Abstract
Previous studies of photosynthetic acclimation to elevated CO2 have focused on the most recently expanded, sunlit leaves in the canopy. We examined acclimation in a vertical profile of leaves through a canopy of wheat (Triticum aestivum L.). The crop was grown at an elevated CO2 partial pressure of 55 Pa within a replicated field experiment using free-air CO2 enrichment. Gas exchange was used to estimate in vivo carboxylation capacity and the maximum rate of ribulose-1,5-bisphosphate-limited photosynthesis. Net photosynthetic CO2 uptake was measured for leaves in situ within the canopy. Leaf contents of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), light-harvesting-complex (LHC) proteins, and total N were determined. Elevated CO2 did not affect carboxylation capacity in the most recently expanded leaves but led to a decrease in lower, shaded leaves during grain development. Despite this acclimation, in situ photosynthetic CO2 uptake remained higher under elevated CO2. Acclimation at elevated CO2 was accompanied by decreases in both Rubisco and total leaf N contents and an increase in LHC content. Elevated CO2 led to a larger increase in LHC/Rubisco in lower canopy leaves than in the uppermost leaf. Acclimation of leaf photosynthesis to elevated CO2 therefore depended on both vertical position within the canopy and the developmental stage.
The pCO2 is expected to increase from the current 36 to 55 Pa by the late 21st century (Schimel et al., 1996). Instantaneous increases in pCO2 lead to a stimulation in C3 photosynthesis through higher carboxylation efficiency and substrate concentration at the primary carboxylating enzyme, Rubisco (Drake et al., 1997). Acclimation of C3 photosynthesis, especially in the form of decreased activity and amount of Rubisco, has often been observed in crops grown at elevated pCO2 but is rarely sufficient to completely offset the increase in photosynthetic CO2 uptake (Gunderson and Wullschleger, 1994; Sage, 1994; Drake et al., 1997). However, decreased Rubisco content is associated with a decline in N content, since the enzyme accounts for 10% to 25% of leaf N (Field and Mooney, 1986; Evans, 1989; Long and Drake, 1992). Stimulation of photosynthetic CO2 uptake and a decline in N content therefore tend to result in increased photosynthetic N-use efficiency at elevated pCO2 (Drake et al., 1997). As a consequence, photosynthetic acclimation to elevated pCO2 has important implications for crop N use.
During the growth of cereal crops, leaves of the main stem and major tillers emerge into full sunlight at the top of the canopy but then become progressively more shaded as newer canopy elements form in sequence above them. Leaves shaded within crop canopies show changes in the photosynthetic system that are typical of shade-acclimated leaves (Evans, 1993). LHC proteins may increase, but Rubisco content and the RuBP-regeneration capacity tend to decline (Björkman, 1981; Anderson, 1986; Baker and McKiernan, 1988; Evans, 1993). Shaded leaves in a cereal canopy are therefore older and likely have a modified photosynthetic system compared with sunlit leaves at the top of the canopy. Previous studies of photosynthetic acclimation to elevated pCO2 have focused on the most recently expanded and sunlit leaves in the canopy (Hakala and Mela, 1995; Ziska et al., 1996; Vu et al., 1997). However, acclimation could differ between sunlit leaves and older, more shaded leaves deeper within the canopy. If ignored, any differences could lead to important errors in determining the acclimation response of whole crop canopies. Acclimation could occur at the canopy level without being detected in the most recently expanded leaf. An understanding of acclimation in crop canopies will be critical for predicting the effects of increasing pCO2 on crop photosynthesis and N use in the future.
FACE allows direct study of the effects of elevated pCO2 on crops under field conditions without any direct modification of microclimate (Hendrey et al., 1993). Large areas of undisturbed canopy are available in which edge and wall effects and other disturbances typical of the small canopies enclosed within controlled-environment and open-top chambers can be avoided. The FACE wheat (Triticum aestivum L.) project therefore provided an unrivaled opportunity to examine the null hypothesis that photosynthetic acclimation to elevated pCO2 in the form of loss of carboxylation capacity and Rubisco content is identical in a vertical profile of leaves through the canopy. Relationships between acclimation or lack of acclimation and photosynthetic CO2 uptake in situ and leaf N-use efficiency were determined. Photosynthetic acclimation of lower canopy leaves was examined at three stages of crop development under elevated pCO2: inflorescence emergence, when whole-plant sink demand for C and N is low, and two stages of grain development, when C and N are being rapidly exported to the ear (Simpson, 1992).
MATERIALS AND METHODS
FACE System and Wheat Crop
Spring wheat (Triticum aestivum L. cv Yecora Rojo) was grown in a field on the experimental farm of the Maricopa Agricultural Center (University of Arizona, Maricopa, 33.075°N, 111.983°W), following standard cultivation practices for obtaining good yields in this region. The field was sown in December 1992 and 1993 in rows 0.25 m apart. A FACE-application system designed and built by the Brookhaven National Laboratory (Upton, NY) was used to achieve controlled elevation of pCO2 within and above the crop under field conditions (Lewin et al., 1994). The FACE apparatus consisted of 25-m-diameter toroidal plenums that were placed on the soil surface immediately after sowing. Vertical-vent pipes were connected to the plenum at 2-m intervals.
The release of CO2, which was controlled by a central computer, was upwind of the plots and just above plant height to maintain a pCO2 of 55 Pa in the plots (Lewin et al., 1994). The enrichment was maintained continuously from emergence (January 1) until harvest (mid-May). Control plots were prepared in the same way but without any airflow and had a daytime ambient pCO2 of 36 Pa. The experimental design consisted of four replicate blocks, each containing one elevated pCO2 (approximately 55 Pa) FACE ring and one control (approximately 36 Pa) ring. The plots were 22 m in diameter, and blocks were separated by at least 90 m. One-half of each plot was subjected to a drought treatment (Pinter et al., 1996). In this paper we report only the plants grown with adequate water. These well-watered plants were irrigated with underground drip tubes whenever the available water in the root zone decreased below 30%. The amounts of water added in each irrigation were calculated to replace the evapotranspiration that had occurred since the preceding irrigation, after adjustment for any precipitation. The crop received 277 kg N ha−1 and 44 kg P ha−1 in 1992/1993 and 277 kg N ha−1 and 29 kg P ha−1 in 1993/1994. Other nutrients were adjusted to avoid any potential deficiencies.
Since crop emergence occurred on the 1st d of the year, that day is equivalent to the DAE for the crop. Further details of the FACE site, experimental treatments, and cultivation practice for FACE in wheat are provided by Kimball et al. (1995) and Pinter et al. (1996). This study concerns photosynthesis of the three uppermost leaves of the flowering stems of wheat, which form most of the active canopy, the lower leaves having commenced or completed senescence. By convention, leaves of cereal stems are numbered from the base upward. The 7th leaf is the lowest and oldest, the 8th leaf is above and in about the middle of the canopy, and the 9th leaf, the flag leaf, is the youngest and forms the top of the leaf canopy.
Light Penetration in the Canopy
τ was determined at the mean height of the flag, at the 8th and 7th leaves, and at the soil surface. It was estimated by one of two methods, in parallel with leaf gas-exchange measurements: (a) from the hemispherical distribution of light within the canopy under diffuse sky-lighting conditions, using a canopy analyzer (LAI-2000, Li-Cor, Lincoln, NE) following the procedure of Welles and Norman (1991); and (b) at approximately solar noon it was estimated under a cloudless sky (Sunfleck Ceptometer, Decagon Devices, Pullman, WA).
Carboxylation Capacity and RuBP-Limited Photosynthesis
Leaf CO2 and water vapor fluxes were measured in fully controlled microenvironment cuvettes incorporated into two identical, open gas-exchange systems (MPH-1000, Campbell Scientific, Logan, UT) and used to determine the response of light-saturated photosynthesis to variations in the ci. Each of these laboratory systems incorporated an IR gas analyzer (LI-6262, Li-Cor) calibrated for CO2 using a gravimetrically prepared calibration mixture of CO2 in air (±1%, Primary Standard, Matheson Gas Products, Cucamonga, CA), and for water vapor with a dew point generator (LI-610, Li-Cor). Set point pCO2 was provided around the leaf by feedback control of the flows of compressed gases.
Leaf temperature was maintained at 22.5 ± 0.1°C (mean ± 1 sd) by a feedback-control system. Humidity was controlled by regulating the proportion of the chamber inlet flow that was bypassed through a bubbler in water, and leaf vapor pressure deficit was maintained at <1.1 kPa. Chambers were illuminated by linear halogen sources in a parabolic reflector, which provided a constant Q at 1048 ± 42 μmol m−2 s−1. A and ci were determined in real time using the equations of von Caemmerer and Farquhar (1981). The initial, linear slope of the A/ci response was used to estimate Vc, max after the method of Wullschleger (1993), incorporating the temperature correction of Harley et al. (1992). Amax was measured at a pCO2 of 85 Pa in 1 kPa pO2 to ensure that photorespiration was eliminated, and photosynthesis was limited by the maximum rate of RuBP regeneration (von Caemmerer and Farquhar, 1981).
Measurements were made on leaves obtained from an undisturbed part of the canopy in 1994 from DAE 88 to 93 during inflorescence emergence and immediately prior to anthesis and from DAE 106 to 117, the milk/early dough stage of grain filling (hereafter referred to as early grain development). Measurements were also made from DAE 97 to 104 in 1993, the dough-development period of grain filling, hereafter referred to as mid-grain development. Plant material was sampled daily between 7:30 and 9:30 am to avoid effects of photoinhibition, “feedback-limitation,” or water deficit on leaves. Plants were removed with the soil surrounding their main roots and sealed in plastic bags with a small amount of water. Sampled plants were kept in the dark at approximately 10°C until measured; 10°C was similar to the mean night temperature at this time of the year. All measurements were made within 12 h of plant removal from the field on excised leaves cut under water. Rates of CO2 uptake were equivalent to the maximum rates recorded for the same leaves in situ, indicating that there was no loss of photosynthetic capacity resulting from this treatment.
Rubisco and N Contents
A subsample of the leaves used for gas-exchange determination of Vc, max and Amax was rapidly frozen in liquid N2 and then stored at −80°C until subsequent analysis. Total proteins were extracted by grinding leaf tissue (1 or 2 cm2) in 5% SDS and 60 mm Tris, pH 8.0, directly in a microcentrifuge tube containing 0.1 g of glass beads. After an aliquot for protein measurement was removed, DTT and Glc were added to a final concentration of 50 mm and 7.5%, respectively. Total proteins extracted from the same leaf surface area for each treatment and from each leaf position combination were separated by SDS-PAGE. Gels were analyzed by western blot to identify LHC and the large subunit of Rubisco and quantified as described previously (Nie et al., 1995b). A second subsample of leaves was dried at 80°C for at least 48 h and then ground to a powder. N was measured as described previously (Osborne et al., 1997).
Photosynthesis under in Situ Conditions
The diurnal course of leaf CO2 uptake for each leaf category and treatment was established using spot measurements on randomly selected stems with two closed gas-exchange systems (LI-6200, Li-Cor). Each IR gas analyzer was calibrated against a gravimetrically prepared calibration mixture of CO2 in air (Primary Standard, Matheson Gas Products) and chamber humidity sensors were calibrated with a dew point generator (LI-610, Li-Cor) immediately prior to use. Measurements of A were started at a leaf chamber pCO2 of 55 ± 2.7 Pa for leaves grown at elevated pCO2 and at 36 ± 1.8 Pa for control leaves. The equations of von Caemmerer and Farquhar (1981) were used to calculate A. Measurements were made from dawn until dusk at mid-grain development (on DAE 101 in 1993) using parts of the canopy that were previously undisturbed. The central portion of most of the leaves was approximately horizontal, and the leaf cuvette was clamped onto this portion of the leaf and maintained in the horizontal aspect. This portion was chosen to minimize both within-leaf variation in photosynthetic capacity and the effect of leaf angle on incident Q.
Statistical Analysis
The null hypothesis that leaf position in the canopy had no effect on photosynthetic acclimation to elevated pCO2 was examined using two-way ANOVA for each of Vc, max, Amax, Rubisco, LHC, LHC/Rubisco, and N, where sources of variation tested were CO2 treatment, leaf position on the stem, and the interaction between the two. Three-way or repeated-measures ANOVA were not considered appropriate because two samples were taken in 1 year and one in another. The null hypothesis that the profile of τ was identical throughout control and elevated pCO2 canopies was also tested using two-way ANOVA, examining the effects of the same sources of variation. In each case the individual FACE and control plots were treated as the sample (n = 4).
RESULTS
Light Penetration in the Canopy
Two-way ANOVA showed no difference in the profile of τ through the canopy (Table I; inflorescence emergence, F1, 18 = 0.35, not significant at P = 0.05; early grain development, F1, 18 = 0.66, not significant at P = 0.05; and mid-grain development, F1, 3 = 1.20, not significant at P = 0.05).
Table I.
τ of a wheat crop grown in elevated pCO2 (55 Pa) and control (36 Pa) conditions
| Leaf | τa
|
|||||
|---|---|---|---|---|---|---|
| Inflorescence emergence | Early grain development | Mid-grain development | ||||
| 36 Pa | 55 Pa | 36 Pa | 55 Pa | 36 Pa | 55 Pa | |
| Flag | 1.00 ± 0.00 | 1.00 ± 0.00 | 0.69 ± 0.03 | 0.66 ± 0.01 | 0.51 ± 0.03 | 0.49 ± 0.04 |
| 8th | 0.49 ± 0.01 | 0.44 ± 0.07 | 0.22 ± 0.05 | 0.21 ± 0.02 | 0.06 ± 0.01 | 0.06 ± 0.03 |
| 7th | 0.07 ± 0.02 | 0.07 ± 0.03 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 |
Mean values (±1 sd to two decimal places) for τ are shown for the approximate positions of the flag, 8th, and 7th leaves within the crop canopy for three stages of crop development: inflorescence emergence (1994); early grain development (1994); and mid-grain development (1993). τ was estimated as the canopy gap fraction in 1993 and the proportion of incident sunlight in 1994.
τ is dimensionless and has a value of 0 to 1.
Acclimation of Photosynthesis
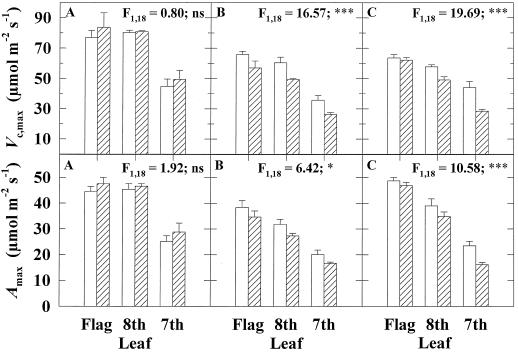
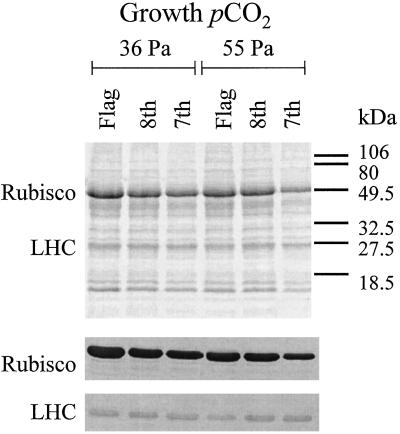
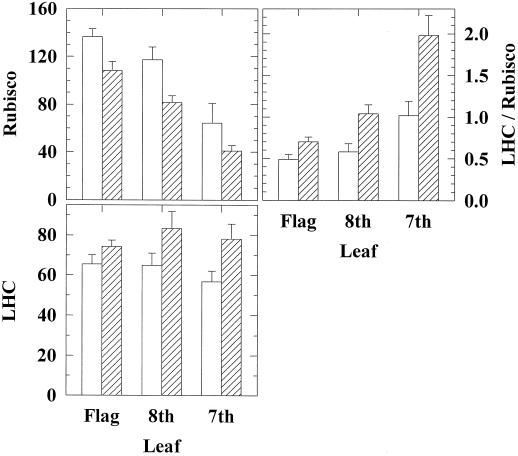
Growth at elevated pCO2 had no effect on Vc, max or Amax prior to anthesis but led to a significant reduction in both during grain development (Fig. 1). An independent and highly significant reduction in Vc, max and Amax occurred with leaf position down the stem at all growth stages examined (Fig. 1). Interaction between elevated pCO2 and leaf position on Vc, max was absent prior to anthesis but was highly significant at mid-grain development (Fig. 1). At mid-grain development, growth in elevated pCO2 had produced a significant decline in the Vc, max of the 7th and 8th leaves relative to controls but not in the flag leaf (Fig. 1). Rubisco contents similarly decreased with leaf position, showing a decline of 50% from the flag to the 7th leaf during early grain development (Figs. 2 and 3). Rubisco contents were significantly lower in the leaves grown in elevated pCO2 (Figs. 2 and 3). LHC showed no significant change with leaf position (Figs. 2 and 3). There was a highly significant effect of CO2 treatment on LHC content (Figs. 2 and 3). The ratio of LHC to Rubisco showed a significantly greater increase with leaf position in elevated pCO2 (Figs. 2 and 3).
Figure 1.
Vc, max and Amax for the flag, 8th, and 7th leaves in wheat grown in elevated pCO2 (hatched bars) and control conditions (white bars). Means (±1 sd) are indicated for the four replicate plots of elevated pCO2 and control during three stages of crop development: A, Inflorescence emergence (1994); B, early grain development (1994); and C, mid-grain development (1993). F values and their significance level for the effect of elevated pCO2 are given. ns, Not statistically significant at the P = 0.05 level; *, statistically significant at the P = 0.05 level; ***, statistically significant at the P = 0.001 level. There was always a significant effect of leaf position on both measures (F2, 18 > 25; P = 0.001) and a significant interaction of elevated pCO2 and leaf position for Vc, max at mid-grain development (F2, 18 = 4.46; P = 0.05).
Figure 2.
Rubisco and LHC proteins during early grain development for the flag, 8th, and 7th leaves in wheat grown in elevated pCO2 and control conditions. Top, Coomassie blue-stained gel. Loaded extracts were obtained from equal leaf areas. The most intensively stained bands are the large-subunit polypeptide of Rubisco at 56 kD and LHC at 27.5 kD. Bottom, Western blot showing the reaction with the large-subunit polypeptide of Rubisco and LHC.
Figure 3.
Rubisco, LHC, and LHC/Rubisco for the flag, 8th, and 7th leaves in wheat grown in elevated pCO2 (hatched bars) and control conditions (white bars). Values are the protein content determined on a leaf-area basis and expressed in arbitrary units of absorbance to a common scale. Means (±1 sd) are indicated for the four replicate plots of elevated pCO2 and control during early grain development (1994). Two-way ANOVA showed that leaf position in the canopy had a highly significant effect on Rubisco (F2, 18 = 27.51; P = 0.001) and LHC/Rubisco (F2, 18 = 23.19; P = 0.001) but had no effect on LHC (F2, 18 = 0.59; not significant). Elevated pCO2 had a highly significant effect on Rubisco (F1, 18 = 13.80; P = 0.01), LHC (F1, 18 = 10.51; P = 0.01), and LHC/Rubisco (F1, 18 = 22.81; P = 0.001). There was a statistically significant interaction between leaf position in the canopy and elevated pCO2 for LHC/Rubisco (F2, 18 = 3.75; P = 0.05).
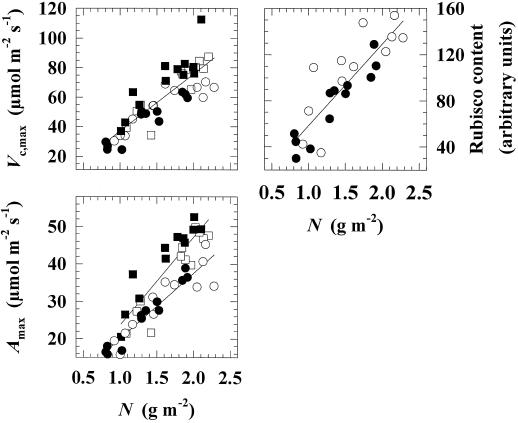
Vc, max, Amax, and Rubisco content were always highly correlated with N, but there was no effect of elevated pCO2 on the relationship, i.e. a decrease in these parameters of photosynthesis was directly in proportion to the decrease in N (Fig. 4). Crop growth stage did not affect the relationship between Vc, max and N or Rubisco and N, but a reduction in the slope of the regression between Amax and N occurred after anthesis (Fig. 4). In the three leaves at mid-grain development, N was 15% lower in elevated pCO2.
Figure 4.
Scatter plots of Vc, max and Amax and Rubisco content against N. There was always a highly significant regression relationship (P = 0.01) between Vc, max, Amax, or Rubisco with N when leaf positions were pooled within pCO2 treatment and crop developmental stage. One-way ANOVA was used to investigate changes in the slope of the regression attributable to growth pCO2 and the stage of crop development. ANOVA showed no change in the slope of regressions between Vc, max and N (F3, 42 = 2.44; not significant at P = 0.05) or Rubisco and N (F1, 22 = 0.11; not significant at P = 0.05). CO2 treatments were therefore pooled for the plotted regression lines. Elevated pCO2 had no effect on the slope of regressions between Amax and N, but a decrease in slope occurred during grain development compared with inflorescence emergence (F3, 42 = 3.43; P = 0.05). CO2 treatments were therefore pooled, but crop developmental stages were separated for regression lines. □, Inflorescence emergence at 36 Pa; ▪, inflorescence emergence at 55 Pa; ○, grain development at 36 Pa; •, grain development at 55 Pa.
Photosynthesis under in Situ Conditions
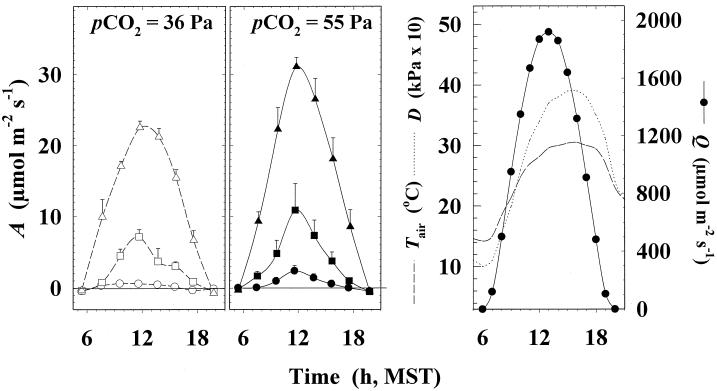
The diurnal course of photosynthesis in situ shows that A in the elevated pCO2 treatment was always equal to or greater than that in the control treatment at all leaf positions (Fig. 5). Average values for Am are shown in Table II. Relative stimulation of Am at elevated pCO2 was greater with increasing depth into the canopy. The increase in total uptake of CO2 over the day caused by elevated pCO2 was 26% and 68% for the flag and 8th leaves, respectively (Table II). In addition, elevated pCO2 caused net CO2 uptake over the day in the 7th leaf to be positive, whereas it was negative in control leaves (Table II).
Figure 5.
Left, Diurnal course of A measured for the flag (▵), 8th (□), and 7th (○) leaves of wheat in control (36 Pa) conditions in the field. Middle, Diurnal course of A measured for the flag (▴), 8th (▪), and 7th (•) leaves of wheat in elevated pCO2 (55 Pa) conditions in the field. Measurements were made on DAE 101, at mid-grain development (1993). Each point is the mean (±1 sd) for six measurements made within a 90-min period in two replicate blocks. MST, U.S. Mountain Standard Time. Right, Meteorological data for the daylight period of DAE 101 (1993) based on hourly average readings from the Arizona Meteorological Network station at Maricopa. Photosynthetically active photon flux density (Q), air temperature (Tair), and vapor pressure deficit (D) show patterns typical of the spring climate in the region (Kimball et al., 1995).
Table II.
Am and A′ (integrated from the curves in Fig. 5) for the flag, 8th, and 7th leaves in wheat grown in control conditions (36 Pa) and in elevated pCO2 (55 Pa)
| Leaf/Treatment | Am | A′ |
|---|---|---|
| Pa | μmol m−2 s−1 | mmol m−2 |
| Flag | ||
| 36 | 21.9 ± 1.1 | 687 |
| 55 | 28.8 ± 2.2 (58)a | 863 (61) |
| 8th | ||
| 36 | 5.4 ± 1.5 | 121 |
| 55 | 9.1 ± 3.1 (31) | 204 (29) |
| 7th | ||
| 36 | 0.5 ± 0.4 | −8 |
| 55 | 1.9 ± 0.7 (12) | 21 (10) |
Means (±sd) for Am are for measurements made 1 h on either side of solar noon of the diurnal time course at mid-grain development (Fig. 5). Each value is the mean net CO2 uptake for a given leaf integrated between 5 am and 8 pm (±sd), corrected for night-time respiration. The integral of night-time respiration was estimated by linear interpolation between measurements made at the beginning and end of the night.
Values in parentheses indicate the absolute increase for each leaf due to elevated pCO2, expressed as a percentage of the sum of the increase for the three leaf positions.
DISCUSSION
Acclimation of Photosynthesis
The decline in Vc, max with growth in elevated pCO2 was affected by the position in the canopy. There was no acclimation of Vc, max in response to elevated pCO2 in the flag leaves, but there was a 35% decrease in the lower leaves during grain filling (Fig. 1). The null hypothesis that photosynthetic acclimation to elevated pCO2 is identical in leaves throughout the canopy should therefore be rejected. We provide clear evidence that acclimation is more pronounced in the more shaded and older leaves of the canopy, an effect that became apparent during grain filling. This has important implications for understanding photosynthetic acclimation to elevated pCO2 in the whole crop. First, studies in which only the most recently expanded, sunlit leaves in a canopy were examined cannot be used to reliably predict acclimation of the whole canopy. Second, patterns of acclimation observed at one crop developmental stage cannot be used to reliably predict acclimation at other stages.
Decreases in Vc, max might in part be explained by the decline in Rubisco content at elevated pCO2 (Figs. 1–3). Rubisco content was lower in all leaves at elevated pCO2, and yet Vc, max was unchanged in the flag leaf, suggesting that the in vivo activation state of the enzyme increased at elevated pCO2 to compensate for decreased quantity (Figs. 1–3). The approximately 20% decrease in Rubisco observed in the flag leaf at elevated pCO2 is consistent with the findings of Nie et al. (1995b) for this stage of the crop. The larger relative decreases in Rubisco observed in the lower leaves with elevated pCO2 were paralleled by decreases in Vc, max.
The decline in Vc, max and Amax with depth into the canopy corresponded with increases in both the degree of shading and age in sequentially formed leaves. LHC remained unchanged with depth into the canopy, suggesting shade acclimation and not senescence (Figs. 2 and 3). In addition, although leaf emergence occurred up to 2 d earlier in elevated pCO2 conditions than in the control (Kimball et al., 1995), acclimation of Vc, max and Amax to elevated pCO2 in shaded leaves could not be explained by the difference in leaf age between CO2 treatments. The 35% reduction in Vc, max in lower leaves at elevated pCO2 compared with the control was greater than the reduction in Vc, max that occurred in any control leaf position between inflorescence emergence and early grain development, a period of 20 d (Fig. 1).
Photosynthetic acclimation to elevated pCO2 has been attributed to greater self-shading in other species, which is caused by a larger leaf area and results in greater shade acclimation (Poorter et al., 1988; Wardlaw, 1990). However, light penetration to each leaf position was not affected by growth pCO2 in the wheat canopy at any of the three developmental stages investigated. Destructive analysis at these stages of development similarly showed no increase in leaf-area index with growth at elevated pCO2 (Pinter et al., 1996). Measurements at different growth stages were made in different years. However, the experimental treatment was the same in both years, and crop responses to pCO2 were apparently similar (Pinter et al., 1996). A similar pattern of acclimation, occurring only after anthesis in the leaves of the lower canopy, was observed in both years.
Photosynthesis under in Situ Conditions
In situ CO2 uptake increased at elevated pCO2 in all leaves, despite the more rapid decline in Vc, max with depth into the canopy (Figs. 1 and 5). Acclimation here may therefore be interpreted as increased efficiency of resource use rather than an adverse reaction to elevated pCO2. The decreases in Vc, max and Amax in the lower leaves with elevated pCO2 will decrease light-saturated CO2 uptake. The actual increase in lower canopy photosynthesis in situ can therefore be attributed to the stimulation of light-limited photosynthesis under elevated pCO2. Increased C gain at elevated pCO2 compared with controls under light limitation was also partly due to a lower light compensation point for leaves, allowing positive net C gain for a longer period in the day (Fig. 5; Long and Drake, 1991; Osborne et al., 1997). Stimulation of light-limited C3 photosynthesis is expected at elevated pCO2, despite reductions in Vc, max or Amax. An increase in the ratio of pCO2 to pO2 suppresses the oxygenation reaction of Rubisco, allowing carboxylation of a greater proportion of the available RuBP pool irrespective of carboxylation or RuBP-regeneration capacities.
Increased leaf photosynthesis throughout the canopy paralleled the increase in daily whole-canopy CO2 uptake, a greater rate of biomass accumulation, a higher rate of grain filling, and a stimulation in harvested grain yield of the plants growing at elevated pCO2 (Kimball et al., 1995; Wechsung et al., 1995; Pinter et al., 1996). The areas of the flag, 8th, and 7th leaves were very similar at the stage of development that we examined. Therefore, the measured rates of photosynthesis would be directly proportional to their absolute contribution to canopy photosynthesis. Considering these three leaves together, which formed the bulk of the canopy, the combined increase in photosynthetic CO2 uptake over 1 d at elevated pCO2 was 280 mmol CO2 m−2. The two lower and shaded leaves accounted for 39% of this increase, emphasizing their importance to the absolute increase in canopy C gain at elevated pCO2 (Table II).
N
Stimulation of photosynthesis occurred despite decreases in N at elevated pCO2, resulting in an increased efficiency of leaf photosynthetic N use (Figs. 3 and 5). N, calculated as Am/N, declined with leaf position. However, the increase with elevated pCO2 relative to controls increased with leaf position. Compared with controls, leaf photosynthetic N use efficiency was 58%, 94%, and 353% higher in flag, 8th, and 7th leaves, respectively, grown at elevated pCO2.
Photosynthetic acclimation through the canopy might be explained by a greater reallocation of N from the leaves to developing grains at elevated pCO2. The onset of acclimation to elevated pCO2 in Rubisco, Vc, max, and Amax after anthesis was accompanied by a reduction in N (Fig. 4). This corresponded to an 85% decrease in mean daily leaf nonstructural carbohydrate contents, suggesting increased sink demand (Nie et al., 1995a). Photosynthetic acclimation to elevated pCO2 is commonly associated with a decrease in N (for review, see Drake et al., 1997), and lower N may be a consequence of reduced investment of N resources in the photosynthetic apparatus (Woodrow, 1994) or of a dilution by increased biomass at elevated pCO2 (Coleman et al., 1993; Sage, 1994). Specific leaf area was unchanged by elevated pCO2 in the present study, removing dilution as a possible explanation.
Changes in Rubisco, Vc, max, and Amax were closely correlated with changes in N (Fig. 4); however, this was not the result of a uniform decrease within the photosynthetic apparatus. Rubisco was lower in leaves grown at elevated pCO2 and showed an increasing decline with leaf position. LHC, the other major protein of the photosynthetic apparatus in terms of the N it accounts for, showed no loss. In the three leaves that form the bulk of the photosynthetic canopy during grain filling, total N was 15% lower than in the controls and Rubisco was approximately 30% lower. Rubisco can account for 25% of leaf N; therefore, up to one-half of the decline in N could be explained by a loss of Rubisco. N is remobilized from leaves to developing wheat grains (Dalling et al., 1976; Simpson et al., 1983; Simpson, 1992). Although there was a slight decrease in the N per unit mass of grain (3%), the absolute quantity of N in the grain at harvest was 5% higher because of the 8% increase in grain yield at elevated pCO2 (A. Giuntoli, unpublished data; Kimball et al., 1995; Pinter et al., 1996). Total plant N content was unchanged at elevated pCO2 (G. Wechsung, personal communication); therefore, increased mobilization from other plant parts had to occur to support the increased amount of N allocated to grain. The grain-filling period was shorter in elevated pCO2 compared with controls, suggesting significant increases in the rate of N mobilization. This would explain acclimatory reductions in leaf N under elevated pCO2. The loss of Rubisco, but not LHC, that accompanied this decrease in N may have functional significance, since at elevated pCO2 less Rubisco but not LHC would be required to support a given CO2-assimilation rate (Woodrow, 1994).
CONCLUSIONS
Most previous studies of the acclimation of leaf photosynthesis to elevated pCO2 have concerned the most recently emerged, upper canopy leaves. This study shows that, even at stages of development at which no marked acclimation to elevated pCO2 is apparent in the upper leaves, acclimation in carboxylation capacity and RuBP-limited photosynthesis occurs in the lower, shaded leaves. Acclimation was accompanied by a decline in Rubisco and an increase in LHC/Rubisco. Loss of photosynthetic capacity was not sufficient to remove an enhancement of photosynthetic activity under elevated pCO2 and resulted in an increased photosynthetic N-use efficiency. Our results could be explained by photosynthetic acclimation to elevated pCO2 in response to increased internal demand for N by the increased mass of developing grain.
ACKNOWLEDGMENTS
We thank Roy Rauschkolb and his staff (Maricopa Agricultural Center, University of Arizona) for help and support and also Martin Parry for the antibodies to Rubisco.
Abbreviations:
- A
net rate of CO2 uptake per unit leaf area
- Am
A at midday in situ
- Amax
CO2- and light-saturated value of A
- A′
daily integral of A
- ANOVA
analysis of variance
- ci
pCO2 in the substomatal cavity
- DAE
days after emergence
- FACE
free-air CO2 enrichment
- LHC
light-harvesting complex
- N
total leaf N content
- pCO2
partial pressure of CO2 in the atmosphere
- pO2
partial pressure of O2 in the atmosphere
- Q
photosynthetically active photon flux density
- RuBP
ribulose-1,5-bisphosphate
- τ
canopy transmittance, i.e. the proportion of light at the canopy surface penetrating to a given level
- Vc
max, maximum RuBP-saturated rate of carboxylation in vivo
Footnotes
This work was supported by a studentship to C.P.O. from the Natural Environment Research Council of the United Kingdom, the Carbon Dioxide Research Program of the Office of Health and Environmental Research of the U.S. Department of Energy, and by the U.S. Department of Agriculture, Agricultural Research Service.
LITERATURE CITED
- Anderson JM. Photoregulation of the composition, function, and structure of thylakoid membranes. Annu Rev Plant Physiol. 1986;37:93–136. [Google Scholar]
- Baker NR, McKiernan M. Modifications to the photosynthetic apparatus of higher plants in response to changes in the light environment. Biol J Linn Soc. 1988;34:193–203. [Google Scholar]
- Björkman O. Responses to different quantum flux densities. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, editors. Encyclopedia of Plant Physiology: Physiological Plant Ecology I (New Series), Vol 12A. Berlin: Springer-Verlag; 1981. pp. 57–107. [Google Scholar]
- Coleman JS, McConnaughay KDM, Bazzaz FA. Elevated CO2 and plant nitrogen-use: is reduced tissue nitrogen concentration size-dependent? Oecologia. 1993;93:195–200. doi: 10.1007/BF00317671. [DOI] [PubMed] [Google Scholar]
- Dalling MJ, Boland G, Wilson JH. Relationship between acid protease activity and redistribution of nitrogen during grain development in wheat. Aust J Plant Physiol. 1976;3:721–730. [Google Scholar]
- Drake BG, Gonzàlez-Meler MA, Long SP. More efficient plants? A consequence of rising atmospheric CO2. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:607–637. doi: 10.1146/annurev.arplant.48.1.609. [DOI] [PubMed] [Google Scholar]
- Evans JR. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia. 1989;78:9–19. doi: 10.1007/BF00377192. [DOI] [PubMed] [Google Scholar]
- Evans JR. Photosynthetic acclimation and nitrogen partitioning within a lucerne canopy. I. Canopy characteristics. Aust J Plant Physiol. 1993;20:55–67. [Google Scholar]
- Field C, Mooney HA. The photosynthesis-nitrogen relationship in wild plants. In: Givnish TJ, editor. On the Economy of Plant Form and Function. Cambridge, UK: Cambridge University Press; 1986. pp. 25–55. [Google Scholar]
- Gunderson CA, Wullschleger SD. Photosynthetic acclimation in trees to rising atmospheric CO2: a broader perspective. Photosynth Res. 1994;39:369–388. doi: 10.1007/BF00014592. [DOI] [PubMed] [Google Scholar]
- Hakala K, Mela T (1995) Greenhouse-based open top chamber experiments on spring wheat. In PA Harrison, RE Butterfield, TE Downing, eds, Climate Change and Agriculture in Europe. Assessment of Impacts and Adaptation. Environmental Change Unit, University of Oxford, UK, pp 162–175
- Harley PC, Thomas RB, Reynolds JF, Strain BR. Modelling photosynthesis of cotton grown in elevated CO2. Plant Cell Environ. 1992;15:271–282. [Google Scholar]
- Hendrey GR, Lewin KF, Nagy J (1993) Free air carbon dioxide enrichment: development, progress, results. Vegetatio, 104/105: 17–31
- Kimball BA, Pinter PJ, Jr, Garcia RL, LaMorte RL, Wall GW, Hunsaker DJ, Wechsung G, Wechsung F, Kartschall T. Productivity and water use of wheat under free-air CO2 enrichment. Global Change Biol. 1995;1:429–442. [Google Scholar]
- Lewin KF, Hendrey GR, Nagy J, LaMorte RL. Design and application of a free-air carbon dioxide enrichment facility. Agric For Meteorol. 1994;70:15–29. [Google Scholar]
- Long SP, Drake BG. Effects of the long-term elevation of CO2 concentration in the field on the quantum yield of photosynthesis of the C3 sedge, Scirpus olneyi. Plant Physiol. 1991;96:221–226. doi: 10.1104/pp.96.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SP, Drake BG (1992) Photosynthetic CO2 assimilation and rising atmospheric CO2 concentrations. In NR Baker, H Thomas, eds, Crop Photosynthesis: Spatial and Temporal Determinants. Elsevier Science Publishers B.V., Amsterdam, The Netherlands, pp 69–103
- Nie G-Y, Hendrix DL, Webber AN, Kimball BA, Long SP. Increased accumulation of carbohydrates and decreased photosynthetic gene transcript levels in wheat grown at an elevated CO2 concentration in the field. Plant Physiol. 1995a;108:975–983. doi: 10.1104/pp.108.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie G-Y, Webber AN, Garcia RL, Kimball BA, LaMorte RL, Pinter PJ, Wall GW, Long SP. Effects of free-air CO2 enrichment on the development of the photosynthetic apparatus in wheat, as indicated by changes in leaf proteins. Plant Cell Environ. 1995b;18:855–864. [Google Scholar]
- Osborne CP, Drake BG, LaRoche J, Long SP. Does long-term elevation of CO2 concentration increase photosynthesis in forest floor vegetation? Indian strawberry in a Maryland forest. Plant Physiol. 1997;114:337–344. doi: 10.1104/pp.114.1.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter PJ, Jr, Kimball BA, Garcia RL, Wall GW, Hunsaker DJ, LaMorte RL. Free-air CO2 enrichment: responses of cotton and wheat crops. In: Mooney HA, Koch GW, editors. Carbon Dioxide and Terrestrial Ecosystems. Orlando, FL: Academic Press; 1996. pp. 215–249. [Google Scholar]
- Poorter H, Pot S, Lambers H. The effect of an elevated atmospheric CO2 concentration on growth, photosynthesis and respiration in Plantago major. Physiol Plant. 1988;73:553–559. [Google Scholar]
- Sage RF. Acclimation of photosynthesis to increasing atmospheric CO2: the gas exchange perspective. Photosynth Res. 1994;39:351–368. doi: 10.1007/BF00014591. [DOI] [PubMed] [Google Scholar]
- Schimel D, Alves D, Enting I, Heimann M, Joos F, Raynaud D, Wigley T. Radiative forcing of climate change. In: Houghton JT, Meira Filho LG, Callander BA, Harris N, Kattenberg A, Maskell K, editors. Climate Change 1995. The Science of Climate Change. Cambridge, UK: Cambridge University Press; 1996. pp. 35–71. [Google Scholar]
- Simpson RJ (1992) Carbon and nitrogen budgets within the plant. In NR Baker, H Thomas, eds, Crop Photosynthesis: Spatial and Temporal Determinants. Elsevier Science Publishers, B.V., Amsterdam, The Netherlands, pp 105–129
- Simpson RJ, Lambers H, Dalling MJ. Nitrogen redistribution during grain growth in wheat (Triticum aestivum L.). IV. Development of a quantitative model of the translocation of nitrogen to the grain. Plant Physiol. 1983;71:7–14. doi: 10.1104/pp.71.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Vu JCV, Allen LH, Jr, Boote KJ, Bowes G. Effects of elevated CO2 and temperature on photosynthesis and Rubisco in rice and soybean. Plant Cell Environ. 1997;20:68–76. [Google Scholar]
- Wardlaw IF. Tansley review number 27. The control of carbon partitioning in plants. New Phytol. 1990;116:341–381. doi: 10.1111/j.1469-8137.1990.tb00524.x. [DOI] [PubMed] [Google Scholar]
- Wechsung G, Wechsung F, Wall GW, Adamsen FJ, Kimball BA, Garcia RL, Pinter PJ, Jr, Kartschall T. Biomass and growth rate of a spring wheat root system grown in free-air CO2 enrichment (FACE) and ample soil moisture. J Biogeogr. 1995;22:623–634. [Google Scholar]
- Welles JM, Norman JM. Instrument for indirect measurement of canopy architecture. Agron J. 1991;83:818–825. [Google Scholar]
- Woodrow IE. Optimal acclimation of the C3 photosynthetic system under enhanced CO2. Photosynth Res. 1994;39:401–412. doi: 10.1007/BF00014594. [DOI] [PubMed] [Google Scholar]
- Wullschleger SD. Biochemical limitations to carbon assimilation in C3 plants—a retrospective analysis of the A/Ci curves from 109 species. J Exp Bot. 1993;44:902–920. [Google Scholar]
- Ziska LH, Weerakoon W, Namuco OS, Pamplona R. The influence of nitrogen on the elevated CO2 response in field-grown rice. Aust J Plant Physiol. 1996;23:45–52. [Google Scholar]