Abstract
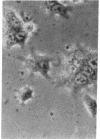
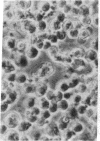
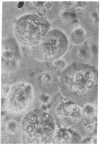
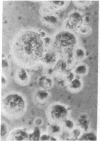
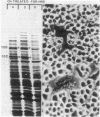
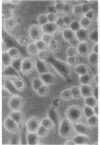
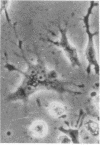
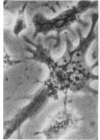
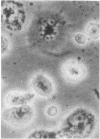
Superinfection of Raji cells with Epstein--Barr virus (EBV) leads to syncytium formation. Studies using metabolic inhibitors and amino acid analogues suggest that the fusion-inducing factor belongs to the early group of virus-specified proteins. Induction of early EBV protein synthesis in Raji cells by using various chemicals also leads to syncytium formation, indicating that the fusion process is not caused by a virion membrane protein introduced into the cells upon infection. The relevance of these findings to the association of EBV with carcinoma of the nasopharynx is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
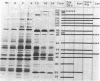
- Bayliss G. J., Nonoyama M. Mechanisms of infection with Epstein-Barr virus. III. The synthesis of proteins in superinfected Raji cells. Virology. 1978 Jun 1;87(1):204–207. doi: 10.1016/0042-6822(78)90173-3. [DOI] [PubMed] [Google Scholar]
- Bayliss G. J., Wolf H. Epstein--Barr virus-induced cell fusion. Nature. 1980 Sep 11;287(5778):164–165. doi: 10.1038/287164a0. [DOI] [PubMed] [Google Scholar]
- Bayliss G. J., Wolf H. The spontaneous and induced synthesis of Epstein-Barr virus antigens in Raji cells immobilized on surface coated with anti-lymphocyte globulin. J Gen Virol. 1981 Jun;54(Pt 2):397–401. doi: 10.1099/0022-1317-54-2-397. [DOI] [PubMed] [Google Scholar]
- Desgranges C., Wolf H., De-Thé G., Shanmugaratnam K., Cammoun N., Ellouz R., Klein G., Lennert K., Muñoz N., Zur Hausen H. Nasopharyngeal carcinoma. X. Presence of epstein-barr genomes in separated epithelial cells of tumours in patients from Singapore, Tunisia and Kenya. Int J Cancer. 1975 Jul 15;16(1):7–15. doi: 10.1002/ijc.2910160103. [DOI] [PubMed] [Google Scholar]
- Glaser R., Farrugia R., Brown N. Effect of the host cell on the maintenance and replication of Epstein-Barr virus. Virology. 1976 Jan;69(1):132–142. doi: 10.1016/0042-6822(76)90200-2. [DOI] [PubMed] [Google Scholar]
- Glaser R., Lang C. M., Lee K. J., Schuller D. E., Jacobs D., McQuattie C. Attempt to infect nonmalignant nasopharyngeal epithelial cells from humans and squirrel monkeys with Epstein-Barr virus. J Natl Cancer Inst. 1980 May;64(5):1085–1090. [PubMed] [Google Scholar]
- Glaser R., Zimmerman J., St Jeor S., Rapp F. Demonstration of a cellular inhibitor of Epstein-Barr and cytomegalovirus synthesis. Virology. 1975 Mar;64(1):289–292. doi: 10.1016/0042-6822(75)90103-8. [DOI] [PubMed] [Google Scholar]
- Glaser R., de Thé G., Lenoir G., Ho J. H. Superinfection epithelial nasopharyngeal carcinoma cells with Epstein-Barr virus. Proc Natl Acad Sci U S A. 1976 Mar;73(3):960–963. doi: 10.1073/pnas.73.3.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampar B., Derge J. G., Nonoyama M., Chang S. Y., Tagamets A., Showalter S. D. Programming of events in Epstein-Barr virus-activated cells induced by 5-iododeoxyuridine. Virology. 1974 Nov;62(1):71–89. doi: 10.1016/0042-6822(74)90304-3. [DOI] [PubMed] [Google Scholar]
- Huang D. P., Ho H. C., Ng M. H., Lui M. Possible transformation of nasopharyngeal epithelial cells in culture with Epstein-Barr virus from B95-8 cells. Br J Cancer. 1977 May;35(5):630–634. doi: 10.1038/bjc.1977.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D. P., Ho J. H., Henle W., Henle G. Demonstration of Epstein-Barr virus-associated nuclear antigen in nasopharyngeal carcinoma cells from fresh biopsies. Int J Cancer. 1974 Nov 15;14(5):580–588. doi: 10.1002/ijc.2910140504. [DOI] [PubMed] [Google Scholar]
- Luka J., Kallin B., Klein G. Induction of the Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology. 1979 Apr 15;94(1):228–231. doi: 10.1016/0042-6822(79)90455-0. [DOI] [PubMed] [Google Scholar]
- RODRIGUEZ J., DEINHARDT F. Preparation of a semipermanent mounting medium for fluorescent antibody studies. Virology. 1960 Oct;12:316–317. doi: 10.1016/0042-6822(60)90205-1. [DOI] [PubMed] [Google Scholar]
- Tovey M. G., Lenoir G., Begon-Lours J. Activation of latent Epstein-Barr virus by antibody to human IgM. Nature. 1978 Nov 16;276(5685):270–272. doi: 10.1038/276270a0. [DOI] [PubMed] [Google Scholar]
- Wolf H., Werner J., zur Hausen H. EBV DNA in nonlymphoid cells of nasopharyngeal carcinomas and in a malignant lymphoma obtained after inoculation of EBV into cottontop marmosets. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):791–796. doi: 10.1101/sqb.1974.039.01.091. [DOI] [PubMed] [Google Scholar]
- Wolf H., zur Hausen H., Becker V. EB viral genomes in epithelial nasopharyngeal carcinoma cells. Nat New Biol. 1973 Aug 22;244(138):245–247. doi: 10.1038/newbio244245a0. [DOI] [PubMed] [Google Scholar]
- zur Hausen H., O'Neill F. J., Freese U. K., Hecker E. Persisting oncogenic herpesvirus induced by the tumour promotor TPA. Nature. 1978 Mar 23;272(5651):373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]