Abstract
The purpose of this study was to determine whether or not the biochemical, functional, and ultrastructural abnormalities produced by brief temporary coronary occlusions (unassociated with necrosis) ever resolve and, if so, when they do. Anesthetized open-chest dogs were subjected to 15 min of coronary artery occlusion followed by 72 hr, 7 days, or 14 days of reperfusion. Serial in vivo myocardial biopsies were performed for measurement of ATP and for ultrastructural analysis. Regional function was evaluated by sonomicrometry. Mean (+/- SEM) myocardial ATP concentration was 36.6 +/- 1.2 nmol/mg of cardiac protein in nonischemic subendocardium and 18.9 +/- 1.5 in ischemic subendocardium after 15 min of ischemia. ATP remainede performed for measurement of ATP and for ultrastructural analysis. Regional function was evaluated by sonomicrometry. Mean (+/- SEM) myocardial ATP concentration was 36.6 +/- 1.2 nmol/mg of cardiac protein in nonischemic subendocardium and 18.9 +/- 1.5 in ischemic subendocardium after 15 min of ischemia. ATP remainede performed for measurement of ATP and for ultrastructural analysis. Regional function was evaluated by sonomicrometry. Mean (+/- SEM) myocardial ATP concentration was 36.6 +/- 1.2 nmol/mg of cardiac protein in nonischemic subendocardium and 18.9 +/- 1.5 in ischemic subendocardium after 15 min of ischemia. ATP remained depressed in the reperfused previously ischemic subendocardium at both 90 min (68% of nonischemic value) and 72 hr (78% of nonischemic value) but returned to normal at 7 days. Regional systolic function and cardiac ultrastructural abnormalities required 7 days for full recovery. Histologic and histochemical analysis did not reveal necrosis at any time. Therefore, biochemical, functional, and ultrastructural abnormalities induced by brief periods of transient coronary occlusion not associated with necrosis do resolve completely but the recovery period is prolonged.
Full text
PDF
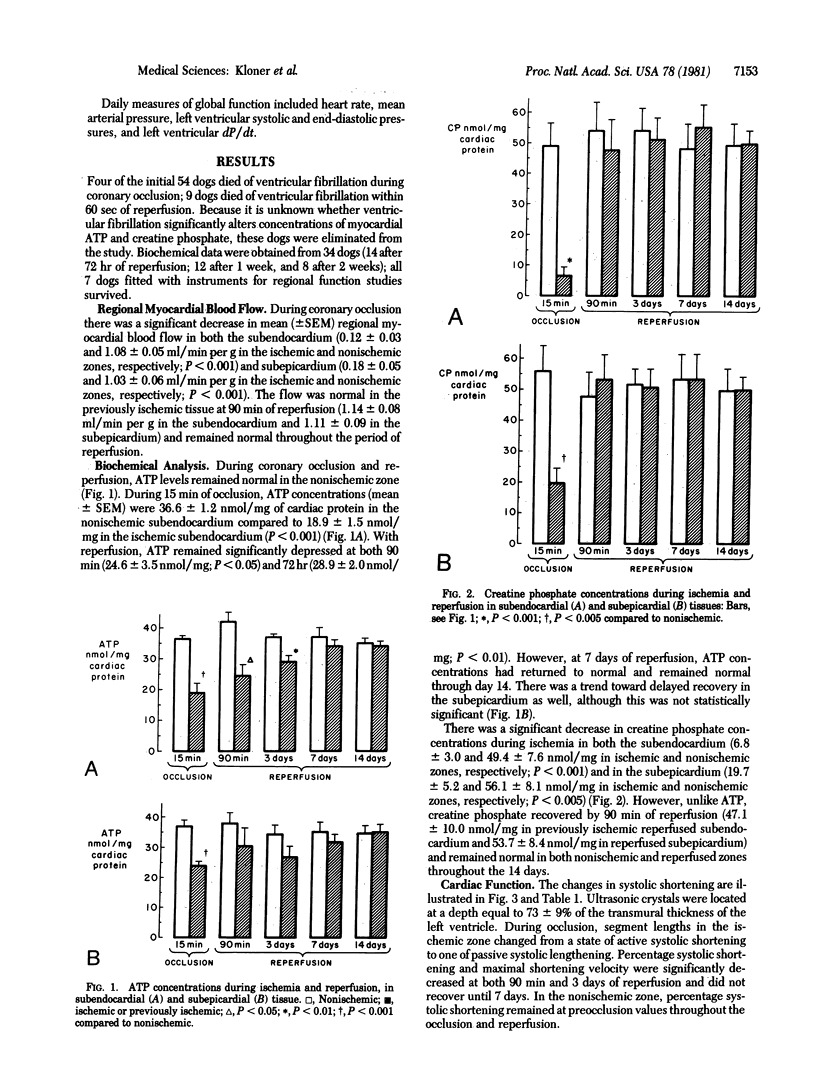
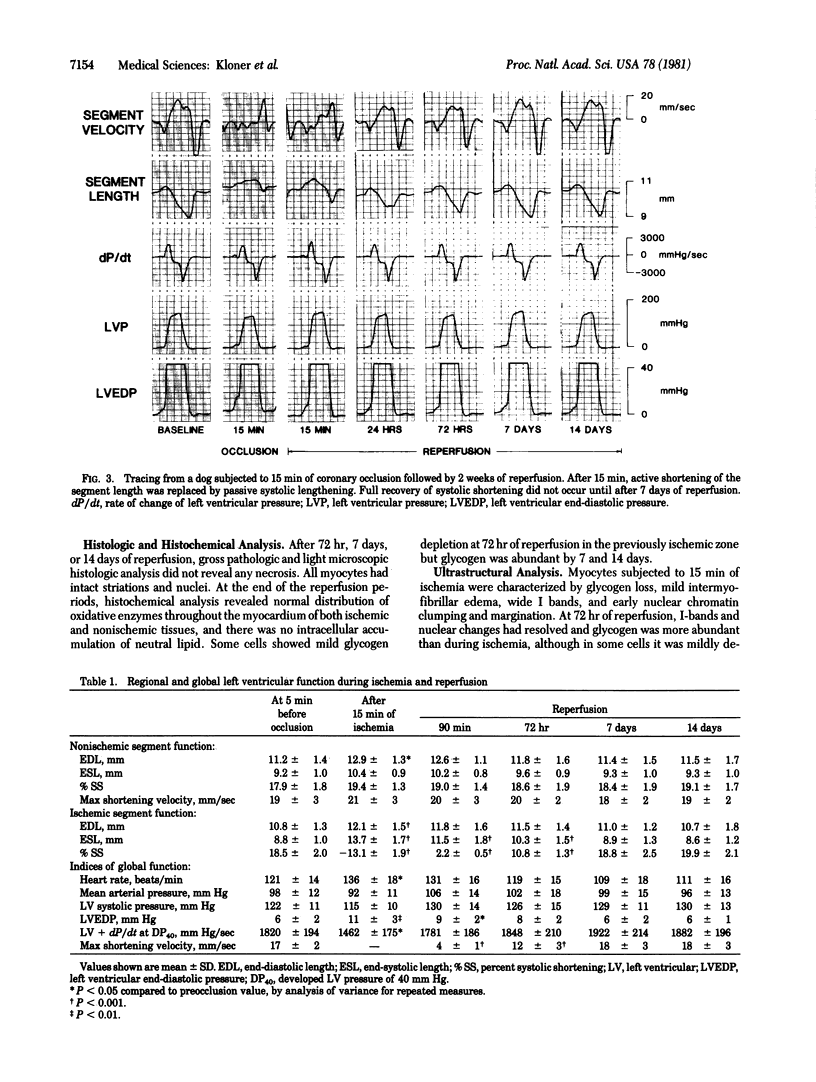


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohn L. H., Gorlin R., Herman M. V., Collins J. J., Jr Aorto-coronary bypass for acute coronary occlusion. J Thorac Cardiovasc Surg. 1972 Oct;64(4):503–513. [PubMed] [Google Scholar]
- Darsee J. R., Kloner R. A., Braunwald E. Time course of regional function after coronary occlusions of 1- to 120-min duration. Am J Physiol. 1981 Mar;240(3):H399–H407. doi: 10.1152/ajpheart.1981.240.3.H399. [DOI] [PubMed] [Google Scholar]
- Darsee J. R., Kloner R. A. The no reflow phenomenon: a time-limiting factor for reperfusion after coronary occlusion? Am J Cardiol. 1980 Nov;46(5):800–806. doi: 10.1016/0002-9149(80)90431-2. [DOI] [PubMed] [Google Scholar]
- DeBoer L. W., Ingwall J. S., Kloner R. A., Braunwald E. Prolonged derangements of canine myocardial purine metabolism after a brief coronary artery occlusion not associated with anatomic evidence of necrosis. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5471–5475. doi: 10.1073/pnas.77.9.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. C., Reed G. E., Meilman H., Silk B. B. Release of nucleosides from canine and human hearts as an index of prior ischemia. Am J Cardiol. 1979 Jan;43(1):52–58. doi: 10.1016/0002-9149(79)90044-4. [DOI] [PubMed] [Google Scholar]
- Ganz W., Buchbinder N., Marcus H., Mondkar A., Maddahi J., Charuzi Y., O'Connor L., Shell W., Fishbein M. C., Kass R. Intracoronary thrombolysis in evolving myocardial infarction. Am Heart J. 1981 Jan;101(1):4–13. doi: 10.1016/0002-8703(81)90376-8. [DOI] [PubMed] [Google Scholar]
- Gudbjarnason S., Puri P. S., Mathes P. Biochemical changes in non-infarcted heart muscle following myocardial infarction. J Mol Cell Cardiol. 1971 Aug;2(3):253–276. doi: 10.1016/0022-2828(71)90058-7. [DOI] [PubMed] [Google Scholar]
- Hearse D. J. Oxygen deprivation and early myocardial contractile failure: a reassessment of the possible role of adenosine triphosphate. Am J Cardiol. 1979 Nov;44(6):1115–1121. doi: 10.1016/0002-9149(79)90177-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Wood J. M., Hanley H. G., Entman M. L., Hartley C. J., Swain J. A., Busch U., Chang C. H., Lewis R. M., Morgan W. J., Schwartz A. Biochemical and morphological correlates of acute experimental myocardial ischemia in the dog. IV. Energy mechanisms during very early ischemia. Circ Res. 1979 Jan;44(1):52–61. doi: 10.1161/01.res.44.1.52. [DOI] [PubMed] [Google Scholar]