Abstract
Background
The authors sought to compare gray matter changes in First Episode Schizophrenia (FES) compared with Fronto-Temporal Lobar Degeneration (FTLD) using meta-analytic methods applied to neuro-imaging studies.
Methods
A systematic search was conducted for published, structural voxel-based morphometric MRI studies in patients with FES or FTLD. Data were combined using anatomical likelihood estimation (ALE) to determine the extent of gray matter decreases and analysed to ascertain the degree of overlap in the spatial distribution of brain changes in both diseases.
Results
Data were extracted from 18 FES studies (including a total of 555 patients and 621 comparison subjects) and 20 studies of FTLD or related disorders (including a total of 311 patients and 431 comparison subjects). The similarity in spatial overlap of brain changes in the two disorders was significant (p = 0.001). Gray matter deficits common to both disorders included bilateral caudate, left insula and bilateral uncus regions.
Conclusions
There is a significant overlap in the distribution of structural brain changes in First Episode Schizophrenia and Fronto-Temporal Lobar Degeneration. This may reflect overlapping aetiologies, or a common vulnerability of these regions to the distinct aetio-pathological processes in the two disorders.
Keywords: Schizophrenia/pathology, FTLD/pathology, Meta-analysis, Magnetic resonance imaging/methods, Humans, Brain/pathology, Brain mapping, Imaging processing, Computer-Assisted/methods
Background
Schizophrenia is a disorder characterised by positive symptoms (hallucinations and delusions), thought disorder and negative symptoms (such as apathy). Brain imaging studies have identified structural changes both early in the presentation of the illness and more extensive changes later in the course of the illness [1]. The distribution of changes has been replicated between studies [2], and may be considered a ‘structural signature’ of schizophrenia within the brain. However, an adequate explanation for this spatial distribution remains elusive. Models have been proposed involving genetic factors coding neuro-protein variants resulting in abnormal development of limbic and frontal-temporal-subcortical networks [3]. However, it remains uncertain which neuro-chemical pathways are responsible and how they cause the spatial changes.
FTLD is characterised by declines in social function, interpersonal conduct, emotional blunting, speech and language abnormalities and loss of insight [4]. It is associated with degeneration of the prefrontal and anterior temporal cortices [5]. However, the topographical distribution of structural brain changes is heterogeneous amongst different patient groups, reflecting behavioral and pathological variants. Although the most common histological feature is tauopathy [6], over half of all patients, including those with a family history of the condition, have no abnormality in the tau gene or protein [7], consistent with pathological and aetiological heterogeneity.
In this study, we investigate whether there is overlap in the distribution of brain changes in First Episode Schizophrenia (FES) and Fronto-Temporal Lobar Degeneration (FTLD). There is evidence for similarities in clinical, neuropsychological and neuroimaging findings in patients with FTLD and schizophrenia [8]. In some cases, patients with FTLD have been diagnosed with a schizophrenia-like psychotic illness years before the dementia diagnosis is made [9]. Echopraxia, echolalia, aprosody of speech, utilisation behavior, ‘negative’ symptoms, self-neglect, and bizarre, compulsive, and stereotyped behaviors are well recognised in both disorders. Executive dysfunction with relative preservation of visual perception and spatial skills [7,10] and deficits in social cognition, theory of mind, empathy and affect recognition have been identified in both disorders [11].
Frontal, temporal and hippocampal atrophy [12,13] and regionally specific reductions in the anterior corpus callosum [14,15] and anterior hippocampus [16,17] have been described in MRI studies of both FTLD and schizophrenia. Frontal hypoperfusion on single photon emission tomography or positron emission tomography constitutes one of the imaging criteria for the diagnosis of frontotemporal dementia [18], and is also one of the most robust functional imaging findings in the schizophrenia literature in patients with chronic and first-episode illness [19]. There is also recent evidence that schizophrenia and FTLD co-occur in some families, suggesting the possibility of a common vulnerability to these disorders [20]. While the pathology of schizophrenia remains uncertain, there have been considerable advances in elucidating the complex and heterogeneous pathology of FTLD [21,22]. We have chosen to examine FES rather than chronic schizophrenia because the structural changes in FES may more accurately reflect the pathological changes of the disorder, and may minimize the confounding effects of long-term medication and other aspects of chronic illness. Different antipsychotics have individual volumetric effects on brain structure [23,24] and therefore, patients with FES were used in order to reduce heterogeneity.
The aim of this review is to determine the distribution of brain changes in FTLD and FES, by employing an established meta-analytic technique (anatomical likelihood estimation, ALE) [25] that is widely used for coordinate-based meta-analyses of neuroimaging data by converting the co-ordinates of peak gray matter changes from multiple published studies into spatial probability maps. However, the accuracy and extent of these maps is dependent on the total number of peak co-ordinates available from published studies. Therefore, this study employs a new statistical approach to investigate the degree of spatial correspondence between the two disorders, taking into account the greater availability of data co-ordinates for FTLD than FES. The comparison of brain changes between an individual MRI scan and maps for different disorders may become increasingly important for early diagnosis, as currently, diagnoses of psychiatric disorders are made on the basis of clinical manifestations and associated psychosocial disturbances. There are current initiatives to encourage the classification of mental disorders for research purposes, such as the RDoC (Research Domain Criteria) approach [26]. Several MRI-based studies have attempted to distinguish between patients and healthy subjects with high accuracy (ranging from 75 to 92%) [27]. Therefore, the statistical technique described in this paper for assessing spatial overlap may have wider clinical utility in the future.
Methods
The PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines were followed to conduct this review [28].
Literature search
A comprehensive keyword search of EMBASE (from 1980), PsycINFO (from 1801) and Medline (from 1950), was conducted using the following search strategy. The following Boolean phrase was used: {([Magnetic Resonance Imaging] OR [MRI]) AND ([Schizophrenia] OR [schizo*] OR [FTLD] OR [Fronto-Temporal Lobar Degeneration) AND ([Voxel] OR [VBM])}. Both free-text and expanded medical subject headings were be used. The search strategy was supplemented using a cited reference search, and by inspecting the reference lists of included articles. The search was conducted in January 2011. No time span was specified for date of publication.
Criteria for inclusion/exclusion
Studies were considered for the review using the following inclusion criteria: 1) they were published in English as a peer-reviewed article (rather than a letter, abstract, or case report); 2) they compared a sample of formally diagnosed subjects with a group of unrelated healthy control subjects; 3) they utilized voxel-based analysis of gray matter in structural MRI scans to investigate differences in whole-brain; and 4) they reported the three-dimensional co-ordinates of changes in stereotactic space.
FTLD studies were considered if they consisted of subjects with Fronto-Temporal Lobar Degeneration or related diagnoses, such as Semantic Dementia. FES studies were considered if they included a group of subjects with schizophrenia or related diagnoses, specifically first episode schizoaffective disorder or psychosis. Only first-episode schizophrenia patients were included, therefore, papers must have documented that patients were experiencing symptoms related to psychosis without a prior diagnosis without administration of prior antipsychotic medication in order to be included in our meta-analysis.
Studies were excluded if: 1) there were insufficient data to extract the number of subjects in each group; 2) there were fewer than five subjects in either the patient group or the comparison group; 3) the comparison groups consisted of patients with minor non-psychiatric illnesses; 4) the patient group in the schizophrenia studies consisted of subjects with child-onset schizophrenia or chronic schizophrenia beyond the stage of first-episode psychosis; 5) the studies used region-of-interest volumetric analyses, or the deformation- or tensor-based volumetric methods for measuring regional brain volumes; and 6) the data contributed to another publication, in which case the publication with the largest group size under study was selected.
Data abstraction
Data were extracted from two independent investigators (BO and IEW) and were double-checked. Information gathered from the studies included the authors, year of publication, demographic variables (number of subjects, age at baseline and gender), illness variables (diagnosis and duration of illness), and the reported stereotactic coordinates related to the comparisons between structural MRI scans of patients and controls from every selected study. Co-ordinates that were reported in the stereotactic space of the Montreal Neurological Institute (MNI) were converted to Talairach coordinates using the Lancaster transform (icbm2tal) in the GingerALE 2.0 program [29]. Talairach co-ordinates that had been generated by the Brett transform applied to statistical parametric mapping MNI co-ordinates were transformed back to MNI space in GingerALE and then to Talairach space using the Lancaster transform.
Statistical analysis
Meta-analyses were performed using the Talairach stereotactic coordinates derived from the included studies. Meta-analyses were carried out using GingerALE 2.0 [29]. This uses the technique of Activation Likelihood Estimation (ALE) [25] and permits weighting of studies in the meta-analysis, e.g. based on sample size. This modification of ALE treats the spatial conjunction of co-ordinates from separate studies as more significant than conjunction of co-ordinates from a single study. The probability values can then be interpreted on an image-wide basis after correction for multiple testing using the False Discovery Rate, a method which controls the proportion of type 1 errors (false positives) among significant results [30].
Descriptive meta-analyses were then performed to identify the distribution of brain changes in FES and FTLD, when compared to control subjects. The overlap of these two distributions was measured.
Spatial overlap testing
We tested the overlap of the FTLD co-ordinates with the FES spatial map generated by ALE using a randomisation method based on our previous work hybridising ALE spatial techniques with GSMA (Genome Scan Meta-Analysis) statistical methods [31].
For each FTLD study, the reported loci of maximal anatomical difference were modeled as the peaks of three-dimensional Gaussian probability density functions with full-width half-maximum of 7 mm, within a brain mask of size N of linear dimension 2 mm. The voxels in this probability image were then ranked from N (highest probability) to 1 (lowest probability), giving voxels of equal probability a mean rank. This created a rank image for each study which was smoothed with a 7 mm Gaussian filter. This image was masked with the First Episode Schizophrenia spatial map generated by ALE and the total value of ranks within the mask was calculated. The total for all studies of ranks within the mask was calculated (‘FES Mask Total’).
A null distribution for the FES Mask Total result was derived by 1000 permutations of the same process, but using an equal number of co-ordinates for each study derived from a random uniform distribution of coordinates within the brain mask. The probability of a FES Mask Total under the null hypothesis was calculated as the proportion of permutations giving a value equal or greater than the actual value.
The data set being tested was included in the ranking of all known outcomes. The significance threshold was set at p < 0.05.
Results
The electronic literature search of the three databases yielded 396 articles, of which 181 were retrieved in full-text format. 56 studies were identified as being potentially appropriate to be included in the meta-analysis, and the inclusion and exclusion criteria was used throughout the selection process to yield 38 articles appropriate for use in the meta-analysis. Figure 1 displays a flow diagram that shows the reasons for exclusion at each stage of the selection process.
Figure 1.
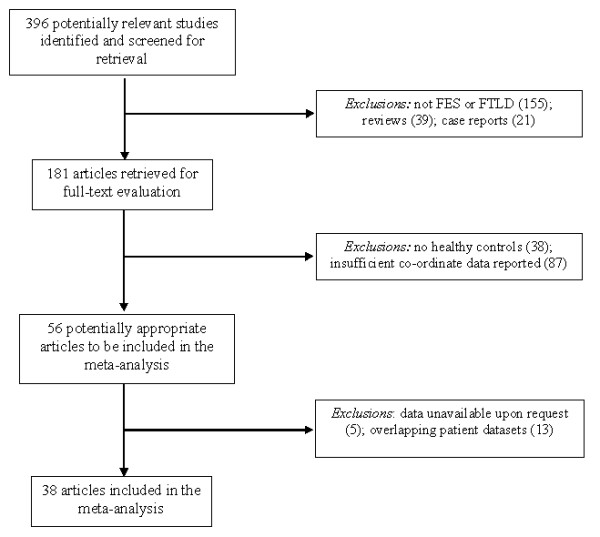
Study flow and reasons for exclusion.
A total of eighteen FES studies and twenty FTLD studies were identified for inclusion in the meta-analysis (Table 1), having published MRI co-ordinates of gray matter changes in FES or FTLD patients and healthy controls [32-69]. The FES studies included a total of 555 patients and 621 comparison subjects, and the FTLD studies included a total of 311 patients and 431 comparison subjects. Among the FES patient group, the smallest size sample was 13 schizophrenia patients [44], and the largest size sample was 68 schizophrenia patients [40]. Among the FTLD patient group, the smallest size sample was 6 patients [50,62], and the largest size sample was 51 patients [60]. All included studies were published from 2000 to 2010.
Table 1.
Studies included in the Meta-analyses
| First author, Year of publication | Disorder type | Number of patients | Number of controls | Mean age of patients (yrs) | Mean age of controls (yrs) |
|---|---|---|---|---|---|
|
FIRST EPISODE SCHIZOPHRENIA |
|
|
|
||
| Berge, 2010
[32] |
PNOS (FE) |
21 |
20 |
24.8 |
25.3 |
| Chua, 2007
[33] |
SZ, SZF, BPSY (FE) |
26 |
38 |
32.0 |
33.0 |
| Douaud, 2007
[34] |
SZ (FE) |
25 |
25 |
16.5 |
16.2 |
| Ebdrup, 2010
[35] |
SZ (FE) |
38 |
43 |
26.2 |
26.9 |
| Jayakumar, 2005
[36] |
SZ (FE) |
18 |
18 |
24.9 |
25.7 |
| Job, 2002
[37] |
SZ (FE) |
34 |
36 |
21.4 |
21.2 |
| Kasparak, 2007
[38] |
SZ (FE) |
22 |
18 |
23.7 |
24.1 |
| Kubicki, 2002
[39] |
SZ (FE) |
16 |
18 |
26.0 |
24.0 |
| Lui, 2009
[40] |
SZ (FE) |
68 |
68 |
24.2 |
24.7 |
| Meda, 2008
[41] |
WPIC |
22 |
21 |
25.1 |
26.2 |
| Molina, 2010
[42] |
SZ (FE) |
30 |
41 |
25.8 |
29.4 |
| Price, 2010
[43] |
SCZ, SZA (FE) |
48 |
47 |
26.2 |
24.8 |
| Salgado-Pineda, 2003
[44] |
SZ (FE) |
13 |
13 |
23.8 |
23.4 |
| Schaufelberger, 2007
[45] |
SZ, SZF (FE) |
62 |
94 |
27.6 |
30.2 |
| Venkatasubramanian, 2010
[46] |
SZ (FE) |
30 |
27 |
30.1 |
27.4 |
| Whitford, 2005
[47] |
SZ (FES) |
41 |
47 |
19.8 |
19.3 |
| Witthaus, 2009
[48] |
SZ (FES) |
23 |
29 |
26.4 |
25.7 |
| Yoshihara, 2008
[49] |
SZ (FES) |
18 |
18 |
15.8 |
15.8 |
|
Total (mean) |
|
555 (30.8) |
621 (34.5) |
(24.4) |
(24.5) |
|
FRONTO-TEMPORAL LOBAR DEGENERATION |
|
|
|
|
|
| Adlam, 2006
[50] |
FTLD: SD, fPPA |
6 |
12 |
62.8 |
65.0 |
| Boccardi, 2005
[51] |
FTLD |
9 |
26 |
62.0 |
69.0 |
| Boxer, 2003
[52] |
FTLD: SD |
11 |
15 |
66.2 |
69.6 |
| Chang, 2005
[53] |
FTLD: FTLD&ALS vs. ALS |
10 |
10 |
64.5 |
49.9 |
| Desgranges, 2007
[54] |
FTLD: SD |
10 |
17 |
65.7 |
65.8 |
| Gee, 2003
[55] |
FTLD |
29 |
12 |
65.1 |
68.5 |
| Gorno-Tempini, 2004
[56] |
FTLD: SD |
10 |
10 |
63.0 |
69.1 |
| Grossman, 2004
[57] |
FTLD |
29 |
12 |
65.1 |
68.5 |
| Kanda, 2008
[58] |
FTLD: bvFTD |
13 |
20 |
64.9 |
65.2 |
| Kim, 2007
[59] |
FTLD |
14 |
61 |
63.3 |
68.0 |
| Libon 2009
[60] |
FTLD: bvFTD |
51 |
43 |
61.3 |
n/av: age-matched |
| Massimo 2009
[61] |
FTLD: disinhibition-predominant |
4 |
10 |
63.6 |
64.1 |
| Mummery 2000
[62] |
FTLD: SD |
6 |
14 |
60.5 |
62.0 |
| Nestor 2003
[63] |
FTLD: PNFA |
7 |
10 |
71.5 |
65.9 |
| Pardini 2009
[64] |
FTLD: bvFTD |
22 |
12 |
60.3 |
n/av: age-matched |
| Pereira 2009
[65] |
FTLD: ubiquitin positive |
9 |
25 |
64.0 |
63.8 |
| Rabinovici 2007
[66] |
FTLD |
18 |
40 |
62.5 |
63.5 |
| Rosen 2002
[67] |
FTLD: bvFTD |
20 |
20 |
61.8 |
62.3 |
| Seeley 2008
[68] |
FTLD: bvFTD CDR2-3 |
15 |
45 |
62.3 |
68.3 |
| Williams 2005
[69] |
FTLD |
18 |
17 |
60.8 |
61.7 |
| Total (mean) | 311 (15.6) | 431 (21.6) | (63.6) | (65.0) | |
All studies using voxel-based morphometry of gray matter compared a patient group with a comparison group. Abbreviations: BPSY, Brief Psychotic Disorder; FE, First episode; PNOS, Psychosis not otherwise specified; SZ, Schizophrenia; SZA, Schizo-affective disorder; SZF, Schizophreniform disorder; WPIC, Patients from the Western Psychiatric Institute and Clinic at the University of Pittsburgh; FTLD, Fronto-temporal Lobar Degeneration; SD, Semantic Dementia; fPPA, Fluent form of Primary Progressive Aphasia; ALS, Amyotrophic Lateral Sclerosis; bvFTD, behavioural / dysexecutive subtype of FTLD; PFNA, Progressive non-fluent Aphasia; CDR, Clinical Dementia Rating (range: 0.5 – 3); n/av, data not available.
ALE meta-analyses
Eighteen FES studies included 185 co-ordinates of gray matter decreases. Meta-analysis of this data identified gray matter decreases in regions including bilateral head of caudate nucleus, bilateral insula, bilateral amygdala/uncus region and bilateral superior temporal region (Figure 2).
Figure 2.
Regions of gray matter change in FES and FTLD subjects. Regions of gray matter decreases in FES subjects compared with controls (red), regions of gray matter decreases in FTLD subjects compared with controls (yellow), and overlapping regions of gray matter decreases controls (orange), displayed on a brain template. The left side of the image represents the left side of the brain. The Talairach level (z co-ordinate) is given above each horizontal slice.
Twenty FTLD studies provided 260 co-ordinates of gray matter decreases. Meta-analysis of this data identified gray matter decreases in regions including bilateral head of caudate nucleus, bilateral insula, bilateral amygdala/uncus region and bilateral superior temporal region (Table 2, Figure 2).
Table 2.
Regions of gray matter decreases in FES and FTLD subjects compared with controls
|
Cluster |
Region |
Talairach coordinates |
Value |
Volume (mm3) |
|||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
|
FIRST EPISODE SCHIZOPHRENIA |
|
||||||
| 1 |
R/L |
Caudate |
2 |
14 |
0 |
0.0046 |
1624 |
| |
R |
Caudate |
10 |
10 |
12 |
0.0017 |
|
| 2 |
L |
Uncus |
−18 |
−2 |
−22 |
0.0031 |
1272 |
| |
L |
Amygdala |
−12 |
0 |
−14 |
0.0028 |
|
| 3 |
R |
Superior temporal gyrus |
48 |
−24 |
10 |
0.0019 |
400 |
| |
R |
Superior temporal gyrus |
48 |
−26 |
16 |
0.0018 |
|
| 4 |
L |
Insula |
−36 |
20 |
6 |
0.0025 |
376 |
| 5 |
L |
Transverse temporal gyrus |
−46 |
−18 |
10 |
0.0021 |
288 |
| 6 |
R |
Middle temporal gyrus |
54 |
−26 |
−2 |
0.0022 |
272 |
| 7 |
L |
Superior temporal gyrus |
−58 |
−26 |
12 |
0.0021 |
272 |
| 8 |
L |
Superior temporal gyrus |
−56 |
2 |
−4 |
0.0021 |
216 |
| 9 |
R |
Insula |
36 |
8 |
10 |
0.0021 |
216 |
| 10 |
R |
Parietal lobe: Postcentral gyrus |
54 |
−20 |
44 |
0.0022 |
216 |
| 11 |
L |
Frontal lobe: Middle frontal gyrus |
−30 |
50 |
4 |
0.002 |
200 |
| 12 |
L |
Frontal lobe: Inferior frontal gyrus |
−22 |
28 |
−6 |
0.0018 |
184 |
| 13 |
L |
Parietal lobe: Postcentral gyrus |
−60 |
−18 |
20 |
0.002 |
184 |
| 14 |
R |
Amygdala / Uncus |
20 |
−4 |
−22 |
0.002 |
168 |
| 15 |
L |
Superior temporal gyrus |
−32 |
14 |
−22 |
0.0019 |
152 |
| 16 |
L |
Inferior temporal gyrus |
−46 |
−14 |
−18 |
0.002 |
152 |
| 17 |
L |
Cingulate gyrus |
−8 |
−4 |
38 |
0.002 |
152 |
| 18 |
R |
Insula / Claustrum |
34 |
−14 |
12 |
0.0018 |
136 |
| 19 |
R |
Medial frontal gyrus (BA 8) |
14 |
34 |
36 |
0.0017 |
136 |
| 20 |
L |
Cingulate gyrus |
0 |
−42 |
38 |
0.0016 |
112 |
| 21 |
R |
Medial frontal gyrus (BA 6) |
6 |
6 |
48 |
0.0017 |
112 |
|
FRONTO-TEMPORAL LOBE DEGENERATION | |||||||
| 1 |
R |
Amygdala / Uncus |
22 |
−4 |
−20 |
0.0029 |
3744 |
| |
R |
Globus Pallidus region |
20 |
2 |
−4 |
0.0024 |
|
| |
R |
Hippocampus / Parahippocampus |
28 |
−20 |
−10 |
0.0015 |
|
| 2 |
L |
Amygdala / Parahippocampus |
−22 |
−6 |
−16 |
0.0027 |
2336 |
| |
L |
Globus Pallidus region |
−18 |
0 |
−6 |
0.0025 |
|
| |
L |
Uncus |
−26 |
−4 |
−28 |
0.002 |
|
| 3 |
L |
Anterior cingulate |
−2 |
10 |
−4 |
0.0024 |
2144 |
| |
L |
Caudate |
−8 |
10 |
10 |
0.0022 |
|
| |
L |
Caudate |
−6 |
2 |
14 |
0.0015 |
|
| 4 |
L |
Frontal lobe: Medial frontal gyrus (BA 9) |
−4 |
48 |
24 |
0.003 |
1256 |
| 5 |
L |
Frontal lobe: Inferior frontal gyrus |
−36 |
24 |
4 |
0.0022 |
936 |
| |
L |
Insula |
−38 |
14 |
0 |
0.0016 |
|
| 6 |
L |
Superior temporal gyrus |
−42 |
12 |
−14 |
0.0021 |
896 |
| |
L |
Superior temporal gyrus |
−44 |
2 |
−12 |
0.0018 |
|
| 7 |
R |
Frontal lobe: Inferior frontal gyrus |
42 |
16 |
−12 |
0.003 |
880 |
| 8 |
L |
Frontal lobe: Middle frontal gyrus |
−44 |
8 |
38 |
0.0032 |
784 |
| 9 |
R |
Frontal lobe: Inferior frontal gyrus |
42 |
16 |
12 |
0.0025 |
768 |
| 10 |
L |
Frontal lobe: Superior frontal gyrus |
−22 |
60 |
2 |
0.0023 |
704 |
| |
L |
Frontal lobe: Superior frontal gyrus |
−24 |
56 |
12 |
0.0017 |
|
| 11 |
R |
Cingulate gyrus |
16 |
26 |
36 |
0.0025 |
576 |
| 12 |
R |
Insula |
42 |
−6 |
−4 |
0.0021 |
568 |
| 13 |
L |
Temporal lobe: fusiform gyrus |
−42 |
−30 |
−18 |
0.0027 |
520 |
| 14 |
L |
Frontal lobe: Middle frontal gyrus |
−22 |
18 |
50 |
0.0027 |
496 |
| 15 |
L |
Anterior cingulate |
−2 |
32 |
16 |
0.002 |
400 |
| 16 |
R |
Caudate |
8 |
12 |
12 |
0.0024 |
376 |
| 17 |
R |
Middle temporal gyrus |
48 |
2 |
−28 |
0.0024 |
312 |
| 18 |
L |
Frontal lobe: Inferior frontal gyrus |
−40 |
52 |
2 |
0.0024 |
232 |
| 19 |
L |
Frontal lobe: Middle frontal gyrus |
−28 |
34 |
−14 |
0.002 |
224 |
| 20 |
L |
Insula |
−42 |
2 |
0 |
0.0017 |
216 |
| 21 |
L |
Parietal lobe (BA 7) |
−20 |
−46 |
58 |
0.0023 |
216 |
| 22 |
L |
Frontal lobe: Medial frontal gyrus (BA 10) |
0 |
54 |
−4 |
0.0018 |
192 |
| 23 |
R |
Superior temporal gyrus |
28 |
14 |
−30 |
0.0016 |
176 |
| 24 |
L |
Uncus |
−34 |
−16 |
−34 |
0.0017 |
136 |
| 25 |
R |
Cerebellum: Anterior lobe |
48 |
−36 |
−28 |
0.0023 |
136 |
| 26 |
R |
Frontal lobe: Middle frontal gyrus |
36 |
44 |
8 |
0.0017 |
120 |
| 27 | R | Uncus | 28 | −10 | −32 | 0.0016 | 112 |
Common changes in FES and FTLD
Gray matter decreases were present in both disorders in regions including bilateral caudate head, left insula and bilateral uncus region (Figure 3).
Figure 3.

Gray matter deficits in FES subjects (red) and FTLD subjects (yellow). Congruent changes in both disorders are shown in orange. Brain changes are shown in three-dimensional views with brain sections removed (A, B), a sagittal plane (C), an axial plane (D) and a coronal plane (E). The Talairach co-ordinate for each plane is given above each image (C, D, E) (P = posterior, A = anterior, S = superior, L = left).
The spatial congruence between the disorders was estimated by percentage overlap. The FTLD map of gray matter decreases comprised 2450 voxels in a brain mask of 201069 voxels. The FES map of gray matter decreases comprised 888 voxels in a brain mask of 201069 voxels. The overlap between the FTLD and FES maps was 124 voxels. Therefore the overlap of FTLD within FES was 14% (compared to an expected overlap of 1.2% if the FTLD map was randomly distributed in the brain mask). The overlap of FES within FTLD was 5% (compared to an expected overlap of 0.4% if the FES mask was randomly distributed in the brain mask). Applying the spatial overlap testing method, the overlap of the FTLD co-ordinates with the FES spatial map generated by ALE was significant (p = 0.001).
Discussion
In this study, we identified overlap in the distribution of brain changes in First Episode Schizophrenia (FES) and Fronto-Temporal Lobar Degeneration (FTLD). We found that the two disorders involved gray matter deficits in common regions (p = 0.001). These included the basal ganglia (bilateral caudate head), paralimbic (left insula) and limbic (bilateral uncus) regions, as shown in Figure 4. To our knowledge, this is the first meta-analysis that compares neuro-structural profiles between FES and FTLD. Our study presents a novel method using ALE analyses to derive a statistical test for the chance overlap of the spatial distribution of the two disorders.
Figure 4.
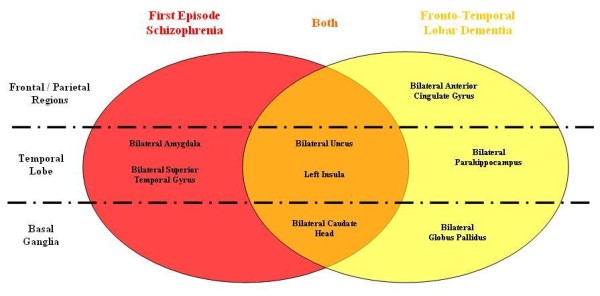
Venn diagram to summarise the distinct and overlapping regions of gray matter deficits found in FES and FTLD.
One approach to investigating the spatial distribution of brain changes in schizophrenia involves conceptualising the disorder as affecting several neural sub-systems which are individually affected in other neuro-psychiatric disorders with genetic or syndromal links to schizophrenia. For example, there is evidence that bipolar disorder and schizophrenia share susceptibility genes, and gray matter deficits of bipolar disorder overlap with those of schizophrenia but are more limited to paralimbic regions involved in emotion regulation [70-72]. Temporal lobe epilepsy is also associated with psychotic episodes with similarities to schizophrenia and is associated with gray matter deficits in limbic regions, overlapping with those of schizophrenia [73-75]. Autistic spectrum disorders and schizophrenia share gray matter deficits in the limbo-striato-thalamic circuitry [76], possibly reflecting shared genetic [77,78] and environmental [79-82] risk factors.
Previous results of meta-analyses and structural neuro-imaging studies of FES and FTLD
These results confirm those of previous meta-analyses of the two disorders, using smaller samples of primary studies. Several previous meta-analyses using ‘region-of-interest’ analyses have examined gray matter deficits in schizophrenia [83,84], with consistent findings of medial temporal lobe atrophy [85-88]. Patients with FES also have smaller whole-brain volume, with greater lateral ventricular volume [89]. Frontal [90,91] and temporal [37] volumes have been reported to be smaller at first episode, and basal ganglia are also affected early in the illness [33,92]. The ALE method has also been used to investigate brain structural abnormalities in schizophrenia derived from studies using voxel-based morpometry analyses [1,93]. In a recent meta-analysis by Chan et al., patients with FES had lower gray matter volumes in frontal, temporal, striatal, and cerebellar regions compared with both control subjects and people who are at genetically high-risk of developing schizophrenia [94]. They mapped progressive changes from the high-risk stage to the first-episode stage, clarifying potential markers for disease risk (anterior cingulate and right insula volume reduction) and for disease onset (caudate volume reduction) [94].
In FTLD, a meta-analysis of voxel-based morphometry studies found predominant frontal and temporal lobe involvement, with specific patterns of atrophy in the three clinical subtypes of FTLD, namely, FTD (frontotemporal dementia), SD (semantic dementia) and PNFA (progressive non-fluent aphasia) [95]. Correspondingly, diagnostic criteria for FTLD create a ‘triple dissociation’ of these subtypes with a high diagnostic accuracy clinically [96]. The clinical characteristics of each subtype of FTLD correspond well with the neuroanatomical deficits found.
Functional significance of implicated regions and symptoms of FES and FTLD
The involvement of the basal ganglia (bilateral gray matter decreases in the caudate head in both disorders) agrees with Middleton and Strick’s prediction that abnormalities within cortico-striatal circuits may underlie neuropsychiatric symptoms [97,98]. Anatomical studies have revealed discrete connections between the basal ganglia and the cerebral cortex, reciprocally interconnecting a large and diverse set of cortical areas [99]. Dysregulation and abnormal dopaminergic transmission in these loops may contribute to hyperkinetic movements as well as cognitive impairments [100-102]. Robbins [103] proposed that the heterogeneous range of core symptoms associated with psychosis, appearing to be associated with a range of structural and functional abnormalities, might be explained by a frontal-striatal hypothesis, where an altered balance in the flow of information between through the basal ganglia could explain the seemingly disparate symptoms and characteristics of schizophrenia and psychotic episodes in FTLD. Our results indicate that the basal ganglia loop in particular incorporating the head of the caudate nucleus, are affected by FES and FTLD, which may at least partly explain their shared symptoms.
Our findings reveal that both disorders are characterised by reduced volumes of various paralimbic and limbic structures. Neuroanatomists and cytoarchitectologists have grouped the regions shown to be affected in FES and FTLD, namely the superior temporal gyrus (temporal pole), rostral and caudal anterior cingulate, posterior cingulate, orbital frontal cortex, insula, and parahippocampal regions, into the paralimbic cortex [104,105]. There is documented evidence to support paralimbic dysfunction in various psychiatric disorders, namely psychopathy [106], bipolar disorder [72], psychotic symptoms in depressive disorder [107] and attention deficit hyperactivity disorder [108]. Our results also indicate that both disorders are characterised by reduced caudate size, lesions of which cause impairments in learning, memory [109] and behaviour through the selection of appropriate sub-goals based on an evaluation of action-outcomes [110]. This may reflect the common symptomatology between the two disorders.
Explanation for congruence
Various explanations need to be considered for the overlap in gray matter deficits between the two disorders. Firstly, the result could be due to coincidence. However, we have applied a statistical test to the spatial congruence which suggests that this is highly unlikely (the p value for the null hypothesis of a random distribution of FTLD co-ordinates within the FES spatial map was p = 0.001). Secondly, the symptoms of neuropsychiatric disorders are related to the anatomy of the brain pathology. Therefore, the selection of schizophrenia and FTLD, which share certain symptoms (as described above) may constrain the anatomical findings towards certain brain regions, in the absence of any other more meaningful connection between the neuropathology of the two disorders. The evidence that schizophrenia and FTLD co-occur in some families [6] suggests the possibility of a more fundamental connection between the two disorders. Thirdly, the neuropathology of the two disorders may be (at the neurochemical pathway level) distinct but the common network identified in this study may be ‘a final common pathway’ in the pathological process of both disorders, or a common reaction to such processes.
Limitations
There are limitations of this meta-analysis. Firstly, by meta-analysing co-ordinates of maximum change from primary studies there is a loss of spatial information. This reduces the spatial resolution of the results. Secondly, as more primary studies are published the distribution of changes in each disorder may become more extensive as there is improved power to detect changes. Thirdly, there are alternative approaches for investigating spatial overlap of two disorders. For example, Yu et al., used a post-hoc meta-analytic estimation of the extent to which gray matter compares with controls in bipolar disorder and schizophrenia by using a modification of the ALE method [71]. Lastly, the mean age of patients with FES was 24.4 years, whereas in FTLD, the mean age was 63.6 years (Table 1). There are no validated methods to account for these differences in an ALE meta-analysis, and it is possible that some of the results may have been affected by “normal” structural brain aging processes [111]. Anatomical likelihood estimation analyses are a relatively novel and changing technique, and as time progresses, standardisation of meta-analysis techniques will help researchers evaluate results from different studies more objectively.
Future directions
In the future, localized gray matter deficits detected via the above analyses may be combined with those identified in activation studies of cognitive deficits in schizophrenia and FTLD, in order to understand the correlation between functional and structural connectivity in both disorders. For example, the structural MRI gray matter deficits could be used as nodes for a network analysis [112], as can now be done on individual scans [113], which may be utilized to investigate and compare functional connectivity changes in FES and FTLD.
Conclusions
In summary, we reviewed data from 18 FES studies and 20 studies of FTLD that used voxel-based morphometry to identify common structural brain abnormalities. The brain regions found to be significantly affected included gray matter deficits in the bilateral caudate, left insula and bilateral uncus regions. The overlap in distribution of the disorders does not necessarily indicate a fundamental sharing of neurochemical pathology between FES and FTLD. However, we propose that the emerging genetic, pathological and clinical typology of FTLD may provide a model for the deconstruction of subtypes of schizophrenia.
Competing interests
BO, IEW and SML disclose that they have no competing interests. EB is employed half-time by GlaxoSmithKline (GSK) and half-time by the University of Cambridge and is a stockholder in GSK.
Authors’ contributions
BO and IEW devised the study and wrote the first draft of the manuscript. BO ascertained the studies and extracted the data. IEW checked and analysed the data. EB and SML participated in the design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Bayanne Olabi, Email: b.olabi@sms.ed.ac.uk.
Ian Ellison-Wright, Email: ian.ew@inbox.com.
Ed Bullmore, Email: etb23@cam.ac.uk.
Stephen M Lawrie, Email: s.lawrie@ed.ac.uk.
Acknowledgements
The listed authors were solely involved the in designing the study, data acquisition, analysis and interpretation, as well as drafting and revising the manuscript, with no external contributions. SML has received financial support from Pfizer (formerly Wyeth) in relation to imaging studies of people with schizophrenia, but this played no role in influencing this study.
References
- Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165(8):1015–23. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Tandon R, Boutros NN, Nasrallah HA. Schizophrenia, "just the facts": what we know in 2008 Part 3: neurobiology. Schizophr Res. 2008;106(2–3):89–107. doi: 10.1016/j.schres.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Goghari VM, Sponheim SR, MacDonald AW 3rd. The functional neuroanatomy of symptom dimensions in schizophrenia: a qualitative and quantitative review of a persistent question. Neurosci Biobehav Rev. 2010;34(3):468–86. doi: 10.1016/j.neubiorev.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/WNL.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Mohandas E, Rajmohan V. Frontotemporal dementia: An updated overview. Indian J Psychiatry. 2009;51(Suppl 1):S65–9. [PMC free article] [PubMed] [Google Scholar]
- Taniguchi S, McDonagh AM, Pickering-Brown SM, Umeda Y, Iwatsubo T, Hasegawa M, Mann DM. The neuropathology of frontotemporal lobar degeneration with respect to the cytological and biochemical characteristics of tau protein. Neuropath Appl Neurobiol. 2004;30:1–18. doi: 10.1046/j.0305-1846.2003.00481.x. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden J, Mann D. Frontotemporal dementia. Lancet Neurol. 2005. pp. 771–80. [DOI] [PubMed]
- Momeni P, Wickremaratchi MM, Bell J, Arnold R, Beer R, Hardy J, Revesz T, Neal JW, Morris HR. Familial early onset frontotemporal dementia caused by a novel S356T MAPT mutation, initially diagnosed as schizophrenia. Clin Neurol Neurosurg. 2010;112(10):917–20. doi: 10.1016/j.clineuro.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Walterfang M, Mocellin R, Pantelis C, McLean C. Frontotemporal dementia presenting as schizophrenia-like psychosis in young people: clinicopathological series and review of cases. Br J Psychiatry. 2009;194(4):298–305. doi: 10.1192/bjp.bp.108.057034. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Barnes TR, Nelson HE, Tanner S, Weatherley L, Owen AM, Robbins TW. Frontal-striatal cognitive deficits in patients with chronic schizophrenia. Brain. 1997;120(Pt 10):1823–43. doi: 10.1093/brain/120.10.1823. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, Levkovitz Y. The neuroanatomical basis of affective mentalizing in schizophrenia: comparison of patients with schizophrenia and patients with localized prefrontal lesions. Schizophr Res. 2007;90(1–3):274–83. doi: 10.1016/j.schres.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Abukmeil SS. Brain abnormality in schizophrenia. A systematic and quantitative review of volumetric magnetic resonance imaging studies. Br J Psychiatry. 1998;172:110–20. doi: 10.1192/bjp.172.2.110. [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Wood SJ, Wong MT, McGorry PD, Yung A, Phillips L, Smith D, Brewer W, Proffitt T, Desmond P, Pantelis C. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63(2):139–49. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- Kaufer DI, Miller BL, Itti L, Fairbanks LA, Li J, Fishman J, Kushi J, Cummings JL. Midline cerebral morphometry distinguishes frontotemporal dementia and Alzheimer's disease. Neurology. 1997;48(4):978–85. doi: 10.1212/WNL.48.4.978. [DOI] [PubMed] [Google Scholar]
- Walterfang M, Wood AG, Reutens DC, Wood SJ, Chen J, Velakoulis D, McGorry PD, Pantelis C. Morphology of the corpus callosum at different stages of schizophrenia: cross-sectional study in first episode and chronic illness. Br J Psychiatry. 2008;192(6):429–34. doi: 10.1192/bjp.bp.107.041251. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Frisoni GB, Kononen M, Mikkonen M, Beltramello A, Geroldi C, Bianchetti A, Trabucchi M, Soininen H, Aronen HJ. Hippocampus and entorhinal cortex in frontotemporal dementia and Alzheimer’s disease: a morphometric MRI study. Biol Psychiatry. 2000;47:1056–63. doi: 10.1016/S0006-3223(99)00306-6. [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Stuart GW, Wood SJ, Smith DJ, Brewer WJ, Desmond P, Singh B, Copolov D, Pantelis C. Selective bilateral hippocampal volume loss in chronic schizophrenia. Biol Psychiatry. 2001;50:531–9. doi: 10.1016/S0006-3223(01)01121-0. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol. 2001;58:1803–9. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Pantelis C. What have we learned from functional imaging studies in schizophrenia? The role of frontal, striatal and temporal areas. Aust N Z J Psychiatry. 1996;30:195–209. doi: 10.3109/00048679609076095. [DOI] [PubMed] [Google Scholar]
- Schoder D, Hannequin D, Martinaud O, Opolczynski G, Guyant-Maréchal L, Le Ber I, Campion D. Morbid risk for schizophrenia in first-degree relatives of people with frontotemporal dementia. Br J Psychiatry. 2010;197:28–35. doi: 10.1192/bjp.bp.109.068981. [DOI] [PubMed] [Google Scholar]
- Pickering-Brown SM. The complex aetiology of frontotemporal lobar degeneration. Exp Neurol. 2007;206(1):1–10. doi: 10.1016/j.expneurol.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Aswathy PM, Jairani PS, Mathuranath PS. Genetics of frontotemporal lobar degeneration. Ann Indian Acad Neurol. 2010;13(Suppl 2):S55–62. doi: 10.4103/0972-2327.74246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goghari VM, Lang DJ, Khorram B, Götz J, Vandorpe RA, Smith GN, Kopala LC, Barr AM, Honer WG. Anterior internal capsule volumes increase in patients with schizophrenia switched from typicalantipsychotics to olanzapine. J Psychopharmacol. 2011;25(5):621–9. doi: 10.1177/0269881110363314. [DOI] [PubMed] [Google Scholar]
- Molina V, Reig S, Sanz J, Palomo T, Benito C, Sánchez J, Pascau J, Desco M. Changes in cortical volume with olanzapine in chronic schizophrenia. Pharmacopsychiatry. 2007;40(4):135–9. doi: 10.1055/s-2007-981479. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SE, Cuthbert BN. Research Domain Criteria: cognitive systems, neural circuits and dimensions of behaviour. Dialogues Clin Neurosci. 2012;14(1):29–37. doi: 10.31887/DCNS.2012.14.1/smorris. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Takahashi T, Orikabe L, Mozue Y, Kawasaki Y, Nakamura K, Sato Y, Itokawa M, Yamasue H, Kasai K, Kurachi M, Okazaki Y, Suzuki M. Classification of first-episode schizophrenia patients and healthy subjects by automated MRI measures of regional brain volume and cortical thickness. PLoS One. 2011;6(6):e21047. doi: 10.1371/journal.pone.0021047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven KJF, Simonsen KL, McIntyre LM. Implementing false discovery rate control: increasing your power. Oikos. 2005;108:643–647. doi: 10.1111/j.0030-1299.2005.13727.x. [DOI] [Google Scholar]
- Ellison-Wright I, Ellison-Wright Z, Bullmore E. Structural brain change in Attention Deficit Hyperactivity Disorder identified by meta-analysis. BMC Psychiatry. 2008;8:51. doi: 10.1186/1471-244X-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergé D, Carmona S, Rovira M, Bulbena A, Salgado P, Vilarroya O. Gray matter volume deficits and correlation with insight and negative symptoms in first-psychotic-episode subjects. Acta Psychiatr Scand. 2011;123(6):431–9. doi: 10.1111/j.1600-0447.2010.01635.x. [DOI] [PubMed] [Google Scholar]
- Chua SE, Cheung C, Cheung V, Tsang JT, Chen EY, Wong JC, Cheung JP, Yip L, Tai KS, Suckling J, McAlonan GM. Cerebral grey, white matter and CSF in never-medicated, first-episode schizophrenia. Schizophr Res. 2007;89:12–21. doi: 10.1016/j.schres.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, Vickers J, James S, Voets N, Watkins K, Matthews PM, James A. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130:2375–86. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- Ebdrup BH, Glenthøj B, Rasmussen H, Aggernaes B, Langkilde AR, Paulson OB, Lublin H, Skimminge A, Baaré W. Hippocampal and caudate volume reductions in antipsychotic-naive first-episode schizophrenia. J Psychiatry Neurosci. 2010;35(2):95–104. doi: 10.1503/jpn.090049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar PN, Venkatasubramanian G, Gangadhar BN, Janakiramaiah N, Keshavan MS. Optimized voxel-based morphometry of gray matter volume in first-episode, antipsychotic-naive schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:587–91. doi: 10.1016/j.pnpbp.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Job DE, Whalley HC, McConnell S, Glabus M, Johnstone EC, Lawrie SM. Structural gray matter differences between first-episode schizophrenics and normal controls using voxel-based morphometry. Neuroimage. 2002;17:880–9. doi: 10.1006/nimg.2002.1180. [DOI] [PubMed] [Google Scholar]
- Kasparek T, Prikryl R, Mikl M, Schwarz D, Ceskova E, Krupa P. Prefrontal but not temporal grey matter changes in males with first-episode schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:151–7. doi: 10.1016/j.pnpbp.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Shenton ME, Salisbury DF, Hirayasu Y, Kasai K, Kikinis R, Jolesz FA, McCarley RW. Voxel-based morphometric analysis of gray matter in first episode schizophrenia. Neuroimage. 2002;17(4):1711–9. doi: 10.1006/nimg.2002.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui S, Deng W, Huang X, Jiang L, Ma X, Chen H, Zhang T, Li X, Li D, Zou L, Tang H, Zhou XJ, Mechelli A, Collier DA, Sweeney JA, Li T, Gong Q. Association of cerebral deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized voxel-based morphometry and resting state functional connectivity study. Am J Psychiatry. 2009;166(2):196–205. doi: 10.1176/appi.ajp.2008.08020183. [DOI] [PubMed] [Google Scholar]
- Meda SA, Giuliani NR, Calhoun VD, Jagannathan K, Schretlen DJ, Pulver A, Cascella N, Keshavan M, Kates W, Buchanan R, Sharma T, Pearlson GD. A large scale (N = 400) investigation of gray matter differences in schizophrenia using optimized voxel-based morphometry. Schizophr Res. 2008;101(1–3):95–105. doi: 10.1016/j.schres.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina V, Sanz J, Villa R, Pérez J, González D, Sarramea F, Ballesteros A, Galindo G, Hernández JA. Voxel-based morphometry comparison between first episodes of psychosis with and without evolution to schizophrenia. Psychiatry Res. 2010;181(3):204–10. doi: 10.1016/j.pscychresns.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Price G, Cercignani M, Chu EM, Barnes TR, Barker GJ, Joyce EM, Ron MA. Brain pathology in first-episode psychosis: magnetization transfer imaging provides additional information to MRI measurements of volume loss. Neuroimage. 2010;49(1):185–92. doi: 10.1016/j.neuroimage.2009.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado-Pineda P, Baeza I, Perez-Gomez M, Vendrell P, Junque C, Bargallo N, Bernardo M. Sustained attention impairment correlates to gray matter decreases in first episode neuroleptic-naive schizophrenic patients. Neuroimage. 2003;19:365–75. doi: 10.1016/S1053-8119(03)00094-6. [DOI] [PubMed] [Google Scholar]
- Schaufelberger MS, Duran FL, Lappin JM, Scazufca M, Amaro E Jr, Leite CC, De Castro CC, Murray RM, McGuire PK, Menezes PR, Busatto GF. Grey matter abnormalities in Brazilians with first-episode psychosis. Br J Psychiatry Suppl. 2007;51:s117–22. doi: 10.1192/bjp.191.51.s117. [DOI] [PubMed] [Google Scholar]
- Venkatasubramanian G. Neuroanatomical correlates of psychopathology in antipsychotic-naïve schizophrenia. Indian J Psychiatry. 2010;52(1):28–36. doi: 10.4103/0019-5545.58892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford TJ, Farrow TF, Gomes L, Brennan J, Harris AW, Williams LM. Grey matter deficits and symptom profile in first episode schizophrenia. Psychiatry Res. 2005;139:229–38. doi: 10.1016/j.pscychresns.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Witthaus H, Kaufmann C, Bohner G, Ozgürdal S, Gudlowski Y, Gallinat J, Ruhrmann S, Brüne M, Heinz A, Klingebiel R, Juckel G. Gray matter abnormalities in subjects at ultra-high risk for schizophrenia and first-episode schizophrenic patients compared to healthy controls. Psychiatry Res. 2009;173(3):163–9. doi: 10.1016/j.pscychresns.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Yoshihara Y, Sugihara G, Matsumoto H, Suckling J, Nishimura K, Toyoda T, Isoda H, Tsuchiya KJ, Takebayashi K, Suzuki K, Sakahara H, Nakamura K, Mori N, Takei N. Voxel-based structural magnetic resonance imaging (MRI) study of patients with early onset schizophrenia. Ann Gen Psychiatry. 2008;7:25. doi: 10.1186/1744-859X-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlam AL, Patterson K, Rogers TT, Nestor PJ, Salmond CH, Acosta-Cabronero J, Hodges JR. Semantic dementia and fluent primary progressive aphasia: two sides of the same coin? Brain. 2006;129(Pt 11):3066–80. doi: 10.1093/brain/awl285. [DOI] [PubMed] [Google Scholar]
- Boccardi M, Sabattoli F, Laakso MP, Testa C, Rossi R, Beltramello A, Soininen H, Frisoni GB. Frontotemporal dementia as a neural system disease. Neurobiol Aging. 2005;26(1):37–44. doi: 10.1016/j.neurobiolaging.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Rankin KP, Miller BL, Schuff N, Weiner M, Gorno-Tempini ML, Rosen HJ. Cinguloparietal atrophy distinguishes Alzheimer disease from semantic dementia. Arch Neurol. 2003;60(7):949–56. doi: 10.1001/archneur.60.7.949. [DOI] [PubMed] [Google Scholar]
- Chang JL, Lomen-Hoerth C, Murphy J, Henry RG, Kramer JH, Miller BL, Gorno-Tempini ML. A voxel-based morphometry study of patterns of brain atrophy in ALS and ALS/FTLD. Neurology. 2005;65(1):75–80. doi: 10.1212/01.wnl.0000167602.38643.29. [DOI] [PubMed] [Google Scholar]
- Desgranges B, Matuszewski V, Piolino P, Chételat G, Mézenge F, Landeau B, de la Sayette V, Belliard S, Eustache F. Anatomical and functional alterations in semantic dementia: a voxel-based MRI and PET study. Neurobiol Aging. 2007;28(12):1904–13. doi: 10.1016/j.neurobiolaging.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Gee J, Ding L, Xie Z, Lin M, DeVita C, Grossman M. Alzheimer's disease and frontotemporal dementia exhibit distinct atrophy-behavior correlates: a computer-assisted imaging study. Acad Radiol. 2003;10(12):1392–401. doi: 10.1016/S1076-6332(03)00543-9. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, Weiner MW, Miller BL. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55(3):335–46. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, McMillan C, Moore P, Ding L, Glosser G, Work M, Gee J. What's in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer's disease, frontotemporal dementia and corticobasal degeneration. Brain. 2004;127(Pt 3):628–49. doi: 10.1093/brain/awh075. [DOI] [PubMed] [Google Scholar]
- Kanda T, Ishii K, Uemura T, Miyamoto N, Yoshikawa T, Kono AK, Mori E. Comparison of grey matter and metabolic reductions in frontotemporal dementia using FDG-PET and voxel-based morphometric MR studies. Eur J Nucl Med Mol Imaging. 2008;35(12):2227–34. doi: 10.1007/s00259-008-0871-5. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Rabinovici GD, Seeley WW, Halabi C, Shu H, Weiner MW, DeArmond SJ, Trojanowski JQ, Gorno-Tempini ML, Miller BL, Rosen HJ. Patterns of MRI atrophy in tau positive and ubiquitin positive frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry. 2007;78(12):1375–8. doi: 10.1136/jnnp.2006.114231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libon DJ, McMillan C, Gunawardena D, Powers C, Massimo L, Khan A, Morgan B, Farag C, Richmond L, Weinstein J, Moore P, Coslett HB, Chatterjee A, Aguirre G, Grossman M. Neurocognitive contributions to verbal fluency deficits in frontotemporal lobar degeneration. Neurology. 2009;73(7):535–42. doi: 10.1212/WNL.0b013e3181b2a4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimo L, Powers C, Moore P, Vesely L, Avants B, Gee J, Libon DJ, Grossman M. Neuroanatomy of apathy and disinhibition in frontotemporal lobar degeneration. Dement Geriatr Cogn Disord. 2009;27(1):96–104. doi: 10.1159/000194658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, Hodges JR. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47(1):36–45. doi: 10.1002/1531-8249(200001)47:1<36::AID-ANA8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Graham NL, Fryer TD, Williams GB, Patterson K, Hodges JR. Progressive non-fluent aphasia is associated with hypometabolism centred on the left anterior insula. Brain. 2003;126(Pt 11):2406–18. doi: 10.1093/brain/awg240. [DOI] [PubMed] [Google Scholar]
- Pardini M, Huey ED, Cavanagh AL, Grafman J. Olfactory function in corticobasal syndrome and frontotemporal dementia. Arch Neurol. 2009;66(1):92–6. doi: 10.1001/archneurol.2008.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JM, Williams GB, Acosta-Cabronero J, Pengas G, Spillantini MG, Xuereb JH, Hodges JR, Nestor PJ. Atrophy patterns in histologic vs clinical groupings of frontotemporal lobar degeneration. Neurology. 2009;72(19):1653–60. doi: 10.1212/WNL.0b013e3181a55fa2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovici GD, Seeley WW, Kim EJ, Gorno-Tempini ML, Rascovsky K, Pagliaro TA, Allison SC, Halabi C, Kramer JH, Johnson JK, Weiner MW, Forman MS, Trojanowski JQ, Dearmond SJ, Miller BL, Rosen HJ. Distinct MRI atrophy patterns in autopsy-proven Alzheimer's disease and frontotemporal lobar degeneration. Am J Alzheimers Dis Other Demen. 2007;22(6):474–88. doi: 10.1177/1533317507308779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, Feiwell R, Kramer JH, Miller BL. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58(2):198–208. doi: 10.1212/WNL.58.2.198. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, Miller BL, Gorno-Tempini ML. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol. 2008;65(2):249–55. doi: 10.1001/archneurol.2007.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GB, Nestor PJ, Hodges JR. Neural correlates of semantic and behavioural deficits in frontotemporal dementia. Neuroimage. 2005;24(4):1042–51. doi: 10.1016/j.neuroimage.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Bora E, Fornito A, Yücel M, Pantelis C. The effects of gender on grey matter abnormalities in major psychoses: a comparative voxelwise meta-analysis of schizophreniaand bipolar disorder. Psychol Med. 2011;11:1–13. doi: 10.1017/S0033291711001450. [DOI] [PubMed] [Google Scholar]
- Yu K, Cheung C, Leung M, Li Q, Chua S, McAlonan G. Are Bipolar Disorder and Schizophrenia Neuroanatomically Distinct? An Anatomical Likelihood Meta-analysis. Front Hum Neurosci. 2010;4:189. doi: 10.3389/fnhum.2010.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr Res. 2010;117(1):1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Sundram F, Cannon M, Doherty CP, Barker GJ, Fitzsimons M, Delanty N, Cotter D. Neuroanatomical correlates of psychosis in temporal lobe epilepsy: voxel-based morphometry study. Br J Psychiatry. 2010;197(6):482–92. doi: 10.1192/bjp.bp.110.080218. [DOI] [PubMed] [Google Scholar]
- Cascella NG, Schretlen DJ, Sawa A. Schizophrenia and epilepsy: is there a shared susceptibility? Neurosci Res. 2009;63(4):227–35. doi: 10.1016/j.neures.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labate A, Cerasa A, Gambardella A, Aguglia U, Quattrone A. Hippocampal and thalamic atrophy in mild temporal lobe epilepsy: a VBM study. Neurology. 2008;71(14):1094–101. doi: 10.1212/01.wnl.0000326898.05099.04. [DOI] [PubMed] [Google Scholar]
- Cheung C, Yu K, Fung G, Leung M, Wong C, Li Q, Sham P, Chua S, McAlonan G. Autistic disorders and schizophrenia: related or remote? An anatomical likelihood estimation. PLoS One. 2010;5(8):e12233. doi: 10.1371/journal.pone.0012233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbach JP, van der Zwaag B. Contact in the genetics of autism and schizophrenia. Trends Neurosci. 2009;32:69–72. doi: 10.1016/j.tins.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Goodman AB. A family history study of schizophrenia spectrum disorders suggests new candidate genes in schizophrenia and autism. Psychiatr Q. 1994;65:287–297. doi: 10.1007/BF02354305. [DOI] [PubMed] [Google Scholar]
- McGrath J, Eyles D, Mowry B, Yolken R, Buka S. Low maternal vitamin D as a risk factor for schizophrenia: a pilot study using banked sera. Schizophr Res. 2003;63:73–78. doi: 10.1016/S0920-9964(02)00435-8. [DOI] [PubMed] [Google Scholar]
- Cannell JJ. Autism and vitamin D. Med Hypotheses. 2008;70:750–759. doi: 10.1016/j.mehy.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophr Bull. 2006;32:200–202. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess S. Autism in children with congenital rubella. J Autism Child Schizophr. 1971;1:33–47. doi: 10.1007/BF01537741. [DOI] [PubMed] [Google Scholar]
- Sommer I, Ramsey N, Kahn R, Aleman A, Bouma A. Handedness, language lateralisation and anatomical asymmetry in schizophrenia: meta-analysis. Br J Psychiatry. 2001;178:344–351. doi: 10.1192/bjp.178.4.344. [DOI] [PubMed] [Google Scholar]
- Konick LC, Friedman L. Meta-analysis of thalamic size in schizophrenia. Biol Psychiatry. 2001;49:28–38. doi: 10.1016/S0006-3223(00)00974-4. [DOI] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PWR, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Poulin P, Hansen KT, Jolic D. Searching the schizophrenic brain for temporal lobe deficits: a systematic review and meta-analysis. Psychol Med. 2000;30:491–504. doi: 10.1017/S0033291799002172. [DOI] [PubMed] [Google Scholar]
- Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry. 1998;55:433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- Chance SA, Esiri MM, Crow TJ. Amygdala volume in schizophrenia: post-mortem study and review of magnetic resonance imaging findings. Br J Psychiatry. 2002;180:331–338. doi: 10.1192/bjp.180.4.331. [DOI] [PubMed] [Google Scholar]
- Vita A, De Peri L. Hippocampal and amygdala volume reductions in first-episode schizophrenia. Br J Psychiatry. 2007;190:271. doi: 10.1192/bjp.190.3.271. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananth H, Popescu I, Critchley HD, Good CD, Frackowiak RSJ, Dolan RJ. Cortical and subcortical gray matter abnormalities in schizophrenia determined through structural magnetic resonance imaging with optimized volumetric voxel-based morphometry. Am J Psychiatry. 2002;159:1497–1505. doi: 10.1176/appi.ajp.159.9.1497. [DOI] [PubMed] [Google Scholar]
- Salgado-Pineda P, Baeza I, Pérez-Gómez M, Vendrell P, Junqué C, Bargalló N, Bernardo M. Sustained attention impairment correlates to gray matter decreases in first episode neuroleptic-naive schizophrenic patients. Neuroimage. 2003;19:365–375. doi: 10.1016/S1053-8119(03)00094-6. [DOI] [PubMed] [Google Scholar]
- Leung M, Cheung C, Yu K, Yip B, Sham P, Li Q, Chua S, McAlonan G. Gray matter in first-episode schizophrenia before and after antipsychotic drug treatment. Anatomical likelihood estimation meta-analyses with sample size weighting. Schizophr Bull. 2011;37(1):199–211. doi: 10.1093/schbul/sbp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RC, Di X, McAlonan GM, Gong QY. Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: an activation likelihood estimation meta-analysis of illness progression. Schizophr Bull. 2011;37(1):177–88. doi: 10.1093/schbul/sbp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter ML, Raczka K, Neumann J, YvesVon Cramon D. Towards a nosology for frontotemporal lobar degenerations - a meta-analysis involving 267 subjects. Neuroimage. 2007;36(3):497–510. doi: 10.1016/j.neuroimage.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Lund T, Groups M. Clinical and neuropathological criteria for frontotemporal dementia. J Neurol Neurosurg Psychiatry. 1994;57:416–418. doi: 10.1136/jnnp.57.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn. 2000;42:183–200. doi: 10.1006/brcg.1999.1099. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Rev. 2000;31:236–250. doi: 10.1016/S0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Delong MR, Wichman T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64(1):20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- Gao WJ, Wang Y, Goldman-Rakic PS. Dopamine modulation of perisomatic and peridendritic inhibition in prefrontal cortex. J Neurosci. 2003;23:1622–1630. doi: 10.1523/JNEUROSCI.23-05-01622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassady SL, Adami H, Moran M, Kunkel R, Thaker GK. Spontaneous dyskinesia in subjects with schizophrenia spectrum personality. Am J Psychiatry. 1998;155(1):70–5. doi: 10.1176/ajp.155.1.70. [DOI] [PubMed] [Google Scholar]
- Fish B, Marcus J, Hans SL, Auerbach JG, Purdue S. Infants at risk for schizophrenia: Sequelae of a genetic neurointegrative defect. Arch Gen Psychiatry. 1992;49:221–235. doi: 10.1001/archpsyc.1992.01820030053007. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The case of frontostriatal dysfunction in schizophrenia. Schizophr Bull. 1990;16(3):391–402. doi: 10.1093/schbul/16.3.391. [DOI] [PubMed] [Google Scholar]
- Garey LJ. Brodmann's Localisation in the Cerebral Cortex. London: Smith-Gordon; 1994. [Google Scholar]
- Mesulam MM. Principles of Behavioural and Cognitive Neurology. New York: Oxford University Press; 2000. [Google Scholar]
- Kiehl KA. A cognitive neuroscience perspective on psychopathy: evidence or paralimbic system dysfunction. Psychiatry Res. 2006;142(2–3):107–28. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaf CR, Yamada A, Garrido GE, Buchpiguel CA, Akamine S, Castro CC, Busatto GF. Psychotic symptoms in major depressive disorder are associated with reduced regional cerebral blood flow in the subgenual anterior cingulate cortex: a voxel-based single photon emission computed tomography (SPECT) study. J Affect Disord. 2002;68(2–3):295–305. doi: 10.1016/s0165-0327(00)00365-7. [DOI] [PubMed] [Google Scholar]
- Rubia K. "Cool" inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus "hot" ventromedial orbitofrontal-limbic dysfunction in conduct disorder: a review. Biol Psychiatry. 2011;69(12):e69–87. doi: 10.1016/j.biopsych.2010.09.023. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM. The role of the basal ganglia in learning and memory: neuropsychological studies. Behav Brain Res. 2009;199(1):53–60. doi: 10.1016/j.bbr.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Prog Neurobiol. 2008;86(3):141–55. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Vemuri P, Wiste HJ, Weigand SD, Knopman DS, Trojanowski JQ, Shaw LM, Bernstein MA, Aisen PS, Weiner M, Petersen RC, Jack CR Jr. Alzheimer's disease neuroimaging initiative: Serial MRI and CSF biomarkers in normal aging, MCI, and AD. Neurology. 2010;75(2):143–51. doi: 10.1212/WNL.0b013e3181e7ca82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. Neuroimage. 2010;53(4):1197–207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- Tijms BM, Seriès P, Willshaw DJ, Lawrie SM. Similarity-based extraction of individual networks from gray matter MRI scans. Cereb Cortex. 2012;22(7):1530–41. doi: 10.1093/cercor/bhr221. [DOI] [PubMed] [Google Scholar]



