Abstract
Tomato (Lycopersicon esculentum) plants were transformed with gene constructs containing a tomato alcohol dehydrogenase (ADH) cDNA (ADH 2) coupled in a sense orientation with either the constitutive cauliflower mosaic virus 35S promoter or the fruit-specific tomato polygalacturonase promoter. Ripening fruit from plants transformed with the constitutively expressed transgene(s) had a range of ADH activities; some plants had no detectable activity, whereas others had significantly higher ADH activity, up to twice that of controls. Transformed plants with fruit-specific expression of the transgene(s) also displayed a range of enhanced ADH activities in the ripening fruit, but no suppression was observed. Modified ADH levels in the ripening fruit influenced the balance between some of the aldehydes and the corresponding alcohols associated with flavor production. Hexanol and Z-3-hexenol levels were increased in fruit with increased ADH activity and reduced in fruit with low ADH activity. Concentrations of the respective aldehydes were generally unaltered. The phenotypes of modified fruit ADH activity and volatile abundance were transmitted to second-generation plants in accordance with the patterns of inheritance of the transgenes. In a preliminary taste trial, fruit with elevated ADH activity and higher levels of alcohols were identified as having a more intense “ripe fruit” flavor.
The tomato (Lycopersicon esculentum) is a universally important food, its popularity deriving at least in part from its attractive color, flavor, and versatility. It is an important source of vitamins and minerals (Bittenbender and Kelly, 1988) and is also the primary dietary source of lycopene, a potent antioxidant associated with resistance to several forms of human cancers, in particular cancer of the prostate (Levy et al., 1995; Caperle et al., 1996; Clinton et al., 1996).
Tomatoes are used either fresh or as a range of processed products. An important quality of both the fresh and processed fruit is flavor, comprising mainly sugars, acids, and, of particular importance in fresh fruit, volatile compounds. Despite the efforts of tomato breeders, fresh tomatoes often do not meet the high standards of flavor required by the consumer. Many breeders are now concentrating on improving sugar and acid levels and vine-ripened fruit are becoming increasingly available. However, little attention is being given to improving the flavor and aroma characteristics produced by the volatile compounds.
The development of flavor and aroma volatiles in the ripening tomato fruit has been studied extensively (Kazeniac and Hall, 1970; Buttery et al., 1971, 1987, 1988, 1989; Dirinck et al., 1976; McGlasson et al., 1987; Baldwin et al., 1991; Linforth et al., 1994). Approximately 400 volatile compounds have been found in the ripening fruit (Baldwin et al., 1991), but of these only a small number have been identified as important components of flavor and aroma. These include Z-3-hexenal, Z-3-hexenol, 2-E-hexenal, hexanal, 3-methylbutanal, 3-methylbutanol, β-ionone, 1-penten-3-one, 2-isobutylthiazole, 6-methyl-5-hepten-2-one, methyl salicylate, geranylacetone, E-2-heptenal, isobutyl cyanide, and 2-phenylethanol (Dirinck et al., 1976; Buttery et al., 1987, 1989). These flavor volatiles are formed by several different pathways, including the deamination and decarboxylation of amino acids (3-methylbutanal and 3-methylbutanol; Yu et al., 1968) and lipid oxidation of unsaturated fatty acids (hexanal, hexanol, and the hexenals and hexenols; Galliard et al., 1977; Hatanaka et al., 1986).
The tomato ADH 2 enzyme (alcohol:NAD+ oxidoreductase; EC 1.1.1.1) is one of two ADH enzymes described in tomato. It has been implicated in the interconversion of the aldehyde and alcohol forms of flavor volatiles (Sieso et al., 1976; Bicsak et al., 1982) and has been shown to accumulate in the fruit during ripening (Bicsak et al., 1982; Longhurst et al., 1990; Chen and Chase, 1993) and to have appropriate substrate specificities in vitro (Bicsak et al., 1982), but direct proof of its role has not been obtained. The tomato ADH 1 enzyme is found only in pollen, seeds, and young seedlings (Tanksley, 1979) and apparently is not associated with functions in the ripening fruit.
The accumulation of the ADH 2 enzyme late in ripening, combined with the coincident large increase in flavor volatiles in the fruit and the enzyme's putative role in interconversion of the volatile aldehydes and alcohols, has led to the suggestion that ADH may play an important role in flavor development (Longhurst et al., 1990).
To test the putative role of the enzyme in the reduction of some flavor aldehydes to alcohols and to examine its possible involvement in the development of flavor in the tomato fruit, we produced a number of transgenic tomato plants with modified levels of ADH 2 activity in the ripening fruit. Analysis of volatiles from fruit with enhanced, normal, or reduced levels of ADH 2 was carried out by GC-MS to determine the effects on the relative amounts of volatile aldehydes and alcohols and on the flavor of fruit from representative plants (determined by a taste panel). The results indicated that ADH is involved in the interconversion of aldehydes and alcohols in tomatoes and that this affects the flavor of the fruit.
MATERIALS AND METHODS
Tomato (Lycopersicon esculentum Mill. cv AC) plants were grown under controlled conditions in a greenhouse and in compliance with regulations for the contained growth of transgenic plants as specified by the Australian Genetic Manipulation Advisory Committee. Harvesting of fruit was randomized so that environmental or positional effects such as slight variations in light intensity or position of fruit on the vine were minimized.
Construction of Adh Transgenes
The Adh cDNA used for the construction of the Adh transgene was modified by PCR using the tomato Adh 2 cDNA pTADH 2 (Longhurst et al., 1994) as a template. The 1.5-kb PCR product includes a 5′ untranslated region 91 bp upstream of the ATG start codon and a 320-bp untranslated region 3′ of the TAA stop codon. The integrity of the sequences was confirmed by sequence analysis. Two Adh 2 transgenes were constructed. The first construct, PJR-ADH, consists of the Adh 2 cDNA ligated into the binary vector PJR1. The PJR1 vector (Smith et al., 1988) is a derivative of Bin 19 (Bevan, 1984; Frisch et al., 1995) and contains the constitutive CaMV 35S promoter and the nopaline synthase 3′ terminator sequence. The second construct, PRD-ADH, consists of the Adh 2 cDNA ligated into the binary vector PRD. The PRD binary vector was derived from Bin 19 and contains a tomato-fruit-specific PG promoter (4.8 kb) and PG terminator (1.8 kb; Nicholass et al., 1995). The Adh 2 cDNA fragment was inserted into the BsaI and SpeI sites of the PRD binary vector.
Tomato Transformation
The constructs PJR-ADH and PRD-ADH were transferred from Escherichia coli DH5α to Agrobacterium tumefaciens LBA4404 by triparental mating via E. coli HB101 and pRK2013. Tomato transformations were carried out according to the method of Bird et al. (1988). Southern-blot analysis of DNA from young leaf tissue was used to estimate the number of Npt II and Adh 2 inserts integrated into each plant, and plants were allowed to grow to maturity and set fruit. Fruit were harvested at the first color change (Br) and 2 (Br+2) and 7 d (Br+7) after the Br stage, and ADH activity in the pericarp tissue was determined. For maintenance of the primary transformants and to provide sufficient fruit for subsequent analyses, multiple cuttings of selected plants were vegetatively propagated.
Genomic Southern-Blot Analyses
Genomic DNA was extracted from young leaves by the method of Thomas et al. (1993). Seven micrograms of each of the genomic DNAs was digested with either HindIII or XmnI and was fractionated by electrophoresis on 0.7% agarose-TBE (89 mm Tris/borate and 2 mm EDTA, pH 8.0) gels. The DNA was transferred onto nylon membranes (Zetaprobe, Bio-Rad) as described by the manufacturer. The filters were hybridized sequentially with 32P-labeled probes corresponding to the Npt II and Adh 2 cDNA regions of the transgene. Hybridization was according to the procedure recommended for Zetaprobe at 65°C for 16 h. The filters were washed twice with 2× SSC, 0.1% SDS at 65°C for 15 min each, followed by two washes of 10 min each with 0.1× SSC, 0.1% SDS at 65°C. The membranes were blotted dry and analyzed by phosphor imaging. The numbers of Npt II and Adh 2 inserts were estimated from the number of bands of hybridization with the respective probes together with their intensities (HindIII). Digestion with XmnI excised the introduced Adh 2 cDNA from the inserted gene construct(s). In this case, the number of copies of the Adh 2 gene inserted was estimated from the intensity of hybridization to the inserted gene(s) relative to the intensity of hybridization to the endogenous gene.
Extraction and Assay of ADH
Tomato pericarp tissues and whole-fruit tissues were extracted and assayed according to the method of Longhurst et al. (1990). Protein concentration was measured using a protein quantification kit (Bio-Rad). Enzyme activities are given in units per milligram of protein, where 1 unit is the amount of enzyme required to produce 1 μmol NADH min−1.
Volatile Analyses
The method used for isolation of headspace volatiles was modified from that of Buttery et al. (1987) and included a short, room-temperature incubation of the macerated tomato tissue before inactivation of endogenous enzymes by the addition of CaCl2. This step was included to simulate the development of volatiles in sliced and chewed tomato. SPME absorption was used to collect headspace volatiles for fractionation and analysis by GC-MS.
Fruit were harvested at the Br+7 stage. Pericarp tissue (10 g) was taken from freshly harvested fruit, sliced, and then briefly macerated using a Polytron homogenizer (model PT2000, Kinematica AG, Littau, Switzerland). The slurry was allowed to stand at room temperature for exactly 3 min, after which 3.3 g of solid CaCl2 was added to inhibit further enzyme activity. Uniformly labeled, deuterated hexanol (1 μL at a concentration of 80 nmol μL−1) was added as an internal standard. An aliquot of the mixture (5.5 g) was transferred to a 10-mL headspace vial sealed with a silicon/Teflon septum. The vial was incubated at 40°C for 30 min. Sampling of the headspace was carried out by insertion of a SPME fiber (65 μm, Carbowax-divinylbenzene, Supelco, Bellefonte, PA) for 30 min while incubation of the vial continued at 40°C. The absorbed sample was analyzed on a gas chromatograph (series 6809, Hewlett-Packard) fitted with a capillary DB-wax column (30 m × 0.25 mm i.d. × 0.25 μm, J & W Scientific, Folsom, CA) and individual peaks were identified by MS. Peak areas were measured by integration and were normalized against the internal deuterated hexanol standard. Tabulated data are peak areas × 10−6.
Taste Trial
To comply with the requirements of the Australian Genetic Manipulation Advisory Committee, seeds were removed from the tomatoes before taste testing. Tomatoes from several vegetatively propagated clones of each of the primary transformed plants of interest were harvested between 7 and 9 d after ripening. The tomatoes were matched on the basis of size. Each tomato was quartered, and the locular tissue containing the seeds was removed. Each quarter was used by each panelist for each of the four attributes in the following order: ripe flavor, green flavor, sweetness, and acidity. Four sets of samples were presented to each panelist, each in a different random order. Each set was used to rank only one of the attributes. Panelists were asked to rank the samples on each of the four attributes. Samples with the greatest intensity of the attribute were given a rank of 1, whereas the least intense was ranked 4. An orthogonal Latin-square design was used to balance out carryover effects. This design required 12 assessments (panelists). However, because of insufficient samples, only 11 assessments were made in this trial.
Seven of the 11 panelists were trained oenologists with extensive experience in wine flavor and acid-balance assessment (mean experience, 10 years). One panelist, although not formally trained, had 3 years of regular experience in assessing these attributes. The three remaining panelists were laboratory employees. Although all panelists knew the general purpose of the tasting, i.e. a comparison of flavor-modified tomatoes, none was aware of the exact nature of the samples.
RESULTS
Transformation with Adh 2 Constructs and Initial Screening
Tomato explants were transformed with constructs containing the tomato Adh 2 cDNA as shown in Figure 1, and transformed plants from each experiment were selected for analysis.
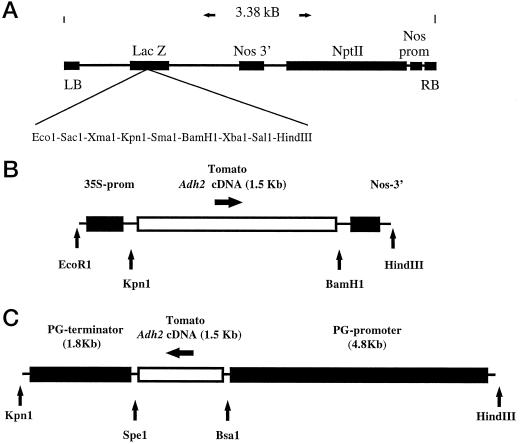
Figure 1.
Constructs PJR-ADH and PRD-ADH. A, General map of the T-DNA region of the binary vector Bin 19 (Bevan, 1984; Frisch et al., 1995) showing the Npt II-selectable marker gene and the polylinker site. B, For construction of the vector PJR-ADH, the tomato Adh 2 cDNA was ligated between the CaMV 35S promoter (prom) and the nopaline synthase (Nos) 3′ terminator, which were inserted into the polycloning site of the Bin 19 vector as described in Methods. C, For construction of the PRD-ADH vector, the tomato Adh 2 cDNA was ligated between the promoter and 3′ regions of the tomato PG, which were inserted into the polycloning site of the Bin 19 vector as described in Methods.
Two types of transformation construct were used. Both contained the tomato Adh 2 cDNA in a sense orientation relative to the construct promoter. In one set of experiments the CaMV 35S promoter was used to provide constitutive expression of the cDNA. In the other the tomato PG gene promoter (Bird et al., 1988; Nicholass et al., 1995) was used to provide fruit-ripening-specific expression of the cDNA.
ADH activity was measured in pericarp, locular, and whole-fruit tissues. Activity was found to vary in the different tissues but was highest in pericarp tissue (data not shown). Because of this, and because of the uniform composition of the tissue, only pericarp activities are presented here.
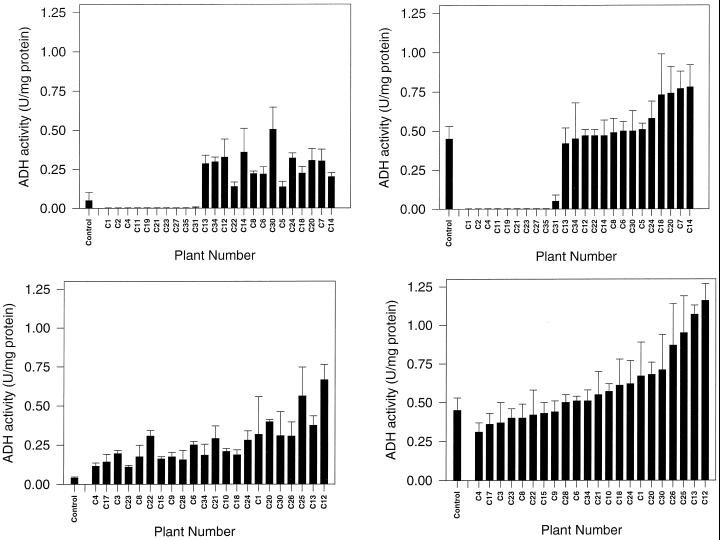
Constitutive expression of the introduced cDNA(s) resulted in both enhanced and inhibited accumulation of the ADH 2 enzyme in the ripening fruit (Fig. 2). Enhanced levels of accumulation were most evident in Br fruit, but a number of fruit continued to show significantly (P < 0.05) enhanced levels at Br+7. About 40% of the plants analyzed had fruit in which ADH 2 expression was completely inhibited.
Figure 2.
ADH specific activities in pericarp from Br, Br+2, and Br+7 fruit from transformed tomato plants. The top two histograms show results for plants transformed with the PJR-ADH construct containing the tomato Adh 2 cDNA coupled with the constitutive CaMV 35S promoter (left Br, right Br+7). The bottom two histograms show results from plants transformed with the PRD-ADH construct containing the tomato Adh 2 cDNA coupled with the tomato fruit-ripening-specific PG promoter (left Br+2, right Br+7). Results are arranged in order of increasing activity in Br+7 fruit and are indicated in the same order in the histograms on the left. Three fruit were averaged for each data point. Control in each histogram is a mean ± sd obtained from three fruit (each control point) from separate, untransformed plants. U, Units.
In the second set of experiments, in which the introduced cDNA(s) was expressed in a fruit-ripening-specific manner, ADH activities at Br+2 ranged from approximately that in control fruit to six to seven times control levels, whereas activities at Br+7 ranged from approximately control fruit levels to two to three times control levels (Fig. 2). With the fruit-specific promoter none of the transgenic fruit had significantly lower ADH activity than the controls (Fig. 2).
ADH Activities during Ripening
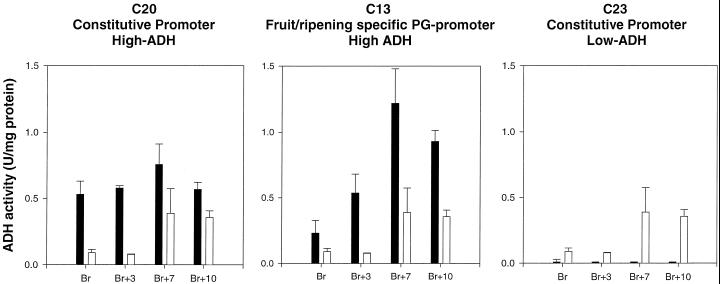
Seven plants showing significant modifications in ADH activities in fruit pericarp were selected for more extensive analysis during fruit ripening and for analysis of fruit volatiles. Cuttings from the selected plants were propagated to provide sufficient material and fruit of appropriate ages. Plants C20 and C23 contained constructs with constitutive expression of the inserted cDNA(s). Fruit from these plants showed, respectively, either enhanced ADH activity or minimal activity in fruit throughout ripening relative to levels in fruit from untransformed control plants (Fig. 3). Plant C13 contained Adh 2 cDNAs regulated by the fruit-ripening-specific PG promoter. Fruit from the C13 plant showed significantly enhanced levels of ADH activity, which continued to increase up to Br+7 and then declined.
Figure 3.
ADH activities in pericarp tissue are shown for ripening fruit from three transgenic plants and are compared with ADH activities in fruit from an untransformed plant. Plant C13 was transformed with the Adh 2 cDNA coupled with the fruit-ripening-specific PG promoter. Plants C20 and C23 were transformed with constructs containing the CaMV 35S promoter. White bars, Untransformed fruit; black bars, transformed fruit. Three fruit were averaged for each data point.
Comparative Activities in Fruit from T0 Plants
In a separate series of experiments, ADH levels were determined in pericarp tissue of Br+7 fruit from the seven selected T0 plants (Table I). ADH activities in fruit from the three plants containing one or two constitutively expressed transgenes, C7, C14, and C20, were significantly higher than in control, untransformed fruit. In contrast, activity was almost undetectable in fruit from the three plants containing three or more constitutively expressed transgenes, C4, C11, and C23. Consistent with activities determined during fruit ripening (Fig. 3), the highest ADH activities were found in Br+7 fruit from the C13 plant (Table I), which contained about six transgenes regulated by the fruit-specific tomato PG promoter.
Table I.
Number of introduced genes, ADH specific activities, and relative abundances of some volatiles in fruit from transformed and control plants
| Plant | Genes Inserted (Adh 2/Np II) | ADH Specific Activity | Hexanal | Z-3-Hexanal | E-2-Hexanal | Hexanol | Z-3-Hexanol | Geraniol | E-2-Octenal | 1-Octen-3-ol | Citral |
|---|---|---|---|---|---|---|---|---|---|---|---|
| units mg−1 protein | relative abundance | ||||||||||
| C7 (n = 4) | 1/1 | 0.67 ± 0.16a | 8.48 ± 1.60b | 8.42 ± 1.07b | 14.38 ± 3.24b | 0.25 ± 0.09b | 3.08 ± 0.28a | 0.72 ± 0.34 | 0.43 ± 0.01 | 0.09 ± 0.01 | 0.30 ± 0.10 |
| C14 (n = 3) | 1/1 | 0.56 ± 0.04c | 9.13 ± 0.31b | 7.28 ± 0.40b | 13.57 ± 1.76b | 0.40 ± 0.05c | 4.12 ± 0.79b | 0.71 ± 0.16 | 0.50 ± 0.09 | 0.07 ± 0.02 | 0.90 ± 0.14 |
| C20 (n = 5) | 2/2 | 0.76 ± 0.15c | 9.94 ± 1.98b | 7.73 ± 1.28b | 15.99 ± 1.72c | 0.52 ± 0.17a | 6.02 ± 1.58c | 0.62 ± 0.14 | 0.57 ± 0.06 | 0.11 ± 0.02 | 0.69 ± 0.28 |
| C13 (n = 3) | 6/7 | 1.26 ± 0.31a | 6.95 ± 0.89a | 7.67 ± 1.42b | 12.92 ± 2.78b | 0.29 ± 0.08b | 4.48 ± 1.27b | 0.49 ± 0.10 | 0.45 ± 0.03 | 0.08 ± 0.01 | 0.38 ± 0.10 |
| AC1–3 (n = 10) | – | 0.375 ± 0.15 | 9.43 ± 1.44 | 7.76 ± 1.57 | 12.37 ± 2.03 | 0.22 ± 0.07 | 2.51 ± 0.46 | 0.89 ± 0.25 | 0.61 ± 0.09 | 0.10 ± 0.03 | 0.70 ± 0.22 |
| C4 (n = 3) | 4/3 | 0.01 ± 0.016d | 9.62 ± 0.85b | 9.28 ± 1.22b | 11.68 ± 2.62b | 0.02 ± 0.025d | 0.15 ± 0.08d | 0.81 ± 0.13 | 0.56 ± 0.1 | 0.12 ± 0.03 | 0.6 ± 0.01 |
| C11 (n = 4) | 8/? | 0.006 ± 0.01d | 8.45 ± 0.44b | 7.57 ± 0.72b | 15.88 ± 1.42c | 0.04 ± 0.02d | 0.065 ± 0.08d | 0.6 ± 0.09 | 0.52 ± 0.05 | 0.10 ± 0.02 | 0.35 ± 0.07 |
| C23 (n = 3) | 3/5 | 0.011 ± 0.02d | 10.09 ± 2.27b | 8.71 ± 2.46b | 10.82 ± 3.23b | 0.02 ± 0.002d | 0.057 ± 0.05d | 0.92 ± 0.15 | 0.54 ± 0.20 | 0.09 ± 0.03 | 0.44 ± 0.15 |
The number of inserted genes was estimated by quantitating the hybridization to genomic DNA of probes for Npt II and for the Adh 2 cDNA, as detailed in Methods. Headspace volatiles were extracted from Br+7 fruit, collected by SPME absorption, and analyzed by GC-MS. Peak areas were determined by integration and were normalized against the area of a deuterated hexanol standard introduced during maceration of the tissue. Averaged ADH activities from pericarp tissue and volatile abundances were derived from the same sets of fruit, and were derived separately from the data presented in Figure 2. Data from individual transformed plants were compared with pooled data from the control plants (AC1, 2, and 3) using Welch's t test, which does not assume equal variances: Statistical analyses of geraniol, E-2-octenal, 1-octen-3-ol, and citral are not included because no correlation with ADH activity was evident.
Significant (P < 0.05).
Not significant.
Very significant (P < 0.01).
Extremely significant (P < 0.001).
Analysis of Adh mRNA
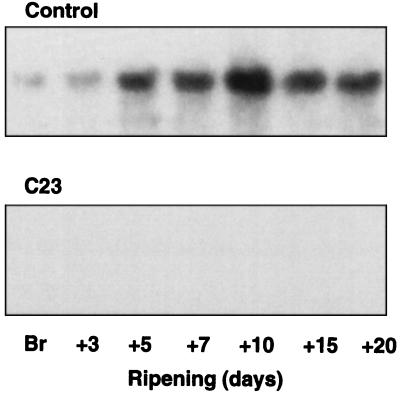
Hybridization analysis was used to examine gene activity relative to ADH activity in one of the plants in which ADH activity was suppressed. Hybridization analysis of Adh mRNA in pericarp tissue of fruit from one of the untransformed plants (AC2), showed mRNA abundance increasing in the fruit up to about Br+10, then declining slightly (Fig. 4). In contrast, no Adh mRNA was detectable in ripening fruit from the C23 plant, in which ADH activity was minimal. This observation indicates that inhibition of ADH activity in this plant is a result of gene silencing or cosuppression (Jorgensen, 1990).
Figure 4.
Hybridization of 32P-labeled tomato Adh 2 cDNA to RNA from pericarp of ripening fruit from a control plant and a low-ADH transgenic plant (C23). Each lane contains 10 μg of total RNA. Fractionation, transfer to membrane, and hybridization were as described by Longhurst et al. (1994).
Transgene Inheritance and Segregation in T1 Plants
T1 populations were generated from all seven of the transgenic plants described above. Inheritance of the transgenes was determined by Southern-blot analysis. Twenty-five T1 progeny from the plants with single inserts, C7 and C14, segregated in an approximately Mendelian fashion. The C7 T1 plants had a ratio of azygous:hemizygous:homozygous plants of 5:12:8 and the C14 T1 plants had a ratio of 8:11:6. Similarly, 40 T1 progeny obtained from the plant with a double insert, C20, segregated in an approximately Mendelian fashion, with a distribution of 9:24:7. We saw no evidence of separation of the two inserts, suggesting that they were closely linked. T1 progeny from the primary transgenic plants with multiple inserts had approximately Gaussian distributions (Table II), with some abnormalities resulting in part from analysis of small populations, but in some cases, also from insertion of clusters of transgenes. For example, C11 hybridization profiles (not shown) and T1 distribution suggest that five or six of the estimated eight transgenes in the T0 plant have been inserted at a single genetic locus.
Table II.
Segregation of T1 plants
| Parent T0 | No. of Inserts | No. of T1 Analyzed | T1 Segregationa |
|---|---|---|---|
| C13 | 6 | 16 | 3/1, 4/3, 6/3, 7/4, 8/3, 12/2 |
| C4 | 4 | 39 | 0/2, 1/9, 2/1, 3/2, 4/10, 5/6, 6/3, 7/2, 8/4 |
| C11 | 8 | 40 | 0/7, 6/3, 7/3, 8/5, 9/6, 10/3, 11/2, 12/3, 13/2, 14/3, 16/3 |
| C23 | 3 | 30 | 0/1, 1/9, 2/10, 3/3, 4/3, 5/2, 6/2 |
The number of transgene inserts in the T1 plants was determined by Southern-blot analysis and quantitation of number and intensity of bands of hybridization relative to hybridization to the endogenous genes.
No. of inserts/no. of T1.
ADH Activity in T1 Fruit
The T1 progeny of the C7, C14, and C20 T0 plants were of particular interest to us because of their simple genetic characteristics and their enhanced ADH levels (Table I). ADH activities were determined in Br+7 fruit from all of the T1 progeny of these three T0 plants. Data for each class of T1, azygous, hemizygous, and homozygous, were pooled and are presented as means ± sd in Table III. ADH activities from control, untransformed fruit (AC2), grown and harvested at the same time as the T1 fruit, are included in Table III. The enhanced ADH character was evident in most of the T1 plants containing one or two transgenes, but activity was almost completely suppressed in fruit from all of the homozygous C20 T1 plants. In addition, ADH activity was also suppressed in fruit from 6 of the 24 hemizygous C20 T1 progeny.
Table III.
ADH activities in Br+7 fruit from T0 and T1 plants
| Plant | N × na | Inserts | ADH Specific Activity |
|---|---|---|---|
| no. | units mg−1 | ||
| AC2 (control) | 1 × 5 | 0 | 0.381 ± 0.10 |
| C7 | |||
| T0 | 1 × 3 | 1 | 0.602 ± 0.06b |
| T1 azygous | 5 × 3 | 0 | 0.342 ± 0.10c |
| T1 hemizygous | 12 × 3 | 1 | 0.528 ± 0.10b |
| T1 homozygous | 8 × 3 | 2 | 0.652 ± 0.13d |
| C14 | |||
| T0 | 1 × 8 | 1 | 0.598 ± 0.10d |
| T1 azygous | 8 × 3 | 0 | 0.402 ± 0.06c |
| T1 hemizygous | 11 × 3 | 1 | 0.581 ± 0.09d |
| T1 homozygous | 6 × 3 | 2 | 0.642 ± 0.09d |
| C20 | |||
| T0 | 1 × 5 | 2 | 0.583 ± 0.11b |
| T1 azygous | 9 × 3 | 0 | 0.291 ± 0.09c |
| T1 hemizygous | 18 × 3 | 2 | 0.575 ± 0.07b |
| 6 × 3 | 2 | 0.003 ± 0.003d | |
| T1 homozygous | 7 × 3 | 4 | 0.002 ± 0.003d |
ADH activity was determined in pericarp tissue from three Br+7 fruit of each plant. Pooled activities for each class of plant were compared with the activity of control (AC2) Br+7 fruit grown and harvested during the same period of time. Statistical comparison was with Welch's t test.
N = Number of different plants, n = number of fruit per plant.
Significant (P < 0.05).
Not significant.
Very significant (P < 0.01).
Analysis of Volatiles in T0 Fruit
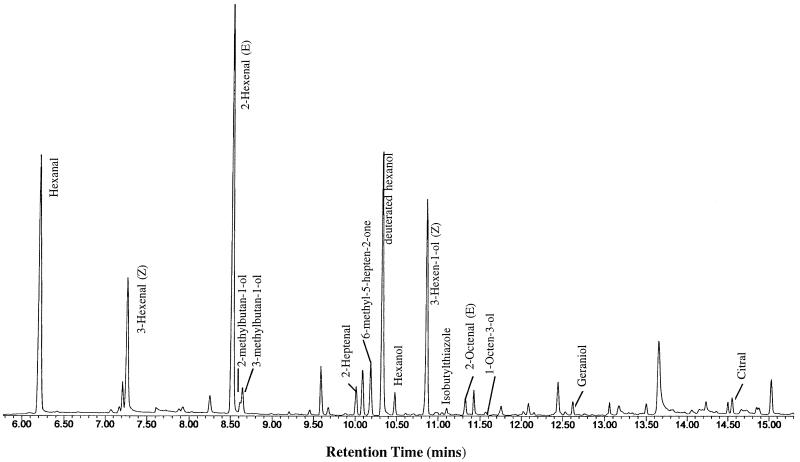
Analysis of headspace volatiles in fruit with modified ADH activities was carried out on Br+7 fruit from the seven selected T0 plants. SPME was used as a quick and convenient method for measuring headspace volatiles from individual fruit. This method revealed some 15 major peaks of volatiles and many minor peaks (Fig. 5). A number of the volatiles identified in Figure 5 are among those considered to be important to flavor and aroma development. Of particular interest to us were the hexanal/hexanol and hexenal/hexenol compounds, peak areas of which are listed in Table I, together with areas of some other aldehydes and alcohols detected.
Figure 5.
Volatiles isolated from headspace above macerated whole-fruit tissue. Typical elution profile of compounds detected by GC analysis of headspace volatiles.
Comparison of areas of individual peaks between tissue from control, high-ADH, and low-ADH fruit generally showed little variation. Peak areas of the aldehyde Z-3-hexenal did not vary greatly between the various plants, whereas variations in the levels of E-2-hexenal were detected only in fruit from the C20 and C11 plants. Similarly, the area of the aldehyde hexanal varied little between plants except in C13, in which it was significantly lower relative to controls (Table I). In contrast, levels of the alcohols hexanol and Z-3-hexenol did show significant variation between plants with differing ADH levels (Table I). Relative to levels in control fruit, hexanol levels were significantly higher in fruit from two of the high-ADH plants, C14 and C20. Z-3-Hexenol levels were also higher in fruit from two of the high-ADH plants, but in this case the significantly increased levels were in C7 and C20. Both hexanol and Z-3-hexenol levels were significantly lower in fruit from all three low-ADH plants. E-2-Hexenol was not detected in any of the fruit.
Analysis of Volatiles in T1 Fruit
Analysis of headspace volatiles was undertaken for fruit from a number of hemizygous and homozygous T1 progeny of the three T0 plants of greatest interest, C7, C14, and C20. During the course of these analyses the abundances of some of the headspace volatiles (particularly hexanol and Z-3-hexenol) obtained from fruit from each plant were observed to vary significantly in fruit harvested several weeks apart. The variations may have resulted from differences in growing conditions, and to compensate for this the results in Table IV are grouped so that each group includes data from control and T0 fruit that were grown along with the T1 fruit and harvested and analyzed during the same period. For brevity, the analyses of hexanal, hexanol, Z-3-hexenal, E-2-hexenal, and Z-3-hexenol are presented in Table IV as ratios of the aldehyde to alcohol. Because Z-3-hexenal isomerizes to E-2-hexenal (Kazeniac and Hall, 1970) and because we detected no E-2-hexenol, the ratio of (Z-3 + E-2)-hexenal to Z-3-hexenol has been used.
Table IV.
ADH activities and ratios of aldehydes:alcohols for populations of Br+7 T0 and T1 fruit
| Plant | Inserts | Fruit Sampled | ADH Activity | Hexanal/ol | Hexenal/ol Z-3 + E-2/Z-3 |
|---|---|---|---|---|---|
| no. | units mg−1 | relative abundance | |||
| Control AC1,2,3a | 10 | 0.38 ± 0.15 | 49.2 ± 7.9 | 8.7 ± 1.3 | |
| C7 T0a | 1 | 4 | 0.67 ± 0.16d | 36.8 ± 10.7g | 7.4 ± 1.2g |
| C14 T0a | 1 | 3 | 0.56 ± 0.04e | 23.4 ± 3.8f | 5.2 ± 1.0e |
| C20 T0a | 2 | 5 | 0.76 ± 0.15e | 20.1 ± 4.7f | 4.2 ± 1.1f |
| C13 T0a | 6 | 3 | 1.26 ± 0.31d | 25.2 ± 8.0d | 4.8 ± 1.4d |
| C4 T0a | 4 | 3 | 0.01 ± 0.016f | ∞f | 113 ± 75.5g |
| C11 T0a | 8 | 4 | 0.006 ± 0.01f | 235 ± 114d | ∞f |
| C23 T0a | 3 | 3 | 0.011 ± 0.02f | 471 ± 126d | ∞f |
| Control AC2b | 0 | 4 | 0.230 ± 0.03 | 287 ± 75 | 103 ± 38 |
| C7 T0b | 1 (Hemizygous) | 6 | 0.657 ± 0.05f | 80 ± 4d | 11 ± 1d |
| C7 T1b | 1 (Hemizygous) | 4 | 0.537 ± 0.11d | 75 ± 13d | 11 ± 3d |
| P41 | 1 (Hemizygous) | 2 | 0.658 ± 0.11g | 84 ± 11d | 10 ± 1d |
| P31 | 2 (Homozygous) | 6 | 0.672 ± 0.13f | 70 ± 6d | 11 ± 5d |
| P35 | 2 (Homozygous) | 4 | 0.630 ± 0.14d | 76 ± 15d | 11 ± 6d |
| Control AC2b | 0 | 4 | 0.230 ± 0.03 | 287 ± 75 | 103 ± 38 |
| C14 T0b | 1 (Hemizygous) | 3 | 0.598 ± 0.10d | 81 ± 1d | 11 ± 1d |
| C14 T1b | |||||
| P23 | 2 (Homozygous) | 5 | 0.724 ± 0.45g | 56 ± 14e | 11 ± 6d |
| P35 | 2 (Homozygous) | 4 | 0.510 ± 0.03f | 69 ± 7d | 9 ± 1d |
| Control AC2c | 0 | 4 | 0.435 ± 0.03 | 192 ± 27 | 49 ± 10 |
| C20 T0c | 2 (Hemizygous) | 6 | 0.722 ± 0.08f | 114 ± 17e | 20 ± 3d |
| C20 T1c | |||||
| P16 | 2 (Hemizygous) | 4 | 0.694 ± 0.05f | 129 ± 32d | 17 ± 4e |
| P33 | 2 (Hemizygous) | 4 | 0.647 ± 0.11d | 120 ± 13e | 19 ± 2e |
| P45 | 2 (Hemizygous) | 6 | 0.656 ± 0.05f | 136 ± 18d | 23 ± 4d |
| P18 | 2 (Hemizygous)cosuppressed | 3 | 0.01 ± 0.01f | ∞ | 404 ± 44e |
Data sets were collected at different times. Each data set includes data for control fruit (AC) grown and harvested during the same period.
Data collected June to August 1996.
Data collected July to September 1997.
Data collected February to March 1998. Within columns, comparisons were made between data for the transgenic fruit and the appropriate control fruit using Welch's t test.
Significant (P < 0.05).
Very significant (P < 0.01).
Extremely significant (P < 0.001).
Not significant.
A comparison of the ratios obtained in the later analyses with those derived from the initial analyses of the T0 plants shows large differences, mostly attributable to differences in abundance of the alcohols. However, within each of the four sets of data a consistent correlation can be seen between the increased levels of ADH in the T0 and T1 fruit and lower aldehyde-to-alcohol ratios, indicating increased levels of the alcohols. In the one cosuppressed plant analyzed, C20 T1 P18 (hemizygous), the barely detectable levels of ADH in the fruit correlated with zero or barely detectable levels of the alcohols, giving rise to high aldehyde-to-alcohol ratios (infinitely high in the case of the hexanal-to-hexanol ratio, because hexanol was undetectable in one of the three fruit analyzed).
Flavor Testing: Taste Trial
Deseeded fruit tissue from three of the T0 plants and a control plant was taste tested by a panel of 11 individuals, most of whom had training and extensive taste panel experience. The panel found a significant increase in ripe-fruit flavor in fruit from the C20 plant in which ADH levels were constitutively enhanced relative to fruit from other transgenic plants and from control plants (Table V). Other attributes did not vary significantly between fruit from the various plants; however, a slight correlation between lowered green-tomato character and increased sweetness of the C20 fruit relative to the other fruit was noted. The flavor characteristics of fruit from the C13 plant did not differ from those of control fruit.
Table V.
Taste trial of transgenic tomatoes
| Characteristic | C20 | C13 | Control (untransformed) | C23 | P | lsd 5% |
|---|---|---|---|---|---|---|
| Ripe flavor | 14a | 34b | 28b | 34b | 0.002 | 10 |
| Green flavor | 34a | 23a | 28a | 25a | 0.288 | 10 |
| Sweetness | 18a | 36b | 24ab | 32b | 0.014 | 10 |
| Acidity | 25a | 29a | 30a | 26a | 0.819 | 10 |
Tomatoes from the selected plants were harvested at optimal ripeness, between Br+7 and Br+9. Pericarp tissue from the fruit was tasted by 11 panelists as described in Methods. Samples with greatest intensity of attribute were given a ranking of 1, whereas those with the least were ranked 4. Summed scores are shown with low rank sums, implying a high intensity of that attribute. In a row, scores followed by a common letter are not significantly different (probability is shown in column P). Rank sums were analyzed by Friedman's test and lsd was determined using the method of Skillings and Mack (1981). The lowest rank sum for ripe tomato flavor consisted of 10 “1” rankings and a single “4” ranking.
DISCUSSION
By introducing tomato Adh 2 cDNA constructs coupled to either a constitutive promoter or a fruit-ripening-specific promoter, we have produced a number of T0 and T1 transgenic tomato plants with modified levels of ADH 2 activity in their ripening fruit. The introduction of the Adh 2 cDNA under the control of the constitutive promoter resulted in a spectrum of T0 plants, including those with enhanced levels of ADH 2 activity in the ripening fruit and other tissues (J. Speirs and E. Lee, unpublished data) and plants with barely detectable levels of ADH 2 activity in the ripening fruit. Hybridization analysis (Fig. 4) related the suppressed ADH activity in fruit from one of the transgenic plants to an absence of Adh mRNA in the fruit, indicating that the introduced transgene had induced gene silencing or co-suppression (Jorgensen, 1990). Transgenic plants containing constructs with the tomato PG promoter produced fruit showing enhanced levels of ADH 2 activity. In fruit from these plants, ADH 2 activity increased as the fruit ripened (C13; Fig. 3), consistent with the fruit-ripening specificity of the PG promoter (Bird et al., 1988; Nicholass et al., 1995).
The production of tomato plants that bear fruit with significantly different ADH activities in their tissues has allowed us to examine the role of ADH in the production of volatiles associated with flavor in the ripening fruit. We found some quantitative variation in headspace volatiles between fruit, but we also found consistent differences in the balance between some of the volatile aldehydes and alcohols in fruit from plants with differing ADH levels. Of particular interest was the interconversion of the 6-C alcohols and aldehydes, which has been linked indirectly with the ADH 2 enzyme (Sieso et al., 1976; Bicsak et al., 1982; Longhurst et al., 1990; Chen and Chase, 1993).
Constitutive Promoter: High-ADH Plant C20
Associated with the increased ADH activity in fruit from plant C20 were increases in the alcohol forms of the hexanal/hexanol and hexenal/hexenol volatiles (Table I), giving rise to reduced ratios of aldehyde to alcohol (Table IV). These low ratios are consistent with an increased conversion of the 6-C aldehydes to their alcohols by the increased ADH activity in the transformed fruit, which is, to our knowledge, the first direct evidence that the tomato ADH 2 enzyme mediates interconversion of hexanal/hexanol and the Z-3- form of hexenal/hexenol in the ripening fruit.
Fruit-Specific Promoter: High-ADH Plant C13
In fruit from the C13 plant ADH activity was marginally higher during development relative to control fruit (J. Speirs and E. Lee, unpublished data), but increased to approximately three times the level in control fruit during ripening (Fig. 3). A small, nonsignificant increase in Z-3-hexenol was found in fruit from this plant (Table I), resulting in a decrease in hexenal-to-hexenol ratios (Table IV). However, no increase in hexanol was observed, whereas a decrease in hexanal was evident (Table I). Although these changes result in small but significant reductions in both hexanal-to-hexanol and (Z-3 + E-2)-hexenal-to-Z-3-hexenol ratios, similar to those evident in the C20 fruit, the mechanism appears to be different. Assuming that ADH is involved, the high ADH activity attained in the fruit, or the specific timing of its increase in the tissues, may affect the mechanism. Possibilities include feedback from the alcohol end of the pathway to the primary events associated with the conversion of free fatty acids to linoleic acid, or some mechanism for increased efflux of the alcohol product. It may also be that the high ADH levels influence some remote pathway that has an indirect influence on the levels of hexanal/hexanol.
Constitutive Promoter: Low-ADH Plant C23
ADH activity was reduced to barely detectable levels in fruit of plant C23. Also barely detectable in these fruit were hexanol and Z-3-hexenol (Table I), with the resulting aldehyde-to-alcohol ratios for C23 fruit differing grossly from those of control fruit (Tables I and IV). Reduction of ADH 2 activity therefore resulted in the inhibition of the conversion of hexanal and Z-3-hexenal to their corresponding alcohols, providing further direct evidence of at least one of the roles of ADH 2 in the ripening fruit.
Inheritance in T1 Plants
Inheritance of the transgene(s) in small populations of T1 plants derived from selfing of the seven T0 plants conformed to expected patterns of Mendelian inheritance of a single genetic locus (plants C7, C14, and C20) or approximated Gaussian distributions in progeny from the plants with more than two inserts (Table II).
Analysis of ADH activities and volatiles in the T1 plants was restricted to the progeny of the C7, C14, and C20 T0 plants because of their simple genetic characteristics and enhanced ADH levels (which were of particular interest). Azygous, hemizygous, and homozygous progeny from these plants had ADH activities consistent with the inheritance of the phenotype endowed by the transgene. That is, azygous progeny had levels of activity equivalent to those of controls grown and harvested during the same period, and hemizygous or homozygous progeny had enhanced activities (or cosuppressed activities) relative to the same controls (Table III).
Inheritance of modified aldehyde and alcohol phenotypes in the T1 fruit was confirmed in a small number of representative progeny from the three T0 plants, C7, C14, and C20 (Table IV). Within groups of control and transgenic T0 and T1 plants grown and analyzed during the same period a consistent trend was evident relating high ADH activity to increased abundances of the alcohols hexanol and Z-3-hexenol. The relationship between ADH activity and changes in the balance between aldehyde and alcohol is shown in Table IV, where the lower ratios of hexanal-to-hexanol and (Z-3 + E-2)-hexenal-to-Z-3-hexenol are mainly indicative of increased levels of the alcohols and the higher ratios are indicative of decreased levels of the alcohols.
The variability in the ratios of aldehyde to alcohol that we have found between sets of plants grown and analyzed at different periods during the year is not fully understood. It may reflect, to an extent, changes in the growing environment. However, because the pathway leading from free fatty acids to the 6-C aldehydes and alcohols is operative mainly in disrupted tissue (Galliard and Matthews, 1977; Hatanaka et al., 1992), an association between growing environment and extracellular conversion of aldehydes to alcohols is difficult to envisage. This is currently under investigation.
Our results indicate that modification of ADH 2 activity in the tomato fruit affects the balance between the 6-C aldehydes and alcohols. The effects are most marked when ADH 2 activity is reduced. The less-pronounced consequences of increasing ADH 2 activity may indicate an upper limit to the tolerated concentration of the alcohols, which is regulated by feedback or by further metabolism, or limitations in the reducing potential of NADH. It is also possible that the upper limit of ADH activity is a function of gene activity. The introduction of a single, constitutively regulated Adh transgene enhanced the activity of the enzyme in the fruit, whereas two transgenes gave rise to a further small increase in most cases. However, 6 of the 24 hemizygous C20 T1 progeny examined that contained two transgenes were cosuppressed, suggesting that the combined activities of the endogenous genes plus the two transgenes constituted a threshold at or above which gene suppression was possible or probable. This is supported by the cosuppression observed in all the T0 and T1 plants containing more than two constitutively expressed transgenes (Tables I and III). The same threshold was not evident in the C13 plant containing six transgenes regulated by a fruit-specific promoter. One explanation may be that gene silencing or cosuppression, if it occurs in the ripening C13 fruit, occurs subsequent to the synthesis of the active enzyme, the residual activity of which masks the more long-term effects throughout the sampling period.
We were unable to detect the E-2-hexenol volatile in SPME-headspace analyses of any of the tomato extracts despite confirming that the compound could be detected by our sampling procedure. Low levels of the aldehyde E-2-hexenal relative to its Z-3-isomer have been reported in tomato fruit (Dirinck et al., 1976; Buttery et al., 1987, 1988, 1989; Baldwin et al., 1991), and an absence or low activity of the cis-3-trans-2 isomerase has been postulated as a reason for this (Galliard et al., 1977). The relatively high levels of E-2-hexenal observed in all of our samples, therefore, were unexpected and may have resulted from nonenzymic isomerization during volatile collection and processing. The apparent absence of E-2-hexenol would therefore result from the accumulation of the E-2-aldehyde subsequent to the inactivation of the ADH enzyme.
Also missing in the headspace analyses was 3-methylbutanal. In this case, however, standards of the volatile were also undetectable by our methods. Direct analysis of headspace volatiles has been undertaken and does show ADH-related modulation of the ratio of 3-methylbutanal to 3-methylbutanol (A. Taylor, personal communication). These results will be published elsewhere.
It was of interest to determine the specificity of ADH 2 on other aldehydes and alcohols readily detectable in headspace analyses of the various fruit. Although some inter- and intrafruit variation was observed in the relative abundances of octenal and octenol, there was no evidence of ADH 2 being involved in their interconversion. Production of the alcohol from the unstable free radical of linoleic acid and an unstable hemiacetal intermediate has been postulated (Hoffman, 1962), and it is possible that dehydrogenases are not involved in regulating the balance between this alcohol and its aldehyde.
Another aldehyde/alcohol combination of interest was citral (trans-3,7-dimethyl-2,6-octadien-1-al)/geraniol (trans-3,7-dimethyl-2,6-octadien-1-ol). Bicsak et al. (1982) reported the tomato ADH enzyme as having “appreciable activity” in vitro on the terpene geraniol, and suggested that the NAD+-dependent tomato enzyme was associated with interconversion of citral and geraniol in the tomato fruit, with the equilibrium being in favor of aldehyde formation. In strawberry (Yamashita et al., 1978), pea (Eriksson, 1968; Leblová and Mancal, 1975), potato, and orange (Potty and Bruemmer, 1970), this interconversion is believed to be mediated by an NADP+-preferring terpene ADH (Bicsak et al., 1982). In addition, Kazeniac and Hall (1970) reported an absence of geraniol in extracts of tomato, although its isomer, linalool, was found. In contrast, we detected significant amounts of geraniol in the tomato fruit and, although there is considerable variation in ratios of citral to geraniol between individual fruit and between plants, there is no evidence of the ratio being influenced by variations in the activity of the ADH 2 enzyme.
The tomato ADH 2 enzyme accumulates naturally during the ripening/softening of the fruit (Bicsak et al., 1982; Longhurst et al., 1990), being most abundant late in ripening. Associated with the increase in enzyme is an increase in abundance of its mRNA (Chen and Chase, 1993; Longhurst et al., 1994). The mechanism for these increases is as yet unclear. It has been speculated (Longhurst et al., 1990) that changes in cytoplasmic pH accompanying changes in cytoplasmic ion concentrations (Vickery and Bruinsma, 1973), which result from membrane leakage in the softening fruit, might be responsible. Another suggested mechanism (Longhurst et al., 1994) is enhanced transcription of the Adh 2 gene in response to a slight lowering of internal O2 concentrations, again as a consequence of the softening process. Analysis of ADH activity levels in firm-fruit varieties (cvs Sunny and Floradade) and soft-fruit varieties (cvs 83G38, Momotaro, and Castlemart) has suggested a loose connection between fruit softness and ADH activity (Longhurst et al., 1990). If fruit softness affects ADH activity, which, as shown here, influences the natural balances of flavor-associated aldehydes and alcohols in the ripening fruit, then a correlation between fruit firmness and flavor development can be postulated.
Modification in Flavor
In a preliminary taste trial, fruit from the C20 T0 high-ADH plant were evaluated by a panel as having significantly enhanced ripe-fruit flavor relative to control fruit (Table V). Despite the preliminary nature of the trial and the small size of the panel, the results are encouraging, particularly in that 10 of the 11 panelists selected the C20 fruit. The C20 fruit also tended to have a lower intensity of the green-fruit attribute and a higher intensity of the sweetness attribute, but these trends were not statistically significant. The C13 T0 high-ADH fruit and the C23 T0 low-ADH fruit did not appear to differ in flavor characteristics from control fruit. Both the C20 and C13 fruit had increased levels of ADH activity relative to control fruit, approximately 2.0 and 3.4 times, respectively. However, the resulting increase in levels of the 6-C alcohols, hexanol and Z-3-hexenol, was greater in the C20 fruit than in the C13 fruit. As discussed above, this may be attributable to enhanced ADH levels being constitutive and maintained throughout the development of the C20 fruit, while being limited to the ripening fruit in the C13 plant. However, because the bulk of the pathway from free fatty acids to the 6-C aldehydes and alcohols occurs only in macerated tissue, the reason for the difference is not clear.
The improved flavor characteristics of the C20 fruit, therefore, appear to be related to increased levels of the alcohols, particularly the Z-3-hexenol, because hexanol is a weak odorant not considered important for flavor (Buttery et al., 1987). Kazeniac and Hall (1970) underlined the importance of the aldehydes to the fresh tomato flavor and suggested that reduction of the aldehydes allowed the contribution of the alcohols to predominate and resulted in development of a “processed” or “enzymic” flavor. In contrast, our findings stress the importance of the alcohols and suggest that a balance between aldehydes and alcohols is essential to the development of the ripe-fruit flavor.
All the fruit compared in the taste trial were equally ripe, between Br+7 and Br+9. Although the ripening process in tomato fruit is generally coordinated, such features as color and flavor development can be uncoupled, e.g. by genetic manipulation and in a variety of mutants (compare with Tucker, 1993). It is possible, therefore, that increasing the ADH level in the transgenic fruit enhanced the rate of flavor development rather than the overall extent of flavor development. Although we consider this unlikely it would still be beneficial because it would impart a richer flavor to less-ripe fruit, thus improving their consumer acceptability.
Wang et al. (1996) have reported the introduction of a functional yeast Δ–9 desaturase gene into tomato. The introduced gene affected unsaturation of fatty acids in the fruit, which resulted in increased abundances of several of the volatiles discussed above. In fruit from one transgenic plant, hexanal and Z-3-hexenal concentrations were 2.7- and 2-fold higher, respectively, than levels in control fruit, whereas hexanol and Z-3-hexenol concentrations were both increased about 4-fold relative to controls.
The increasing ability to modify individual stages of this important pathway greatly increases the prospects for improving the flavor characteristics of tomato and possibly other fruits.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Richard Gawel, University of Adelaide, for conducting the taste trial, to Dr. Rachel Drake for advice on analysis of transformants, and to Sue Maffei for helping to establish the system for volatile analyses. We would also like to thank the Australian Horticultural Research and Development Corporation for financial support.
Abbreviations:
- AC
Ailsa Craig
- ADH
alcohol dehydrogenase
- Br
breaker
- CaMV
cauliflower mosaic virus
- PG
polygalacturonase
- SPME
solid-phase microextraction
Footnotes
This work was supported by a grant from the Australian Horticultural Research and Development Corporation.
LITERATURE CITED
- Baldwin EA, Nisperos-Carriedo MO, Moshonas MG. Quantitative analysis of flavour and other volatiles and for certain constituents of two tomato cultivars during ripening. J Am Soc Hortic Sci. 1991;116:265–269. [Google Scholar]
- Bevan MW. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984;12:8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicsak TA, Kann LR, Reiter A, Chase T., Jr Tomato alcohol dehydrogenase: purification and substrate specificity. Arch Biochem Biophys. 1982;216:605–615. doi: 10.1016/0003-9861(82)90250-8. [DOI] [PubMed] [Google Scholar]
- Bird CR, Smith CJS, Ray JA, Moureau P, Bevan MJ, Bird AS, Hughes S, Morris PC, Grierson D, Schuch W. The tomato polygalacturonase gene and ripening specific expression in transgenic plants. Plant Mol Biol. 1988;11:651–662. doi: 10.1007/BF00017465. [DOI] [PubMed] [Google Scholar]
- Bittenbender HC, Kelly JF (1988) Improving the nutritional quality of vegtables through plant breeding. In E Karmas, RS Harris, eds, Nutritional Evaluation of Food Processing, Ed 3. Wiley, New York, pp 659–686
- Buttery RG, Seifert RM, Guardagni DG, Ling LC. Characterization of additional volatile components of tomato. J Agric Food Chem. 1971;19:524–529. [Google Scholar]
- Buttery RG, Teranishi R, Flath RA, Ling LC. Fresh tomato volatiles. In: Teranishi R, Buttery RG, Shahidi F, editors. Flavour Chemistry: Trends and Developments. ACS Symposium Series 338. Washington, DC: American Chemical Society; 1989. pp. 213–222. [Google Scholar]
- Buttery RG, Teranishi R, Ling LC. Fresh tomato aroma volatiles: a quantitative study. J Agric Food Chem. 1987;35:540–544. [Google Scholar]
- Buttery RG, Teranishi R, Ling LC, Flath RA, Stern DJ. Quantitative studies on origins of fresh tomato volatiles. J Agric Food Chem. 1988;36:1247–1250. [Google Scholar]
- Caperle M, Maiani G, Azzini E, Conti EMS, Raguzzini A, Ramazzotti V, Crespi M. Dietary profiles and anti-oxidants in a rural population of central Italy with a low frequency of cancer. Eur J Cancer Prev. 1996;5:197–206. doi: 10.1097/00008469-199606000-00008. [DOI] [PubMed] [Google Scholar]
- Chen A-RS, Chase T., Jr Alcohol dehydrogenase 2 and pyruvate decarboxylase induction in ripening and hypoxic tomato fruit. Plant Physiol Biochem. 1993;31:875–885. [Google Scholar]
- Clinton SK, Emenhiser C, Schwartz SJ, Bostwick DG, Williams AW, Moore BJ, Erdman JW. Cis-trans lycopene isomers, carotenoids, and retinol in the human prostate. Cancer Epidemiology Biomarkers & Prevention. 1996;5:823–833. [PubMed] [Google Scholar]
- Dirinck P, Schreyen L, van Wassenhove F, Schamp N. Flavour quality of tomatoes. J Sci Food Agric. 1976;27:499–508. [Google Scholar]
- Eriksson CE. Alcohol: NAD oxidoreductase (E.C.1.1.1.1.) from peas. J Food Sci. 1968;33:525–532. [Google Scholar]
- Frisch DA, Harris-Haller LW, Yokubaitis NT, Thomas TL, Hardin SH, Hall TC. Complete sequence of the binary vector Bin 19. Plant Mol Biol. 1995;27:405–409. doi: 10.1007/BF00020193. [DOI] [PubMed] [Google Scholar]
- Galliard T, Matthew JA, Wright AJ, Fishwick MJ. The enzymatic breakdown of lipids to volatile and non-volatile carbonyl fragments in disrupted tomato fruits. J Sci Food Agric. 1977;28:863–868. [Google Scholar]
- Hatanaka A, Kajiwara T, Matsui K, Kitamura A. Expression of lipoxygenase and hydroperoxide lyase activities in tomato fruits. Z Naturforsch. 1992;47c:369–374. [Google Scholar]
- Hatanaka A, Kajiwara T, Sekiya J. Fatty acid hyperoxide lyase in plant tissues: volatile aldehyde formation from linoleic and linolenic acid. In: Parliament TH, Croteau R, editors. Biogeneration of Aroma. ACS Symposium Series 317. Washington, DC: American Chemical Society; 1986. pp. 167–175. [Google Scholar]
- Hoffman G. 1-Octen-3-ol and its relation to the oxidative cleavage products from esters of linoleic acid. J Am Oil Chem Soc. 1962;39:439–444. [Google Scholar]
- Jorgensen R. Altered gene expression in plants due to trans interaction between homologous genes. Trends Biotechnol. 1990;8:340–344. doi: 10.1016/0167-7799(90)90220-r. [DOI] [PubMed] [Google Scholar]
- Kazeniac SJ, Hall RM. Flavour chemistry of tomato volatiles. J Food Sci. 1970;35:519–530. [Google Scholar]
- Leblová S, Mancal P. Characterization of plant alcohol dehydrogenase. Physiol Plant. 1975;34:246–249. [Google Scholar]
- Levy J, Bosin E, Feldman B, Giat Y, Miinster A, Danilenko M, Sharoni Y. Lycopene is a more potent inhibitor of human cancer cell proliferation than either alpha-carotene or beta-carotene. Nutr Cancer. 1995;24:257–266. doi: 10.1080/01635589509514415. [DOI] [PubMed] [Google Scholar]
- Linforth RST, Savary I, Pattenden B, Taylor AJ. Volatile compounds found in expired air during eating of fresh tomatoes and in the headspace above tomatoes. J Sci Food Agric. 1994;65:241–247. [Google Scholar]
- Longhurst T, Lee L, Hinde R, Brady C, Speirs J. Structure of the tomato Adh 2 gene and Adh 2 pseudogenes, and a study of Adh 2 gene expression in fruit. Plant Mol Biol. 1994;26:1073–1084. doi: 10.1007/BF00040690. [DOI] [PubMed] [Google Scholar]
- Longhurst TJ, Tung HF, Brady CJ. Developmental regulation of the expression of alcohol dehydrogenase in ripening tomato fruits. J Food Biochem. 1990;14:421–433. [Google Scholar]
- McGlasson WB, Last JH, Shaw KJ, Meldrum SK. Influence of the non-ripening mutants rin and nor on the aroma of tomato fruit. HortScience. 1987;22:632–634. [Google Scholar]
- Nicholass FJ, Smith CJS, Schuch W, Bird CR, Grierson D. High levels of ripening-specific reporter gene expression directed by tomato fruit polygalacturonase gene-flanking regions. Plant Mol Biol. 1995;28:423–435. doi: 10.1007/BF00020391. [DOI] [PubMed] [Google Scholar]
- Potty VH, Bruemmer JH. Oxidation of geraniol by an enzyme system from orange. Phytochemistry. 1970;9:1001–1007. [Google Scholar]
- Sieso V, Nicolas M, Seck S, Crouzet J. Constituants volatils de la tomate: mise en evidence et formation par voie enzymatique du trans-hexene-2-ol. Agric Biol Chem. 1976;40:2349–2353. [Google Scholar]
- Skillings JH, Mack GA. On the use of a Friedman-type statistic in balanced and unbalanced block designs. Technometrics. 1981;23:171–177. [Google Scholar]
- Smith CJS, Watson CF, Ray J, Bird CR, Morris PC, Schuch W, Grierson D. Antisense RNA inhibition of polygalacturonase gene expression in transgenic tomatoes. Nature. 1988;334:724–726. [Google Scholar]
- Tanksley SD. Linkage, chromosomal association and expression of Adh-1 and Pgm-2 in tomato. Biochem Genet. 1979;17:1159–1167. doi: 10.1007/BF00504353. [DOI] [PubMed] [Google Scholar]
- Thomas MR, Matsumoto S, Cain P, Scott NS. Repetitive DNA of grape wine: classes present and sequences suitable for cultivar identification. Theor Appl Genet. 1993;86:173–180. doi: 10.1007/BF00222076. [DOI] [PubMed] [Google Scholar]
- Tucker GA. Introduction. In: Seymour GB, Taylor JE, Tucker GA, editors. Biochemistry of Fruit Ripening. New York: Chapman & Hall; 1993. pp. 1–51. [Google Scholar]
- Vickery RS, Bruinsma J. Compartments and permeability for potassium in developing fruits of tomato (Lycopersicon esculentum Mill.) J Exp Bot. 1973;24:1261–1270. [Google Scholar]
- Wang C, Chin C-K, Ho C-T, Hwang C-F, Polashock JJ, Martin CE. Changes in fatty acids and fatty acid-derived flavour compounds by expressing the yeast Δ–9 desaturase gene in tomato. J Agric Food Chem. 1996;44:3399–3402. [Google Scholar]
- Yamashita I, Iino K, Yoshikawa S. Alcohol dehydrogenases from strawberry seeds. Agric Biol Chem. 1978;42:1125–1132. [Google Scholar]
- Yu MH, Salunkhe DK, Olsen LE. Production of 3-methyl-butanal from L-leucine by tomato extracts. Plant Cell Physiol. 1968;9:633–638. [Google Scholar]