Abstract
The nanoparticle industry is expected to become a trillion dollar business in the near future. Therefore, the unintentional introduction of nanoparticles into the environment is increasingly likely. However, currently applied risk-assessment practices require further adaptation to accommodate the intrinsic nature of engineered nanoparticles. Combining a chronic flow-through exposure system with subsequent acute toxicity tests for the standard test organism Daphnia magna, we found that juvenile offspring of adults that were previously exposed to titanium dioxide nanoparticles exhibit a significantly increased sensitivity to titanium dioxide nanoparticles compared with the offspring of unexposed adults, as displayed by lower 96 h-EC50 values. This observation is particularly remarkable because adults exhibited no differences among treatments in terms of typically assessed endpoints, such as sensitivity, number of offspring, or energy reserves. Hence, the present study suggests that ecotoxicological research requires further development to include the assessment of the environmental risks of nanoparticles for the next and hence not directly exposed generation, which is currently not included in standard test protocols.
Introduction
More than 1,000 products, including sunscreens, textiles, and self-cleaning surfaces, either contain engineered nanoparticles or are produced by nanotechnology. As a result, the unintentional introduction of engineered nanoparticles into the environment is increasing [1]. However, potential environmental risks and effects elicited by such nanoparticles on the integrity of ecosystems remain largely unknown [2]. This situation has arisen due to the lack of information regarding current environmental concentrations of nanoparticles, which is primarily caused by the absence of suitable analytical techniques [3]. Models predicting environmental concentrations of nanoparticles are compromised for this reason. A recent study reported a median titanium dioxide nanoparticle (nTiO2) concentration within surface waters in the ng to low µg/L range [4]. Moreover, the test protocols currently utilised for environmental risk assessment of nanoparticles were developed to accommodate traditional chemicals, such as pesticides [5]. As nanoparticles exhibit distinct physical and chemical properties, they often present different behaviours relative to their bulk phases and other chemical stressors [6], [7]. Their rapid aggregation, which causes an accumulation at the bottom of the experimental test units, could adversely affect benthic aquatic organisms [8]. At the same time, their bioavailability in the aqueous phase in ecotoxicological experiments is reduced [9]. These unique properties and associated experimental challenges require modifications of the respective test protocols [5].
Here, we investigated the chronic adverse effects of nTiO2 on the reproduction of Daphnia magna, whose parthenogenetic reproduction cycle is for instance described in detail by Zaffagnini [10], by applying two commercially available products with differing crystalline structure, namely P25 (Evonik, Germany; Figure 1) and A-100 (Crenox, Germany), as an additive-free, size-homogenised, stable suspension. The experimental procedure followed largely the standard test protocol designated by the Organisation for Economic Co-operation and Development (OECD) [11]. However, the experiments were performed in a flow-though system avoiding the accumulation of nTiO2 aggregates at the bottom of the test vessels as a potential source of ecotoxicological effects (Figure 2) [8]. To allow for inferences on potential effects on the next ( = filial) generation, the fifth brood released by the exposed adults was assessed separately for each treatment (0.00, 0.02, 2.00 mg/L) regarding its acute sensitivity to nTiO2 following the respective OECD test protocol [12]. This pathway of effect may be considered as relevant in the context of the present study since similar observations were reported for other chemical stressors such as algae toxins and polyfluorinated substances [13], [14] but also silver nanoparticles [15].
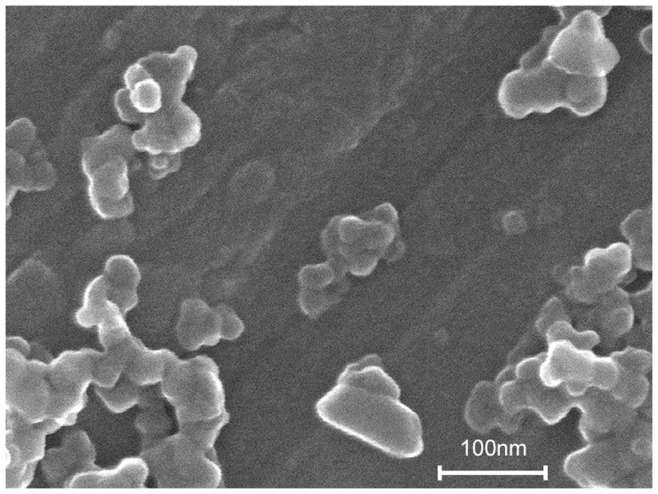
Figure 1. Scanning electron microscope image.
An image of the size-homogenised, stable nTiO2 suspension of the product P25 taken by an scanning electron microscope using 100,000-fold magnification (Hitachi SU8030).
Figure 2. The experimental set-up.
The flow-through testing apparatus, showing four experimental units (volume, 500 mL) with five D. magna each. Approximately 40 mL of the test medium was introduced every other hour slightly below the surface of the water. The old test medium was passively discharged using hydrostatic pressure at the bottom of each vessel. A fine mesh screen (0.1 mm) prevented the loss of recently hatched juvenile daphnids.
Materials and Methods
General Study Design
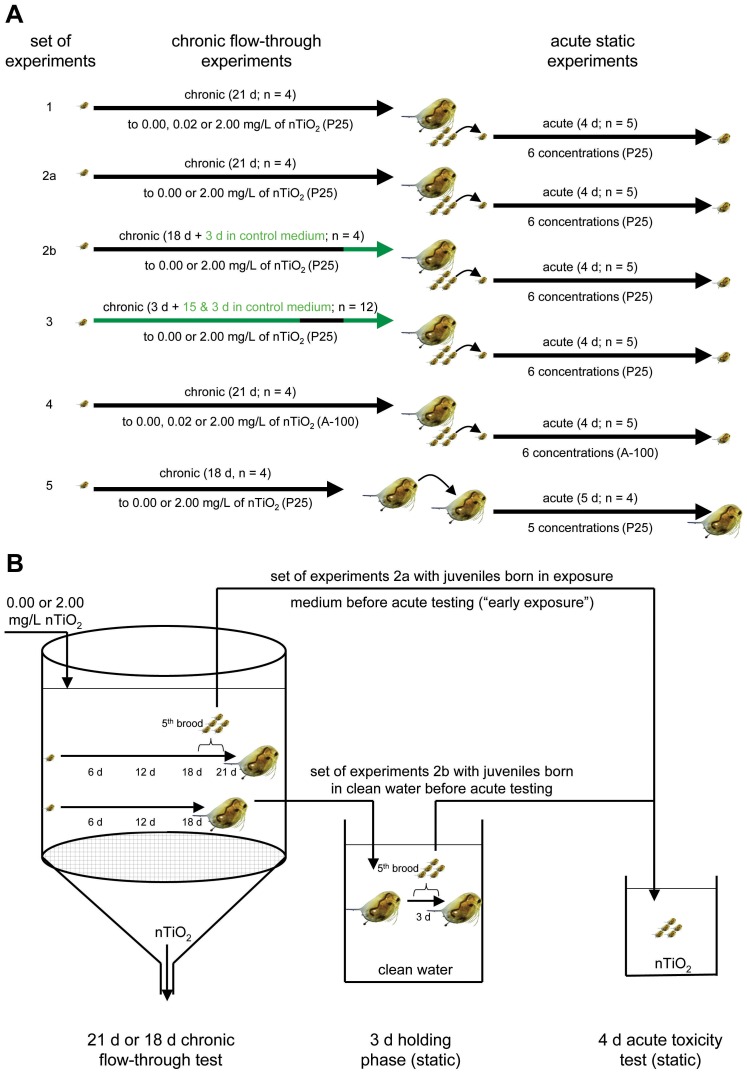
In total, five sets of experiments were performed to assess potential effects of nTiO2 on the next generation of exposed adult D. magna (Figure 3A). During the first set of experiments carried out once, juveniles of the fifth brood released by adult daphnids exposed to 0.00, 0.02 or 2.00 mg/L P25-nTiO2 for 21 days were introduced into acute toxicity experiments. The second set of experiments was performed three times to assess the importance of an “early exposure” of juveniles towards nTiO2 directly after their release (Figure 3B). Therefore, half of the adults of each treatment, including the control, were transferred to test medium not containing P25-nTiO2 approximately 23 hours subsequent to the release of the fourth brood, i.e. after approximately 18 days. Thus, the exposure of newly born juveniles to nanoparticles prior to the initiation of the acute toxicity experiments was avoided. The remaining adults, in contrast, released their juveniles in the respective nTiO2 treatment (as in the first set of experiments). The third set of experiments – performed once – intended to investigate implications on juveniles’ sensitivity potentially driven by the exposure of daphnid’s eggs towards nTiO2 within the brood pouch. Therefore, adult daphnids were exposed to 0.00 and 2.00 mg/L nTiO2 starting with the release of the third brood and lasting until 23 h after the release of the fourth brood resulting in an exposure period of approximately 3 days. This procedure limited any chronic implications potentially transferred from adults to juveniles and represented the maximum time period eggs may have been exposed to nTiO2 during earlier experiments. Subsequently, adults were transferred to clean medium for the release of the fifth brood. To investigate whether the results obtained with P25 may be transferable to other nTiO2 forms, the experimental procedure used for the first set of experiments was carried out with the product A-100 during the fourth set of experiments. The fifth set is not further described in the manuscript. It was utilized to assess the sensitivity of adult daphnids following the 18 days of exposure to 0.00 and 2.00 mg/L nTiO2 (P25), which is displayed in Figure S1.
Figure 3. Experimental design.
(A) Schematic diagram illustrating the experimental procedure of each of the five sets of experiments conducted. (B) Visualised experimental procedure for the assessment of the “early exposure” hypothesis (second set of experiments).
Organisms
D. magna (Clone V, Eurofins-GAB laboratories, Germany) were cultured at 20±1°C with a 16:8 hour (light:dark) photoperiod in ASTM reconstituted with hard freshwater that was enriched with selenium, vitamins (thiamine hydrochloride, cyanocobalamine, biotine) and seaweed extract (Marinure®, Glenside, Scotland). The daphnids were fed with the green algae Desmodesmus sp. on a daily basis (∼200 µg carbon per organism).
Flow-through Tests
The experimental procedure followed the standard test protocol designated by the OECD [11], with minor deviations. Briefly, each replicate consisted of 500 mL test medium and five D. magna that were younger than 24 hours of age at the start of the experiments. The study duration was set at 21 days, and the offspring were counted daily as a measure of the sublethal effect. Most importantly, the experiments were performed in a flow-though system. Therefore, the ASTM medium (148 mL) was mixed with the nTiO2 stock solution (12 mL) immediately before delivery in equal proportions to four independent replicates, each with a volume of 500 mL. The old test medium was passively discharged through a mesh at the bottom of the vessel (Figure 1). This procedure was repeated every other hour, ensuring a complete water exchange within 24 hours. The ASTM medium was renewed daily and amended in an age-dependent manner with food (Desmodesmus sp.; 50–100 µg carbon/test organism). The nTiO2 stock solutions were renewed at 72 h intervals. All of the pumps were equipped as completely as possible with Teflon tubes to minimise the loss of nTiO2 during pumping. The flow-through apparatus avoided the accumulation of nTiO2 aggregates at the bottom of the test vessels, eliminating one potential source of ecotoxicological effects [8]. Moreover, the medium was amended with seaweed extract to simulate dissolved organic matter that is normally present in natural water, which stabilised nTiO2 within the aqueous phase [16]. This procedure ensured a continuous exposure to nTiO2 particles of sizes <150 nm (Table S1). The average particle size was monitored daily during the chronic experiments for the 2.00 mg/L nTiO2 treatment. Moreover, the actual zeta potential of both products in the test medium was analysed. Because even the highest test concentration delivered in this study was still too low to measure the zeta potential, the respective nTiO2 stock suspension was diluted in test medium prior to measurement (Table S1).
Acute Toxicity Experiments
The fifth brood released by the exposed adults was assessed separately for each treatment regarding its sensitivity to nTiO2. The concentration of nTiO2 that resulted in 50% immobility of the juvenile daphnids after 96 hours of exposure (96 h-EC50) was used as the measure of sensitivity. Because this prolonged study duration was recently recommended for nanoparticle testing [9], the OECD test protocol for acute toxicity tests with D. magna [12] was adapted correspondingly, and the daphnids were exposed to 0.00, 0.50, 1.00, 2.00, 4.00 or 8.00 mg/L nTiO2.
Nanoparticle Characterisation
Both of the nTiO2 products used in this study, P25 (Evonik) and A-100 (Crenox), were purchased as powdered reagents. Subsequently, both products were prepared as dispersant- and additive-free, size-homogenised, stable suspensions by stirred media milling [9]. The zeta potential and the actual particle size distribution of both suspensions were determined via electrophoretic mobility and dynamic light scattering (Delsa™ Nano C, Beckman Coulter, Germany), respectively. The concentrations of nTiO2 in the 2.00 mg/L treatment were verified weekly by inductively coupled plasma mass spectrometry [9]. These analyses were supplemented by scanning electron microscope (Hitachi SU8030) imaging for P25 for the verification of particle size.
Statistical Analysis
Immobilisation data gained from the acute toxicity tests were adjusted by Abbott’s formula if necessary and fitted to adequate dose-response models – based on Akaike Information Criterion and expert judgement – in order to determine 96 h-EC50 values using the drc extension package [17] for the statistics program R version 2.13.0. Confidence interval (CI) testing was accomplished to assess for statistically significant differences between 96 h-EC50 values of juveniles released by daphnids exposed to the control and those exposed to nTiO2 obtained during the first, third, fourth and fifth set of experiments [18]. The 96 h-EC50 values of the first and second set of experiments were combined in a meta-analysis assessing for difference between juveniles of the fifth brood released by daphnids not exposed to nTiO2 and those exposed to either 0.02 or 2.00 mg/L P25-nTiO2. Therefore, a fixed effect model based on the standardised effect size Cohen’s d was applied. An effect size was considered to be statistically significant when the respective CI did not include the zero value. Additionally, the respective p-values were computed [19].
Results
First and Second Set of Experiments
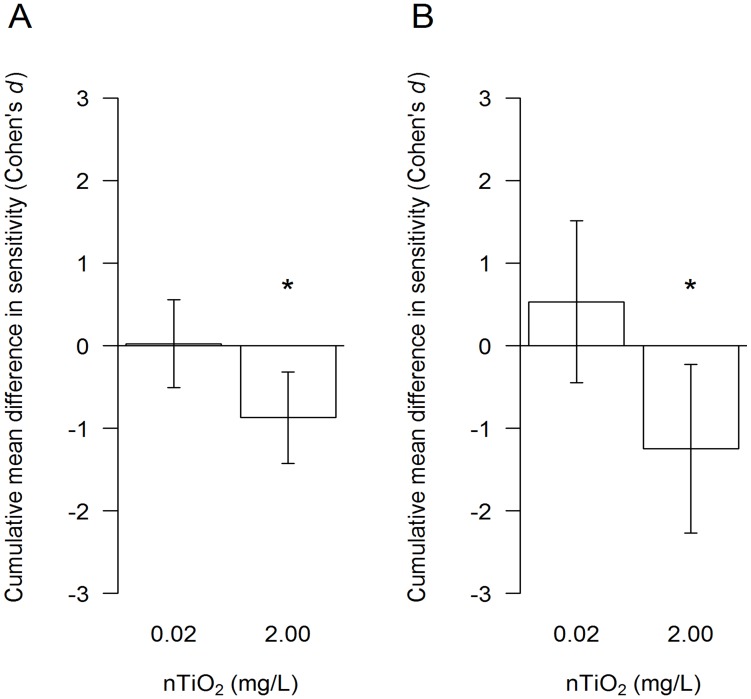
The P25-nTiO2 particles were dispersed within the aqueous phase throughout the whole chronic study duration and displayed a mean size of 135.8 nm, with approximately 30% of the particles <100 nm (Table S1). The nanoparticles did not adversely affect the mean number of offspring released by exposed adults (Figure S2 left). However, subsequent acute toxicity studies (first set of experiments) showed for the fifth brood released by adults exposed to 2.00 mg/L P25-nTiO2 a significantly lower 96 h-EC50 value (difference between 96 h-EC50 values: 4.39 mg/L, 95% CI of the difference 0.62 to 8.15; Figure S3). Additionally, a meta-analysis, which considered all 96 h-EC50 values of the first and the second set of experiments, supported these results (p = 0.0021 with n = 7; Figure 4A). This holds also true for a second meta-analysis that accounted exclusively for the effect sizes of the offspring that originated from the adults exposed to nTiO2 but that were released into nanoparticle-free test medium, which hence avoided the possibility of an early exposure (p = 0.0169 with n = 3; Figure 4B).
Figure 4. The sensitivity of juveniles released by nTiO2-exposed adults.
The cumulative mean (±95% CIs) difference in sensitivity – in terms of 96 h-EC50 values – of the offspring (fifth brood) released by adults exposed to 0.02 or 2.00 mg nTiO2/L and offspring released by control (uncontaminated) daphnids is displayed using the standardised effect size Cohen’s d. (A) The cumulative effect sizes for all bioassays conducted (n = 7) with the fifth brood during the first and second set of experiments. (B) The cumulative effect sizes for acute toxicity experiments conducted with offspring released in the control medium by adults previously exposed to the above-mentioned nTiO2 concentrations during the second set of experiments (n = 3). The statistical significance of a cumulative effect is highlighted by an asterisk (*). Negative effect sizes indicate increased toxicity.
Third Set of Experiments
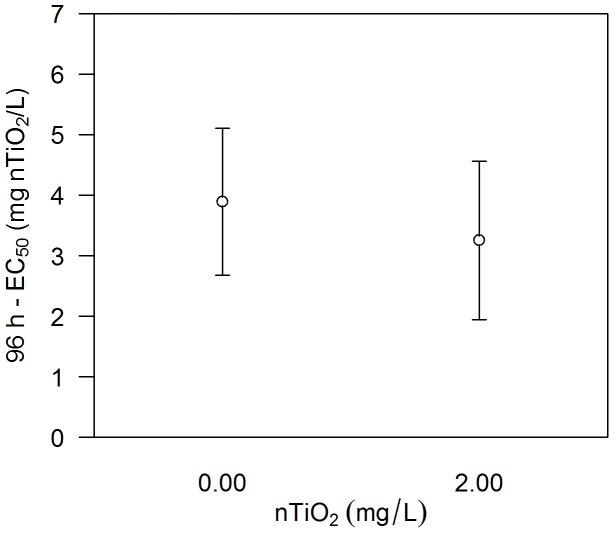
No difference in the sensitivity of the offspring (fifth brood) released by adults exposed for approximately 3 days to 0.00 and 2.00 mg/L nTiO2 was observed (the difference between 96 h-EC50 values: 0.64 mg/L, 95% CI of the difference −1.04 to 2.32; Figure 5).
Figure 5. The sensitivity of juveniles released during the third set of experiments.
96 h- EC50 values with respective 95% CIs of the fifth brood released by adults exposed to 0.00 and 2.00 mg/L P25-nTiO2 during the third set of experiments, which considered exclusively potential implication of nTiO2 exposure within the brood pouch. No statistically significant difference among treatments was observed.
Fourth Set of Experiments
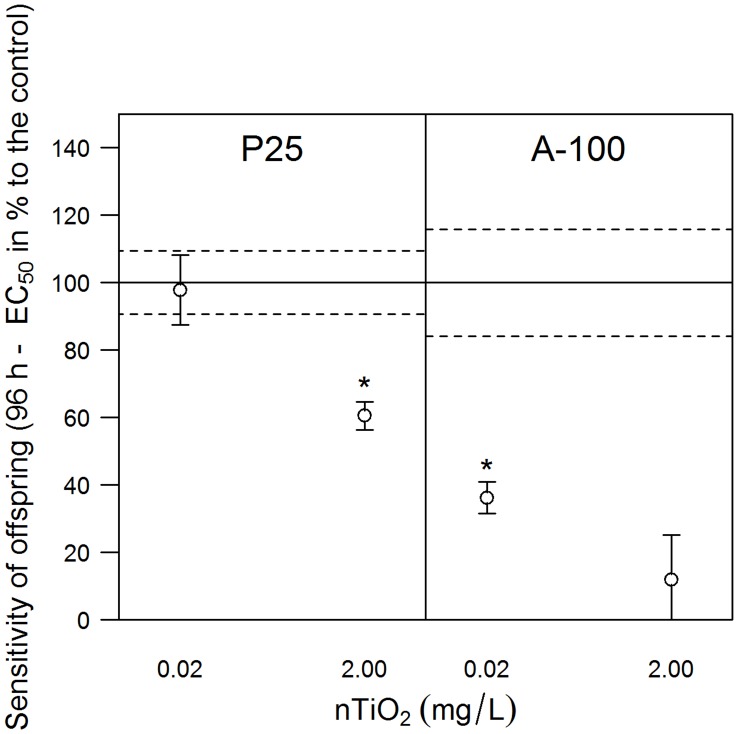
The chronic exposure to A-100 (for particle characteristics see Table S1) did not affect the reproduction of the test species D. magna, at concentrations up to 2.00 mg/L (Figure S2, right). Nevertheless, the offspring released by adults exposed to 0.02 mg/L A-100 were significantly more sensitive than those released by adults from the nanoparticle free control (difference between 96 h-EC50 values: 1.26 mg/L, 95% CI of the difference 0.62 to 1.90; Figure 6, right). The sensitivity of the offspring released by adults exposed to high nTiO2 concentrations was increased by a factor of at least five. However, it was not possible to verify this deviation by statistical analysis due to the lack of suitable quantitative approaches (Figure 6, right).
Figure 6. The sensitivity of juveniles released by adults exposed to different nTiO2 products.
Sensitivity, displayed as percent relative to the 96 h-EC50 of the respective control, of the fifth brood released by adult D. magna exposed to different nTiO2 treatments using the products P25 or A-100. The data displayed for P25 represent the weighted mean values of the seven experiments (first and second set of experiments), each with four replicates per treatment, whereas the 96 h-EC50 for the offspring released from the control parents was 3.13 mg/L nTiO2. For the product A-100, the results of one experiment with four replicates of pre-treatment are displayed (fourth set of experiments). In this situation, the 96 h-EC50 for the offspring released from the control parents was 1.98 mg/L nTiO2. The error bars and dashed lines indicate the standard error. The dashed lines are related to the control. Asterisks (*) denote significant differences between a treatment and the respective control.
Discussion
The first set of experiments showed that the fifth brood released by adults exposed to 2.00 mg/L P25-nTiO2 was significantly more sensitive than the juvenile offspring released by the non-exposed control adults (Figure S3), indicating the possibility of effects passed from the parental to the filial generation of daphnids. However, these results might also be explained by the exposure of the offspring immediately after hatching and prior to their introduction into the acute toxicity tests (Figure 3A). In contrast, the exposure of the juveniles from the nanoparticle-free control commenced up to 24 hours later, with the start of the acute toxicity experiments. To test this “early exposure” possibility, we performed further D. magna reproduction assays. However, half of the adults for each treatment during the second set of experiments (Figure 3B), including the control adults, were transferred to test medium containing no nTiO2 approximately 23 hours subsequent to the release of the fourth brood. The exposure of the newly released fifth brood to nanoparticles prior to the initiation of the acute toxicity experiments was thus avoided. In contrast, the remaining adults released their offspring into the respective treatment conditions. Three additional independent experiments were performed using this experimental set-up. All of the 96 h-EC50 (n = 7) values obtained for the offspring released by adults exposed to nTiO2 were combined into a meta-analysis (Figure 4A), which was based on a fixed effect model using Cohen’s d as a standardised effect size. Furthermore, a second meta-analysis was performed considering exclusively the effect sizes of the offspring that originated from the adults exposed to nTiO2 but that were released into nanoparticle-free test medium (n = 3; Figure 4B). Both meta-analyses revealed a significant increase in the sensitivity of the offspring released by adults exposed to 2.00 mg/L nTiO2 compared to offspring released from adults not suffering from nTiO2 exposure. These results suggest that “early exposure” is insufficient to explain the approximately twofold increase in sensitivity (Figure 6, left) observed in the fifth brood released from the exposed adults. However, the daphnids’ eggs might already be exposed to nTiO2 within the brood pouch, which could potentially affect the sensitivity of the subsequent offspring [20]. To assess this issue, adult daphnids were exposed to 0.00 and 2.00 mg/L nTiO2 starting with the release of the third brood and lasting until at least 23 h after the release of their fourth brood (third set of experiments). This procedure alleviated any long-term implications potentially transferred from adults to offspring and represented the maximum time period over which eggs may have been exposed to nTiO2 during our earlier experiments. Subsequently, the adults were transferred to nTiO2-free medium for the release of the fifth brood. Acute toxicity experiments revealed no difference in the sensitivity of the offspring released by adults exposed to 0.00 and 2.00 mg/L nTiO2 (Figure 5), indicating that the exposure of the eggs during the early phases of development could not account for the observed effects. Consistent with another study [15], which uncovered adverse effects of silver nanoparticles ingested by adult fruit fly Drosophila melanogaster (Meigen) passed on to their offspring, a similar explanation might apply for this study. Comparable observations were also reported for other chemical stressors like algal toxins [13], perfluorooctane sulfonic acid, perfluorooctanoic acid [14], vinclozolin and 5-azacytidine [21]. However, in this study, we have uncovered effects induced by nTiO2 on the next generation of D. magna but not the parental generation, in terms of quantitative ecotoxicological endpoints: sensitivity to nTiO2 after 18 days of exposure, number of offspring, and lipid content (Figure S1; S2, left; S4), while the mechanism resulting in this observation have no yet been understood.
Although this study demonstrates, for the first time, the possibility of nTiO2 effects on the next generation of D. magna in which the parental generation exhibits no obvious effects, whether these effects would also result from exposure to other TiO2 products remains unclear. Therefore, another flow-through experiment followed by acute toxicity experiments was performed with the product A-100 (fourth set of experiments; Figure 3A). Exposure to A-100 also did not affect the reproduction of the test species D. magna, measured as the number of offspring released at a product concentration of up to 2.00 mg/L (Figure S2, right). Dabrunz et al. [9] reported a 96 h-EC50 of 0.74 mg/L for this product. This deviation in effects may be explained by the amendment of the test medium with seaweed extract [16] as well as by the avoidance of nTiO2 deposition on the bottom of the test vessels [8]. Nonetheless, the offspring released by adults that were exposed to even 0.02 mg/L A-100 were significantly more sensitive–by an approximate factor of 3–than those released by the control parents (Figure 6, right). Moreover, the sensitivity of the offspring released by adults exposed to high nTiO2 concentrations was increased by a factor of at least five (Figure 6, right). These results might represent general safety implications of nTiO2 products, although their intensity varies among products and the concentration applied.
Conclusion
Although the test design of this study ensured the bioavailability of the investigated nanoparticles over the whole study duration, the parental D. magna did not exhibit any indications of toxic stress under these standard test conditions. However, the offspring generation of daphnids released by parental Daphnia that were previously exposed to nTiO2 were significantly more sensitive to nTiO2. Finally, the present study suggests that the standardised testing protocols that comprise the foundation of the current environmental risk-assessment approaches for nanoparticles underestimate risks and require modification. Apart from the recently recommended extension of the study duration for acute toxicity testing [9], the OECD test guidelines for the assessment of chronic ecotoxicity need to be improved by considering the effects on the next generation, even on offspring that has never been directly exposed to the agents.
Supporting Information
120 h- EC50 values with respective 95% CIs of adult D. magna following 18 days of exposure to nTiO2 (P25) in the flow-through system (fifth set of experiments). No statistically significant deviations regarding the sensitivity were detected.
(PDF)
Boxplot (bold line represents the median) of the offspring per test organism (n = 20) exposed to P25 (first set of experiments) or A-100 (fourth set of experiments) nTiO2 after 21 days of exposure to 0.00, 0.02 or 2.00 mg nTiO2/L.
(PDF)
96 h-EC50 values with respective 95% CIs of the fifth brood released by adults exposed to P25 nTiO2 during the flow-through experiment (first set of experiments); Asterisk (*) denotes statistically significant difference between the juveniles released from adults exposed to 2.00 mg/L TiO2 and the control based on confidence interval testing (difference between 96 h-EC50 values 4.39 mg/L, 95% CI 0.62 to 8.15).
(PDF)
Lipid content per adult D. magna after 21 days of exposure to P25 nTiO2 (first set of experiments).
(PDF)
Particle characteristics of P25 and A-100: The table displays the 10th and the 90th percentile of the particle size distribution, the mean percentage of particles below a particle size of 100 nm as well as the mean particle size together with the polydispersity index. This index provides information on the range of the particle size distribution. A value above 0.3 indicates unreliability of the measurement, due to masking of small particles by large ones. Additionally the zeta potential of the particles and their measured concentration in the test medium is given. nTiO2 concentrations were measured following Dabrunz et al.
(PDF)
Acknowledgments
The authors thank W. Fey for assistance during ICP-MS analysis, U. Gernert (ZELMI, Technical University Berlin) for the SEM picture, and C. Schilde (Institute of Particle Technology, Technical University Braunschweig) for the provision of stable nanoparticle dispersions. D.J. Baird, M.C. Newman and K. Schwenk are acknowledged for their valuable comments on an earlier draft of this manuscript and A.A. Kotov for advice on the early embryology od Daphnia.
Funding Statement
The Ministry of Science Rhineland-Palatinate (MBWJK) funded this study, which is linked with studies conducted within the research group INTERNANO, which is funded by the German Research Foundation (DFG). Moreover, we acknowledge the Fix-Stiftung, Landau for financial support of the research infrastructure. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Klaine SJ, Koelmans AA, Horne N, Carley S, Handy RD, et al. (2012) Paradigms to assess the environmental impact of manufactured nanomaterials. Environ Toxicol Chem 31: 3–14. [DOI] [PubMed] [Google Scholar]
- 2. Behra R, Krug H (2008) Nanoecotoxicology - Nanoparticles at large. Nat Nanotechnol 3: 253–254. [DOI] [PubMed] [Google Scholar]
- 3. von der Kammer F, Ferguson PL, Holden PA, Masion A, Rogers KR, et al. (2012) Analysis of engineered nanomaterials in complex matrices (environment and biota): general considerations and conceptual case studies. Environ Toxicol Chem 31: 32–49. [DOI] [PubMed] [Google Scholar]
- 4. Gottschalk F, Ort C, Scholz RW, Nowack B (2011) Engineered nanomaterials in rivers - exposure scenarios for Switzerland at high spatial and temporal resolution. Environ Pollut 159: 3439–3445. [DOI] [PubMed] [Google Scholar]
- 5. Handy RD, Cornelis G, Fernandes T, Tsyusko O, Decho A, et al. (2012) Ecotoxicity test methods for engineered nanomaterials: practical experiences and recommendations from the bench. Environ Toxicol Chem 31: 15–31. [DOI] [PubMed] [Google Scholar]
- 6. Owen R, Handy R (2007) Formulating the problems for environmental risk assessment of nanomaterials. Environ Sci Technol 41: 5582–5588. [DOI] [PubMed] [Google Scholar]
- 7. Ferry JL, Craig P, Hexel C, Sisco P, Frey R, et al. (2009) Transfer of gold nanoparticles from the water column to the estuarine food web. Nat Nanotechnol 4: 441–444. [DOI] [PubMed] [Google Scholar]
- 8. Bundschuh M, Zubrod JP, Englert D, Seitz F, Rosenfeldt RR, et al. (2011) Effects of nano-TiO2 in combination with ambient UV-irradiation on a leaf shredding amphipod. Chemosphere 85: 1563–1567. [DOI] [PubMed] [Google Scholar]
- 9. Dabrunz A, Duester L, Prasse C, Seitz F, Rosenfeldt RR, et al. (2011) Biological surface coating and molting inhibition as mechanisms of TiO2 nanoparticle toxicity in Daphnia magna . PLoS One 6: e20112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaffagnini F (1987) Reproduction in Daphnia In Memorie dell'Istituto Italiano di Idrobiologia “Dott. Marco De Marchi”, Peters, R. H.; de Bernardi, R., Eds. Pallanza, 1987; 245–284.
- 11.OECD (2008) Guideline No. 211: Daphnia magna reproduction test.
- 12.OECD (2004) Guideline No. 202: Daphnia sp., acute immobilisation test.
- 13. Ortiz-Rodríguez R, Dao TS, Wiegand C (2012) Transgenerational effects of microcystin-LR on Daphnia magna . J Exp Biol 215: 2795–2805. [DOI] [PubMed] [Google Scholar]
- 14. Ji K, Kim Y, Oh S, Ahn B, Jo H, et al. (2008) Toxicity of perfluorooctane sulfonic acid and perfluorooctanoic acid on freshwater macroinvertebrates (Daphnia magna and Moina macrocopa) and fish (Oryzias latipes). Environ Toxicol Chem 27: 2159–2168. [DOI] [PubMed] [Google Scholar]
- 15. Panacek A, Prucek R, Safarova D, Dittrich M, Richtrova J, et al. (2011) Acute and chronic toxicity effects of silver nanoparticles (NPs) on Drosophila melanogaster . Environ Sci Technol 45: 4974–4979. [DOI] [PubMed] [Google Scholar]
- 16. Hall S, Bradley T, Moore JT, Kuykindall T, Minella L (2009) Acute and chronic toxicity of nano-scale TiO2, particles to freshwater fish, cladocerans, and green algae, and effects of organic and inorganic substrate on TiO2 toxicity. Nanotoxicology 3: 91–97. [Google Scholar]
- 17.Ritz C, Streibig JC (2008) Non linear regression with R. Springer Science & Business Media: New York, USA.
- 18. Wheeler MW, Park RM, Bailer AJ (2006) Comparing median lethal concentration values using confidence interval overlap or ratio tests. Environ Toxicol Chem 25: 1441–1444. [DOI] [PubMed] [Google Scholar]
- 19.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR (2009) Introduction to Meta-Analysis John Wiley & Sons Ltd: West Sussex, UK.
- 20. Baird DJ, Barber I, Soares AMVM, Calow P (1991) An early life-stage test with Daphnia magna straus: An alternative to the 21-day chronic test? Ecotoxicol Environ Saf 22: 1–7. [DOI] [PubMed] [Google Scholar]
- 21. Vandegehuchte MB, Lemière F, Vanhaecke L, Vanden Berghe W, Janssen CR (2010) Direct and transgenerational impact on Daphnia magna of chemicals with a known effect on DNA methylation. Comp Biochem Physiol C 151: 278–285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
120 h- EC50 values with respective 95% CIs of adult D. magna following 18 days of exposure to nTiO2 (P25) in the flow-through system (fifth set of experiments). No statistically significant deviations regarding the sensitivity were detected.
(PDF)
Boxplot (bold line represents the median) of the offspring per test organism (n = 20) exposed to P25 (first set of experiments) or A-100 (fourth set of experiments) nTiO2 after 21 days of exposure to 0.00, 0.02 or 2.00 mg nTiO2/L.
(PDF)
96 h-EC50 values with respective 95% CIs of the fifth brood released by adults exposed to P25 nTiO2 during the flow-through experiment (first set of experiments); Asterisk (*) denotes statistically significant difference between the juveniles released from adults exposed to 2.00 mg/L TiO2 and the control based on confidence interval testing (difference between 96 h-EC50 values 4.39 mg/L, 95% CI 0.62 to 8.15).
(PDF)
Lipid content per adult D. magna after 21 days of exposure to P25 nTiO2 (first set of experiments).
(PDF)
Particle characteristics of P25 and A-100: The table displays the 10th and the 90th percentile of the particle size distribution, the mean percentage of particles below a particle size of 100 nm as well as the mean particle size together with the polydispersity index. This index provides information on the range of the particle size distribution. A value above 0.3 indicates unreliability of the measurement, due to masking of small particles by large ones. Additionally the zeta potential of the particles and their measured concentration in the test medium is given. nTiO2 concentrations were measured following Dabrunz et al.
(PDF)