 years; as a result, anthropometric data were available for only 261 children in this wave (66% of the children with cognitive function data). No differences in BMI at age 6 years, parental characteristics (including education, age, and smoking status), or child characteristics at birth (including birth weight, height, and sex) were found between children with anthropometric data at age 4 years and those without these data (P > 0.20). However, children missing data on BMI at age 4 years had a lower mean score on the general cognitive function test (96 points vs. 99 points) (P < 0.05). Since this precluded the possibility that data were missing completely at random, exclusion of participants without anthropometric measures (complete-case analysis) may have led to bias (31). Therefore, multiple imputation of missing values, which is a widely accepted method that provides correct inferences under weaker assumptions, was performed (see “Statistical analysis” subsection). Underweight children (n = 7) were excluded from the analysis because of the small number of observations and because of the strong differences observed between these children and others with regard to several characteristics.
Informed consent was obtained from all mothers. The study protocol was approved by a local ethics committee and complied with the Declaration of Helsinki.
Study variables
Weight and height were measured with calibrated scales and a wall-mounted stadiometer, respectively, with the children standing in light clothing and barefoot, by 1 trained field worker at ages 4 years and 6 years. BMI was calculated as weight in kilograms divided by the square of height in meters. Using standard procedures, age- and sex-specific BMI z scores were obtained for each child based on how his/her BMI value compared with the referent population of the US National Center for Health Statistics (32). Centers for Disease Control and Prevention software (“NutStat” application, available at www.cdc.gov/epiinfo/) was used to derive z scores based on each child's exact age in 1-month intervals. As recommended, the underweight category was defined as a BMI z score below the 5th percentile of the referent population, healthy weight as a BMI z score between the 5th and 84th percentiles, being at risk of overweight as a BMI z score between the 85th and 94th percentiles, and overweight as a BMI z score greater than or equal to the 95th percentile.
Neuropsychological testing at age 4 years, including assessment of intellectual and motor abilities, was conducted by trained psychologists using the Spanish version of the McCarthy Scales of Children's Abilities (MCSA) (33). The MCSA includes a general cognitive score and 5 conventional sub-area scores: 1) verbal, which refers to those cognitive tasks related to any kind of verbal information processing; 2) quantitative, which takes into account numerical abilities; 3) memory, which includes short-time retention of information (verbal, perceptive, or numerical); 4) perceptive-performance, which refers to those cognitive tasks related to any kind of perceptive information processing, including manual performance; and 5) motor abilities, which includes both fine (i.e., drawing) and gross (i.e., playing with a ball) motor skills. To further improve our understanding, we restructured the MCSA subtests into a new outcome measure, the executive function subscale, incorporating tasks highly associated with this specific neurocognitive function (i.e., the underlying construct between the relation of a brain function to a specific behavior). Executive functions are unique aspects of human cognition because they modulate all other cognitive functions. For example, these functions are necessary for learning new information or for learning and retrieval strategies and are solicited to adapt to new testing situations. In summary, these functions refer to those cognitive tasks critical to nonroutine, goal-oriented situations that are performed by the prefrontal cortex (34, 35). The executive function subscale was created incorporating MCSA subtests 2 (“puzzle solving”), 5 (“number questions”), 6 (“taping sequence”), 14II (“numerical memory”), 15 (“verbal fluency”), 17 (“opposite analogies”), and 18 (“conceptual grouping”), in accordance with the standards of present neuropsychological assessments (36, 37). This new subscale showed good psychometric properties (38).
Information on maternal and paternal education in years, maternal and paternal social class (using the United Kingdom Registrar General's 1990 classification according to parental occupation, by 1988 International Standard Classification of Occupations code (http://www.ilo.org/public/english/bureau/stat/isco/isco88/index.htm)), maternal and paternal age at child's birth, maternal health and obstetric history, maternal height, prepregnancy weight and BMI, maternal tobacco and alcohol use during pregnancy, maternal diet during pregnancy, number of siblings at child's birth, and child's sex was collected after delivery. In subsequent interviews, data were collected on type and duration of breastfeeding, marital status, maternal tobacco consumption, and child's diet at ages 4 years and 6 years. All questionnaires were administered face to face by trained interviewers. Additionally, information relating to the child's gestational age and anthropometric measurements taken at birth was collected from clinical records.
Statistical analysis
Multiple imputation of missing values using chained equations was performed, where 10 completed data sets were generated and analyzed separately, and the results were combined using the standard combination rules for multiple imputation (39). This procedure accounts for the uncertainty added due to the use of imputations.
BMI was treated as a categorical outcome variable (healthy weight/at risk of overweight/overweight). The MCSA scores were treated as continuous variables and were standardized to a mean of 100 points with a standard deviation of 15 to homogenize all of the scales. Associations are reported for an increase equivalent to a 1-standard-deviation change in each cognitive test score.
Prior to model-fitting, we assessed the linearity of the associations between cognitive function scores, maternal prepregnancy BMI, birth weight, birth height, BMI z score at age 4 years, and BMI z score at age 6 years using generalized additive models. All variables showed a linear association except BMI z score at age 4 years, which had a quadratic relation with BMI z score at age 6 years (for gain of linearity, P < 0.001); this reflected the fact that this age is the period of adiposity rebound, during which BMI gains are normative (40). To fully adjust for earlier BMI, we included both BMI z score at age 4 years and BMI z score2 at age 4 years in the models.
Odds ratios for the association between cognitive function at age 4 years and BMI group at age 6 years were assessed using polytomous logistic regression; the reference group was children with a healthy weight. Models were adjusted for the following variables, selected a priori on the basis of previous studies (38, 41, 42): child's exact age, schooling level (grade and trimester) at neuropsychological test administration, psychologist, child's sex, birth weight and height, gestational age, maternal smoking during pregnancy, maternal education, maternal age, prepregnancy height and BMI, breastfeeding, maternal smoking at child's age 4 years and number of siblings at child's age 4 years, child's consumption of sweetened beverages, sweets, and meat at age 4 years, and BMI z score and BMI z score2 at age 4 years.
In order to assess the associations between cognitive function scores at age 4 years and changes in BMI category between ages 4 years and 6 years, we cross-classified children into 4 groups to identify those who: 1) maintained a healthy weight at both ages; 2) maintained an unhealthy weight at both ages (at risk of becoming overweight or overweight); 3) worsened their weight status between ages 4 years and 6 years (shifted from healthy weight to being overweight or at risk of overweight, or from being at risk of overweight to overweight); and 4) improved their weight status between both ages (shifted from being overweight or at risk of overweight to a healthy weight, or from being overweight to being at risk of overweight). Polytomous logistic regression models were fitted; the reference group was those children who maintained a healthy weight status at both ages. Covariates in these models were the same as those used to analyze BMI groups at age 6 years separately.
Finally, as a complementary analysis to separately evaluate associations between cognitive function and weight status at ages 4 years and 6 years, path analysis was used to examine the role of BMI at age 4 years as an intermediate variable in the association between cognitive function at age 4 years and BMI at age 6 years, taking into account the same variables as those used in previous polytomous logistic regression models.
Statistical analyses were conducted using STATA 10.1 (Stata Corporation, College Station, Texas) and SAS 9.1 (path analysis using PROC CALIS; SAS Institute Inc., Cary, North Carolina).
RESULTS
The distributions of child and maternal anthropometric variables and child cognitive function scores are shown in Table 1. At age 6 years, the median BMI was 16.2 (range, 13.5–27.3), and the general cognitive function score at age 4 years had a median of 99 points (range, 53–142).
Table 1.
Distribution of Child and Maternal Anthropometric Variables and Child's Cognitive Function Test Scores in the Menorca Cohort (n = 388), Spain, 1997–2003
| Minimum | 25th Percentile | Median | 75th Percentile | Maximum | Mean | |
| Anthropometric variables | ||||||
| Body mass indexa at age 6 years | 13.5 | 15.2 | 16.2 | 17.7 | 27.3 | 16.7 (2.1)b |
| Body mass index at age 4 years | 12.6 | 15.2 | 16.1 | 16.9 | 21.5 | 16.2 (1.5) |
| Birth weight, g | 780 | 2,905 | 3,200 | 3,535 | 4,880 | 3,189 (503) |
| Birth height, cm | 35 | 48 | 49 | 50 | 55 | 49 (2) |
| Maternal prepregnancy body mass index | 15.3 | 20.6 | 22.0 | 24.4 | 48.5 | 22.8 (3.7) |
| Cognitive function score at age 4 years | ||||||
| General cognitive | 53 | 89 | 99 | 108 | 142 | 100 (15) |
| Verbal | 52 | 90 | 99 | 109 | 140 | 100 (15) |
| Executive function | 54 | 89 | 100 | 109 | 152 | 100 (15) |
| Quantitative | 71 | 90 | 96 | 105 | 154 | 100 (15) |
| Memory | 65 | 91 | 99 | 107 | 142 | 100 (15) |
| Perceptive-performance | 58 | 91 | 98 | 109 | 138 | 100 (15) |
| Motor skills | 39 | 90 | 99 | 110 | 143 | 100 (15) |
Weight (kg)/height (m)2.
Numbers in parentheses, standard deviation.
Overall, 16.7% of children at age 6 years were at risk of becoming overweight and 11.9% were overweight (Table 2). Children who were overweight at age 6 years had lower mean general cognitive, verbal skills, executive function, and quantitative scores at age 4 years (P < 0.05). Mean memory, perceptual performance, and motor skills scores also decreased with worsening weight status, although differences were not statistically significant. However, cognitive function scores at age 4 years were not associated with BMI at age 4 years (P > 0.30). This was observed in spite of a strong association between BMI z score and BMI z score2 at age 4 years with BMI z score at age 6 years (β = 0.62 (95% confidence interval (CI): 0.54, 0.70; P < 0.001) and β = 0.09 (95% CI: 0.03, 0.14; P = 0.001), respectively).
Table 2.
Cognitive Function (Mean Test Score) at Age 4 Years According to Body Mass Index Group at Age 6 Years in the Menorca Cohort (n = 388), Spain, 1997–2003
| Cognitive Function Sub-Area | Body Mass Indexa Group at Age 6 Years |
P Value for Linear Trendb | |||||
| Healthy Weight (n = 277) |
At Risk of Overweight (n = 65) |
Overweight (n = 46) |
|||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | ||
| General cognitive | 95 | 91, 98 | 94 | 89, 99 | 88 | 83, 93 | 0.007* |
| Verbal | 97 | 93, 101 | 96 | 91, 101 | 90 | 84, 95 | 0.007* |
| Executive function | 98 | 94, 101 | 98 | 93, 103 | 90 | 85, 95 | 0.007** |
| Quantitative | 95 | 91, 99 | 97 | 92, 101 | 89 | 84, 94 | 0.048* |
| Memory | 94 | 90, 98 | 96 | 91, 101 | 90 | 85, 96 | 0.286 |
| Perceptual-performance | 94 | 91, 98 | 93 | 88, 98 | 90 | 85, 95 | 0.084 |
| Motor skills | 93 | 89, 96 | 90 | 86, 95 | 90 | 85, 95 | 0.195 |
P < 0.010;
P < 0.001 (comparison between overweight group and healthy weight group).
Weight (kg)/height (m)2.
P value for the 3 categories of body mass index treated as a continuous variable, adjusted for age, school grade, and psychologist, in a linear regression model.
Higher general cognitive function score at age 4 years was associated with reduced odds of being overweight at age 6 years (Table 3, model 1). This association was strengthened after adjustment for BMI at age 4 years (model 2) and after adjustment for covariates, including maternal prepregnancy BMI, children's dietary intakes, and earlier feeding practices (model 3). Among the different cognitive sub-areas, verbal abilities, executive function, quantitative, and memory skills scores maintained strong and significant associations with overweight at age 6 years. All analyses were rerun after excluding infants not born at term (<37 weeks or >42 weeks) (n = 24), with similar results (data not shown).
Table 3.
Associationa Between Cognitive Function at Age 4 Years and Body Mass Index Group at Age 6 Years in the Menorca Cohort (n = 388), Spain, 1997–2003
| Cognitive Function Sub-Area and Model | Body Mass Indexb Group at Age 6 Years |
|||||
| At Risk of Overweight |
Overweight |
|||||
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| General cognitive | ||||||
| Model 1c | 0.96 | 0.72, 1.28 | 0.797 | 0.59 | 0.42, 0.83 | 0.003 |
| Model 2d | 0.85 | 0.60, 1.20 | 0.366 | 0.50 | 0.30, 0.84 | 0.009 |
| Model 3e | 0.87 | 0.59, 1.28 | 0.473 | 0.47 | 0.25, 0.88 | 0.019 |
| Verbal | ||||||
| Model 1 | 0.96 | 0.72, 1.26 | 0.752 | 0.61 | 0.45, 0.85 | 0.003 |
| Model 2 | 0.84 | 0.59, 1.19 | 0.322 | 0.48 | 0.28, 0.83 | 0.010 |
| Model 3 | 0.80 | 0.54, 1.20 | 0.282 | 0.43 | 0.22, 0.83 | 0.013 |
| Executive function | ||||||
| Model 1 | 1.03 | 0.78, 1.37 | 0.816 | 0.56 | 0.40, 0.80 | 0.001 |
| Model 2 | 0.93 | 0.66, 1.30 | 0.668 | 0.47 | 0.28, 0.81 | 0.006 |
| Model 3 | 0.92 | 0.63, 1.34 | 0.683 | 0.44 | 0.23, 0.84 | 0.013 |
| Quantitative | ||||||
| Model 1 | 1.10 | 0.84, 1.45 | 0.478 | 0.56 | 0.37, 0.86 | 0.008 |
| Model 2 | 1.01 | 0.73, 1.39 | 0.964 | 0.54 | 0.32, 0.91 | 0.022 |
| Model 3 | 1.06 | 0.75, 1.51 | 0.724 | 0.50 | 0.26, 0.97 | 0.042 |
| Memory | ||||||
| Model 1 | 1.15 | 0.88, 1.51 | 0.314 | 0.75 | 0.53, 1.04 | 0.085 |
| Model 2 | 1.03 | 0.75, 1.43 | 0.834 | 0.61 | 0.37, 0.99 | 0.048 |
| Model 3 | 1.04 | 0.74, 1.47 | 0.827 | 0.60 | 0.35, 1.03 | 0.035 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Odds ratio for a 1-standard-deviation increase in test score in polytomous logistic regression modeling. The reference group was children with a healthy weight at age 6 years.
Weight (kg)/height (m)2.
Adjusted for age, school grade, and psychologist.
Adjusted for the variables in model 1 plus body mass index z score at age 4 years and body mass index z score2 at age 4 years.
Adjusted for the variables in model 2 plus child's sex, birth weight and height, gestational age, maternal smoking during pregnancy, maternal education, maternal age, prepregnancy height and body mass index, breastfeeding, maternal smoking at child's age 4 years, number of siblings at child's age 4 years, and child's consumption of sweetened beverages, sweets, and meat at age 4 years.
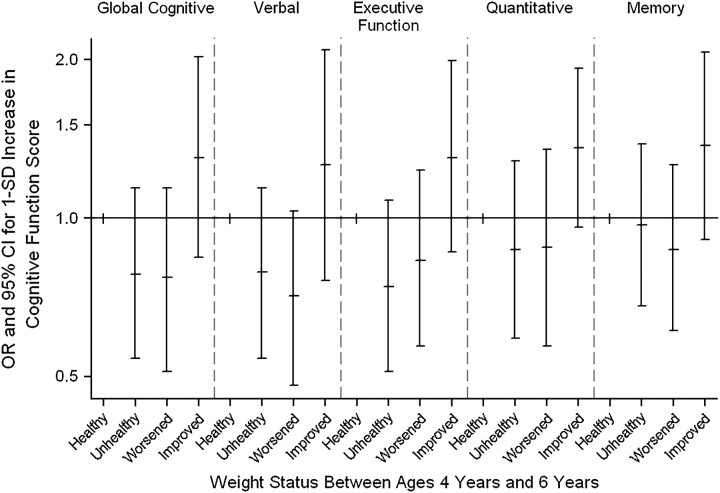
Children with higher general cognitive, verbal, and executive function scores at age 4 years had a lower likelihood of maintaining an unhealthy weight status (at risk of overweight or overweight) between ages 4 years and 6 years, as well as of worsening their weight status over time (incidence of being at risk of overweight or overweight), compared with children who maintained a healthy weight (odds ratio = 0.78, 95% CI: 0.54, 1.14, and odds ratio = 0.77, 95% CI: 0.51, 1.14, respectively) (Figure 1). In contrast, children with higher general cognitive, verbal, executive function, quantitative, and memory scores had a higher likelihood of improving their weight status between both ages than children who maintained a healthy weight status.
Figure 1.
Association between cognitive function at age 4 years (1-standard-deviation (SD) change in cognitive test score) and change in weight status between ages 4 years and 6 years in the Menorca Cohort, Spain, 1997–2003. The reference group (“Healthy”) was children who maintained a healthy weight status between ages 4 years and 6 years. “Unhealthy” children were those who maintained an unhealthy weight status between ages 4 years and 6 years (were at risk of becoming overweight or were overweight). Children in the “Worsened” group were those whose weight status worsened between ages 4 years and 6 years (shifted from healthy weight to at risk of overweight or overweight, or from at risk of overweight to overweight). Children in the “Improved” group were those whose weight status improved between ages 4 years and 6 years (shifted from overweight or at risk of overweight to healthy weight, or from overweight to at risk of overweight). Odds ratios (ORs) were adjusted for age, school grade, psychologist, body mass index (weight (kg)/height (m)2) z score at age 4 years, body mass index z score2 at age 4 years, child's sex, birth weight and height, gestational age, maternal smoking during pregnancy, maternal education, maternal age, prepregnancy height and body mass index, breastfeeding, maternal smoking at child's age 4 years, number of siblings at child's age 4 years, and child's consumption of sweetened beverages, sweets, and meat at age 4 years. Bars, 95% confidence interval (CI).
In path analysis modeling, relations between cognitive function scores at age 4 years and BMI at age 6 years remained similar. BMI at age 4 years was positively associated with BMI at age 6 years. Paths linking BMI at age 4 years to cognitive function scores at age 4 years did not reach significance.
DISCUSSION
The present study is, to our knowledge, the first to have assessed associations between early-life cognitive function scores and overweight in late childhood. Higher general cognitive abilities at age 4 years, particularly executive function, verbal, quantitative, and memory skills scores, were associated with a lower likelihood of being overweight at age 6 years, after adjustment for a large list of covariates, including socioeconomic factors and maternal BMI. Interestingly, despite the strong association observed between cognitive scores at age 4 years and weight status at age 6 years, we did not observe a cross-sectional relation between cognitive function scores and concurrent measures of BMI at age 4 years. Nonetheless, although this suggests that cognitive abilities may influence the development of overweight, we cannot rule out the possibility that this pattern of associations is not due in part to the lower variability in BMI data at age 4 years. Further study using prospective data is needed to gain additional insights on these relations.
Previous studies on obesity and cognitive function conducted during childhood have shown mixed results (3–10, 14–29), but most suffered from 1 or more of the following limitations: a cross-sectional rather than longitudinal approach; lack of adjustment for relevant confounders such as maternal education, smoking, or prepregnancy BMI; the use of raw BMI data rather than z scores or percentiles, which take into account changes in BMI associated with growth and maturation; or the use of raw weight data, which also fails to adequately account for the variable changes in height which occur at different stages of growth and maturation. Our results are based on a birth cohort study with appropriate measures of BMI, assessing child cognitive function with validated scales and incorporating multivariable adjustment for a large array of key factors. Although recognized as an imperfect measure of adiposity, BMI has been established to correlate well with more direct measures of body fat in children as well as in adults (43). Cutoffs used to identify overweight children have been shown to be highly specific to children with high levels of adiposity (44).
A possible explanation for the negative association observed between cognitive dimensions such as executive function at age 4 years and overweight 2 years later may involve the pathophysiology of both the cognitive function and food intake control systems. Executive function, a behavioral marker of prefrontal functioning, encompasses a number of different neuropsychological concepts, including inhibitory control, attention control, working memory, cognitive flexibility, goal setting, and problem solving (36, 37). Thus, executive function could influence the ability to maintain energy balance and control eating impulses. Animal models suggest that overeating may be in part an addiction, where foods may be conceived of as natural drugs (45), especially highly palatable foods (46). Common neural substrates, such as dopamine circuits, underlie both food and drug intake (47). Some neuropsychological studies have shown that substance-dependent persons have impairments in cognitive function, specifically executive control tasks (48). Similar to our findings, Cserjési et al. (16) found that obese children aged 12 years had worse cognitive flexibility and shifting ability than those of normal weight. In another study, Gunstad et al. (5) showed that overweight/obese middle-aged adults had reduced executive function performance compared with those of normal weight. The association with executive function is also consistent with a recent report by Davis et al. (17) showing that young adults with poor decision-making skills tended to have higher BMIs than those who made better decisions. However, given that food choices in young children are made by parents, in contrast to the self-feeding practiced by older children and adults, it is less clear to what extent mechanisms related to poor self-regulation influence excess intakes in young children. Nevertheless, since childhood is a critical period for executive function maturation (36, 49), these results suggest that special attention should be paid to factors influencing this process of maturation given its possible relation with the development of conditions such as overweight.
We also observed a strong association between higher verbal abilities assessed at age 4 years and decreased odds of overweight at age 6 years. The MCSA verbal score is a proxy for crystallized intelligence. Crystallized intelligence depends on education, involves stimuli and concepts available to members of a cultural group, and is related to factual information (36). A number of cross-sectional studies have assessed the relation between this cognitive area and various measures of weight status at different ages, with inconsistent results. Cawley et al. (3) found that obesity was associated with reduced verbal skills in early childhood (age 2–3 years). An earlier cross-sectional study of children at age 5 years showed an unadjusted negative relation between crystallized intelligence and BMI (14). In contrast, another cross-sectional study found no relation between elevated BMI and scores on a test of intellectual function in children and adolescents (19). However, in another study, the same authors showed that obese persons aged 21–82 years had poorer verbal memory performance (18). Finally, Richards et al. (8) found that weight was not associated with verbal intelligence at ages 8 years and 15 years but was negatively associated with verbal ability at age 26 years and with verbal memory at age 43 years, all assessed using cross-sectional data.
Another explanation for the associations observed could involve common environmental factors, such as time spent watching television, leading to both poor cognitive function (50, 51) and overweight (52–54). In our study, we found time spent watching television (more than 2 hours per day) to be significantly associated with both increased overweight and lower cognitive function. However, adjusting for television watching did not attenuate the associations observed between cognitive function at age 4 years and subsequent overweight at age 6 years (data not shown). Further studies exploring the possible mediating role of television in this relation should be carried out.
A limitation of our study is that parental intelligence, mental health, or problematic family functioning was not evaluated; these factors are known to be related to cognitive function, type of diet, and overweight in young children (55, 56). Inclusion of maternal educational level and social class probably reduced any confounding by these factors but may not have eliminated residual confounding. A second limitation was the loss of nearly 40% of the weight and height data at age 4 years, which could have introduced selection bias. There were no differences in either anthropometric measures at age 6 years or socioeconomic variables between children with BMI data at age 4 years and those without it, and analyses including all children produced findings consistent with those obtained using multiple imputation. Another limitation of this study was the absence of several BMI data points between ages 4 years and 6 years, a phase of very rapid child development and weight and height change (40). BMI z scores were used to classify children's weight status, taking into account the normative changes in BMI experienced by children during this period. Nonetheless, it is possible that some children experienced important changes in weight or height that were not observed at the time points measured. Furthermore, information about weight gain during the first months of life was not recorded; rapid gains during this period have been associated with both greater childhood overweight and poor cognitive performance (6, 57). This suggests that rather than a causal relation between cognition and risk of overweight, there may be common underlying etiologies for both rapid growth leading to obesity and poorer neurodevelopment. Nonetheless, while we were unable to fully explore this issue, we were able to adjust for both birth weight and BMI at age 4 years as proxy measures for early growth; these factors did not appear to explain the associations observed. Similarly, adjusting for maternal smoking in pregnancy, another factor associated with both poor cognitive function and overweight in offspring (38, 58, 59), did not appear to explain the relation between child cognition and obesity.
In conclusion, this study found that higher cognitive function at age 4 years, specifically executive function and verbal skills, was associated with decrease risk of being overweight at age 6 years. Although significant associations have been found after adjustment for a large number of potential confounders, further longitudinal studies are required to understand the mechanisms of this association, including the possible role of genetic or other common etiologic factors.
Acknowledgments
Author affiliations: Centre for Research in Environmental Epidemiology (CREAL), Barcelona, Spain (Mònica Guxens, Michelle A. Mendez, Jordi Julvez, Estel Plana, Joan Forns, Xavier Basagaña, Jordi Sunyer); Municipal Institute of Medical Research (IMIM–Hospital del Mar), Barcelona, Spain (Mònica Guxens, Michelle A. Mendez, Jordi Julvez, Estel Plana, Joan Forns, Xavier Basagaña, Jordi Sunyer); CIBER Epidemiología y Salud Pública (CIBERESP), Barcelona, Spain (Mònica Guxens, Michelle A. Mendez, Jordi Julvez, Estel Plana, Joan Forns, Xavier Basagaña, Jordi Sunyer); Àrea de Salut de Menorca, Servei de Salut de les Illes Balears, Menorca, Spain (Maties Torrent); and Universitat Pompeu Fabra, Barcelona, Spain (Jordi Sunyer).
This work was supported by the Spanish Ministry of Health (Instituto de Salud Carlos III (Red INMA grant G03/176 and Red RCESP grant C03/09)) and Fundació ‘La Caixa’ (grant 00/077-00).
The authors gratefully acknowledge Victoria Estraña and Mireia Garcia for their assistance in contacting the families and administering the questionnaires.
Conflict of interest: none declared.
Glossary
Abbreviations
- BMI
body mass index
- CI
confidence interval
- MCSA
McCarthy Scales of Children's Abilities
References
- 1.Wang Y, Lobestein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes. 2006;1(1):11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- 2.Aggoun Y. Obesity, metabolic syndrome, and cardiovascular disease. Pediatr Res. 2007;61(6):653–659. doi: 10.1203/pdr.0b013e31805d8a8c. [DOI] [PubMed] [Google Scholar]
- 3.Cawley J, Spiess CK. Obesity and skill attainment in early childhood. Econ Hum Biol. 2008;6(3):388–397. doi: 10.1016/j.ehb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Elias MF, Elias PK, Sullivan LM, et al. Lower cognitive function in the presence of obesity and hypertension: the Framingham Heart Study. Int J Obes Relat Metab Disord. 2003;27(2):260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- 5.Gunstad J, Paul RH, Cohen RA, et al. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Compr Psychiatry. 2007;48(1):57–61. doi: 10.1016/j.comppsych.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Heinonen K, Räikkönen K, Pesonen AK, et al. Prenatal and postnatal growth and cognitive abilities at 56 months of age: a longitudinal study of infants born at term. Pediatrics. 2008;121(5):e1325–e1333. doi: 10.1542/peds.2007-1172. [DOI] [PubMed] [Google Scholar]
- 7.Li X. A study of intelligence and personality in children with simple obesity. Int J Obes Relat Metab Disord. 1995;19(5):355–357. [PubMed] [Google Scholar]
- 8.Richards M, Hardy R, Kuh D, et al. Birthweight, postnatal growth and cognitive function in a national UK birth cohort. Int J Epidemiol. 2002;31(2):342–348. [PubMed] [Google Scholar]
- 9.Silva A, Metha Z, O'Callaghan FJ. The relative effect of size at birth, postnatal growth and social factors on cognitive function in late childhood. Ann Epidemiol. 2006;16(6):469–476. doi: 10.1016/j.annepidem.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Dai Q, Jackson JC, et al. Overweight is associated with decreased cognitive functioning among school-age children and adolescents. Obesity (Silver Spring) 2008;16(8):1809–1815. doi: 10.1038/oby.2008.296. [DOI] [PubMed] [Google Scholar]
- 11.Cortese S, Angriman M, Maffeis C, et al. Attention-deficit/hyperactivity disorder (ADHD) and obesity: a systematic review of the literature. Crit Rev Food Sci Nutr. 2008;48(6):524–537. doi: 10.1080/10408390701540124. [DOI] [PubMed] [Google Scholar]
- 12.Gustafson D, Rothenberg E, Blennow K, et al. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163(13):1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 13.Gustafson D, Lissner L, Bengtsson C, et al. A 24-year follow-up of body mass index and cerebral atrophy. Neurology. 2004;63(10):1876–1881. doi: 10.1212/01.wnl.0000141850.47773.5f. [DOI] [PubMed] [Google Scholar]
- 14.Azurmendi A, Braza F, Sorozabal A, et al. Cognitive abilities, androgen levels, and body mass index in 5-year-old children. Horm Behav. 2005;48(2):187–195. doi: 10.1016/j.yhbeh.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Chandola T, Deary IJ, Blane D, et al. Childhood IQ in relation to obesity and weight gain in adult life: the National Child Development (1958) Study. Int J Obes (Lond) 2006;30(9):1422–1432. doi: 10.1038/sj.ijo.0803279. [DOI] [PubMed] [Google Scholar]
- 16.Cserjési R, Molnár D, Luminet O, et al. Is there any relationship between obesity and mental flexibility in children? Appetite. 2007;49(3):675–678. doi: 10.1016/j.appet.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Davis C, Levitan RD, Muglia P, et al. Decision-making deficits and overeating: a risk model for obesity. Obes Res. 2004;12(6):929–935. doi: 10.1038/oby.2004.113. [DOI] [PubMed] [Google Scholar]
- 18.Gunstad J, Paul RH, Cohen RA, et al. Obesity is associated with memory deficits in young and middle-aged adults. Eat Weight Disord. 2006;11(1):e15–e19. doi: 10.1007/BF03327747. [DOI] [PubMed] [Google Scholar]
- 19.Gunstad J, Spitznagel MB, Paul RH, et al. Body mass index and neuropsychological function in healthy children and adolescents. Appetite. 2008;50(2-3):246–251. doi: 10.1016/j.appet.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Halkjaer J, Holst C, Sørensen TI. Intelligence test score and educational level in relation to BMI changes and obesity. Obes Res. 2003;11(10):1238–1245. doi: 10.1038/oby.2003.170. [DOI] [PubMed] [Google Scholar]
- 21.Hart CL, Taylor MD, Smith GD, et al. Childhood IQ and cardiovascular disease in adulthood: prospective observational study linking the Scottish Mental Survey 1932 and the Midspan studies. Soc Sci Med. 2004;59(10):2131–2138. doi: 10.1016/j.socscimed.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Jeong SK, Nam HS, Son MH, et al. Interactive effect of obesity indexes on cognition. Dement Geriatr Cogn Disord. 2005;19(2-3):91–96. doi: 10.1159/000082659. [DOI] [PubMed] [Google Scholar]
- 23.Lawlor DA, Clark H, Davey Smith G, et al. Childhood intelligence, educational attainment and adult body mass index: findings from a prospective cohort and within sibling-pairs analysis. Int J Obes (Lond) 2006;30(12):1758–1765. doi: 10.1038/sj.ijo.0803330. [DOI] [PubMed] [Google Scholar]
- 24.Mond JM, Stich H, Hay PJ, et al. Associations between obesity and developmental functioning in pre-school children: a population-based study. Int J Obes (Lond) 2007;31(7):1068–1073. doi: 10.1038/sj.ijo.0803644. [DOI] [PubMed] [Google Scholar]
- 25.Sørensen TI, Sonne-Holm S, Christensen U, et al. Reduced intellectual performance in extreme overweight. Hum Biol. 1982;54(4):765–775. [PubMed] [Google Scholar]
- 26.Sørensen TI, Sonne-Holm S, Christensen U. Cognitive deficiency in obesity independent of social origin. Lancet. 1983;1(8333):1105–1106. doi: 10.1016/s0140-6736(83)91940-2. [DOI] [PubMed] [Google Scholar]
- 27.Sørensen TI, Sonne-Holm S. Intelligence test performance in obesity in relation to educational attainment and parental social class. J Biosoc Sci. 1985;17(4):379–387. doi: 10.1017/s002193200001590x. [DOI] [PubMed] [Google Scholar]
- 28.Teasdale TW, Sørensen TI, Stunkard AJ. Intelligence and educational level in relation to body mass index of adult males. Hum Biol. 1992;64(1):99–106. [PubMed] [Google Scholar]
- 29.D'Hondt E, Deforche B, De Bourdeaudhuij I, et al. Childhood obesity affects fine motor skill performance under different postural constraints. Neurosci Lett. 2008;440(1):72–75. doi: 10.1016/j.neulet.2008.05.056. [DOI] [PubMed] [Google Scholar]
- 30.Polk S, Sunyer J, Muñoz-Ortiz L, et al. A prospective study of Fel d1 and Der p1 exposure in infancy and childhood wheezing. Am J Respir Crit Care Med. 2004;170(3):273–278. doi: 10.1164/rccm.200310-1348OC. [DOI] [PubMed] [Google Scholar]
- 31.Klebanoff MA, Cole SR. Use of multiple imputation in the epidemiologic literature. Am J Epidemiol. 2008;168(4):355–357. doi: 10.1093/aje/kwn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 33.McCarthy D. Manual for the McCarthy Scales of Children’s Abilities. New York, NY: Psychological Corporation; 1972. [Google Scholar]
- 34.Barkley RA. The executive functions and self-regulation: an evolutionary neuropsychological perspective. Neuropsychol Rev. 2001;11(1):1–29. doi: 10.1023/a:1009085417776. [DOI] [PubMed] [Google Scholar]
- 35.Fried PA, Smith AM. A literature review of the consequences of prenatal marihuana exposure. An emerging theme of a deficiency in aspects of executive function. Neurotoxicol Teratol. 2001;23(1):1–11. doi: 10.1016/s0892-0362(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 36.Baron SI. Neuropsychological Evaluation of the Child. 1st ed. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 37.Lezak MD, Howieson DB, Lorin DW, et al. Neuropsychological Assessment. 4th ed. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 38.Julvez J, Ribas-Fitó N, Torrent M, et al. Maternal smoking habits and cognitive development of children at age 4 years in a population-based birth cohort. Int J Epidemiol. 2007;36(4):825–832. doi: 10.1093/ije/dym107. [DOI] [PubMed] [Google Scholar]
- 39.van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18(6):681–694. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 40.Dietz WH. Critical periods in childhood for the development of obesity. Am J Clin Nutr. 1994;59(5):955–959. doi: 10.1093/ajcn/59.5.955. [DOI] [PubMed] [Google Scholar]
- 41.Hawkins SS, Law C. A review of risk factors for overweight in preschool children: a policy perspective. Int J Pediatr Obes. 2006;1(4):195–209. doi: 10.1080/17477160600943351. [DOI] [PubMed] [Google Scholar]
- 42.Rennie KL, Johnson L, Jebb SA. Behavioural determinants of obesity. Best Pract Res Clin Endocrinol Metab. 2005;19(3):343–358. doi: 10.1016/j.beem.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Hall DM, Cole TJ. What use is the BMI? Arch Dis Child. 2006;91(4):283–286. doi: 10.1136/adc.2005.077339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ellis KJ. Selected body composition methods can be used in field studies. J Nutr. 2001;131(suppl):1589S–1595S. doi: 10.1093/jn/131.5.1589S. [DOI] [PubMed] [Google Scholar]
- 45.Hernandez L, Hoebel BG. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci. 1988;42(18):1705–1712. doi: 10.1016/0024-3205(88)90036-7. [DOI] [PubMed] [Google Scholar]
- 46.Small DM, Zatorre RJ, Dagher A, et al. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124(9):1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- 47.Trinko R, Sears RM, Guarnieri DJ, et al. Neural mechanisms underlying obesity and drug addiction. Physiol Behav. 2007;91(5):499–505. doi: 10.1016/j.physbeh.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38(8):1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- 49.Herschkowitz N. Neurological bases of behavioral development in infancy. Brain Dev. 2000;22(7):411–416. doi: 10.1016/s0387-7604(00)00185-6. [DOI] [PubMed] [Google Scholar]
- 50.Kappos AD. The impact of electronic media on mental and somatic children's health. Int J Hyg Environ Health. 2007;210(5):555–562. doi: 10.1016/j.ijheh.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Landhuis CE, Poulton R, Welch D, et al. Does childhood television viewing lead to attention problems in adolescence? Results from a prospective longitudinal study. Pediatrics. 2007;120(3):532–537. doi: 10.1542/peds.2007-0978. [DOI] [PubMed] [Google Scholar]
- 52.Hancox RJ, Poulton R. Watching television is associated with childhood obesity: but is it clinically important? Int J Obes (Lond) 2006;30(1):171–175. doi: 10.1038/sj.ijo.0803071. [DOI] [PubMed] [Google Scholar]
- 53.Marshall SJ, Biddle SJ, Gorely T, et al. Relationships between media use, body fatness and physical activity in children and youth: a meta-analysis. Int J Obes Relat Metab Disord. 2004;28(10):1238–1246. doi: 10.1038/sj.ijo.0802706. [DOI] [PubMed] [Google Scholar]
- 54.Rey-López JP, Vicente-Rodríguez G, Biosca M, et al. Sedentary behaviour and obesity development in children and adolescents. Nutr Metab Cardiovasc Dis. 2008;18(3):242–251. doi: 10.1016/j.numecd.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 55.Anoop S, Saravanan B, Joseph A, et al. Maternal depression and low maternal intelligence as risk factors for malnutrition in children: a community based case-control study from South India. Arch Dis Child. 2004;89(4):325–329. doi: 10.1136/adc.2002.009738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bennett DS, Bendersky M, Lewis M. Children's cognitive ability from 4 to 9 years old as a function of prenatal cocaine exposure, environmental risk, and maternal verbal intelligence. Dev Psychol. 2008;44(4):919–928. doi: 10.1037/0012-1649.44.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dubois L, Girard M. Early determinants of overweight at 4.5 years in a population-based longitudinal study. Int J Obes (Lond) 2006;30(4):610–617. doi: 10.1038/sj.ijo.0803141. [DOI] [PubMed] [Google Scholar]
- 58.Mendez MA, Torrent M, Ferrer C, et al. Maternal smoking very early in pregnancy is related to child overweight at age 5–7 y. Am J Clin Nutr. 2008;87(6):1906–1913. doi: 10.1093/ajcn/87.6.1906. [DOI] [PubMed] [Google Scholar]
- 59.Gilman SE, Gardener H, Buka SL. Maternal smoking during pregnancy and children's cognitive and physical development: a causal risk factor? Am J Epidemiol. 2008;168(5):522–531. doi: 10.1093/aje/kwn175. [DOI] [PMC free article] [PubMed] [Google Scholar]