Abstract
Background
Between 8% and 22% of female carriers of DMD mutations exhibit clinical symptoms of variable severity. Development of symptoms in DMD mutation carriers without chromosomal rearrangements has been attributed to skewed X-chromosome inactivation (XCI) favouring predominant expression of the DMD mutant allele. However the prognostic use of XCI analysis is controversial. We aimed to evaluate the correlation between X-chromosome inactivation and development of clinical symptoms in a series of symptomatic female carriers of dystrophinopathy.
Methods
We reviewed the clinical, pathological and genetic features of twenty-four symptomatic carriers covering a wide spectrum of clinical phenotypes. DMD gene analysis was performed using MLPA and whole gene sequencing in blood DNA and muscle cDNA. Blood and muscle DNA was used for X-chromosome inactivation (XCI) analysis thought the AR methylation assay in symptomatic carriers and their female relatives, asymptomatic carriers as well as non-carrier females.
Results
Symptomatic carriers exhibited 49.2% more skewed XCI profiles than asymptomatic carriers. The extent of XCI skewing in blood tended to increase in line with the severity of muscle symptoms. Skewed XCI patterns were found in at least one first-degree female relative in 78.6% of symptomatic carrier families. No mutations altering XCI in the XIST gene promoter were found.
Conclusions
Skewed XCI is in many cases familial inherited. The extent of XCI skewing is related to phenotype severity. However, the assessment of XCI by means of the AR methylation assay has a poor prognostic value, probably because the methylation status of the AR gene in muscle may not reflect in all cases the methylation status of the DMD gene.
Keywords: Dystrophin, DMD, Symptomatic carrier, Duchenne muscular dystrophy, Becker muscular dystrophy, X-chromosome inactivation
Background
The dystrophinopathies are a group of X-linked muscle diseases caused by mutations in the DMD gene. Clinical phenotypes vary from asymptomatic high CK levels and cramps to severe progressive skeletal and cardiac muscle disorders such as Duchenne (DMD) and Becker (BMD) muscular dystrophies, and X-linked dilated cardiomyopathy (XLCM) [1]. About one third of patients present mental retardation [2]. Most disease-responsible mutations are large intragenic rearrangements (exonic deletions and duplications) that account for 65 to 75% of cases, while the remaining cases are caused by single point mutations or small rearrangements [3,4]. In most patients, the clinical outcome can be predicted according to the reading-frame rule. The majority of DMD patients carry truncating mutations while BMD patients usually carry in-frame mutations allowing the expression of semi-functional dystrophins [1,5].
Female carriers of DMD mutations are usually asymptomatic due to the X-linked inheritance of the disease. However, symptomatic carriers can manifest a wide spectrum of clinical symptoms ranging from myalgia and cramps on exertion to severe disabling DMD-like muscle weakness [6-11]. Onset of symptoms fluctuates from early childhood to the fourth decade. The percentage of carriers with clinical abnormalities varies among the series [7,12]. Hoogerwaard et al. found that 5% present regular myalgia and cramps without muscle weakness, 17% show mild to moderately severe muscle weakness, and 8% present dilated cardiomyopathy, showing an average onset at 33 years [12].
Several disease-causing mechanisms have been implicated in DMD/BMD manifesting carriers. These include X-autosomal translocations disrupting the DMD gene [13], mutations on both DMD alleles [10,14] and co-occurrence of DMD mutations together with other genetic abnormalities such as X-chromosome monosomy [15-17], X-chromosome uniparental disomy [18] as well as male pseudohermaphroditism caused by a mutation in the androgen receptor gene [19]. However, the most frequently reported mechanism to provoke symptoms in DMD carriers is skewed X-inactivation, favouring the expression of the X chromosome with the DMD mutated allele [8,20-22]. Although some studies suggest the use of X-inactivation analysis for prognostic purposes, the results of different reports are controversial [21,23,24].
In this study we aimed to investigate the prognostic value of X-chromosome inactivation in a large series of dystrophinopathy affected females presenting with a wide spectrum of clinical phenotypes.
Materials and methods
Patients
We reviewed our database records of all dystrophinopathy patients. The database includes patients with a clinical history compatible with dystrophinopathy, X-linked family history of myopathy and/or a muscle biopsy showing abnormal dystrophin immunostaining. We identified 24 symptomatic carrier females referred from different centres around Spain. For the present study we included females with a confirmed DMD mutation or a muscle biopsy showing altered dystrophin staining, who manifest at least one of the following symptoms: myalgia, dilated cardiomyopathy, cognitive abnormalities or muscle weakness. We retrospectively collected data regarding clinical features, serum CK levels, cardiological studies and muscle biopsy analysis. Patients were grouped into different phenotype categories on the basis of their clinical course.
Two control groups were selected for X-chromosome inactivation (XCI) studies. The first control group consisted of 40 asymptomatic female carriers who presented 28 different DMD mutations: 20 exonic deletions, 5 exonic duplications, and 3 point mutations. The second control group included 41 confirmed non-carrier females from 28 unrelated DMD/BMD families with known DMD mutations. The study was approved by the Ethical Committe of Hospital de la Santa Creu i Sant Pau (HSCSP), Barcelona. All participants gave their written informed consent.
Muscle biopsy pathological analysis
A muscle biopsy was taken in 17 of the 24 symptomatic carriers. These were processed for routine histological and histochemical techniques, and for dystrophin immunohistochemistry as described elsewhere [25].
Genetic analysis
DNA extracted from peripheral blood underwent DMD mutational analysis using a combination of techniques. Intragenic deletions and duplications were analyzed using MLPA (P034 and P035 Salsa Kit, MRC-Holland). Point mutation detection was done by whole gene sequencing using published primers [26]. When a muscle biopsy was available, total mRNA was extracted and purified from approximately 30 mg of muscle using RNeasy Fibrous Tissue Mini Kit (Qiagen, Hilden, Germany). Subsequently, muscle mRNA was retrotranscribed to cDNA by RT-PCR using polythymine primers (Invitrogen, Carlsbad, NM). Complete DMD cDNA was amplified and sequenced in twenty overlapping fragments using published [27] and self-designed primers. Splicing mutations were analyzed using predictive software (Human Splice Finder and NNSPLICE). Nucleotide positions were determined according to the standard DMD reference sequence (GenBank accession number NM_004006.2). In eight families segregation analysis of the Xp21 locus was performed using microsatellite markers: 5’DYS-1, DXS1242, DXS1243, DXS206, DXS1238, DXS1237, DXS1236, DXS1235 and DXS1234. In four cases (subjects #1, #2, #18 and #19) a karyotype was performed before the DMD molecular analyses; all four cases showed a normal 46XX karyotype.
X-chromosome inactivation (XCI) analysis
Methylation of the HpaII restriction site near a polymorphic (CAG)n repeat in the AR gene (Androgen Receptor) correlates with XCI [28]. We used HpaII digestion followed by QF-PCR to determine the methylation status of parental X chromosomes. Active alleles are digested while the inactive alleles are not. The ratio of undigested parental alleles gives the pattern of X-chromosome inactivation.
For each sample, 500 ng of DNA were digested with HpaII restriction endonuclease (New England Biolabs, Ipswich, MA). Digested products together with non-digested DNA were used as templates for amplification of the AR polymorphic repeat using fluorescence labelled primers. PCR fragments were run in an ABI 3500xL Genetic Analyzer (Applied Biosymtems, Foster City, CA). AR alleles were sized and quantified using Genemapper software (Applied Biosymtems, Foster City, CA). To correct preferential allele amplification, the allele ratio in HpaII digested DNA was normalized using the ratio of non-digested DNA. Monoallelic patients for the AR repeat were reported as “non-informative”. Following previously published criteria, XCI ratios equal to or less than 80:20 were considered “random” patterns while ratios greater than 80:20 were considered “skewed” patterns [29].
XCI studies were performed in lymphocyte DNA from 24 symptomatic carriers, 40 asymptomatic carriers and 41 non-carrier females. To detect familial XCI skewing, analyses were also performed in at least one first-degree female relative (mother, daughter or sister) in 15 of the 23 families with a symptomatic carrier. In 9 symptomatic carriers XCI analysis was also performed on muscle DNA.
XIST minimal promoter mutation analysis
The expression of the non-coding gene XIST is involved in the mechanisms that determine the choice of inactive X chromosome [30]. Mutations c.-43C > A and c.-43C > G, located in the XIST promoter region have been associated to cause XCI skewing [31,32]. XIST minimal promoter was amplified and sequenced in order to detect XCI altering mutations using forward primer 5’ACCCATTGAAGTTGTGACTCCTGGT3’ and reverse primer 5’ACGCCATAAAGGGTGTTGGGGG3’.
Statistical analysis
Statistical comparison of proportion of skewed XCI between two groups was estimated using Fisher's Exact Test. Comparison of XCI ratios between two groups was estimated using paired t test while comparison among groups was done using one-way ANOVA, followed by the Bonferroni post hoc test. P values lower than 0.05 were considered as statistically significant.
Results
Clinical presentation
From a total of 344 female carriers of DMD mutations we identified 24 (7%) patients from 23 unrelated families who presented symptoms associated with dystrophinopathy. Two cases were identified within the same family (subject #13, mother, and subject #14, daughter). Clinical features are summarized in Table 1. Clinical presentations were highly heterogeneous and included: isolated dilated cardiomyopathy (n = 2, 8.3%), isolated cognitive abnormalities (n = 3, 12.5%), myalgia without muscle weakness (n = 4, 16.7%), and mild to severe muscle weakness (n = 15, 62.5%). Patients presenting muscle weakness were grouped into three different phenotype categories: mild BMD-like (n = 7, 29%), severe BMD-like (n = 5, 21%) and DMD-like (n = 2, 8.3%). Subject #3 presented with weakness at the age of 2 years and was too young to be assigned to either DMD-like or severe BMD-like. Muscle weakness was asymmetric in 6 cases (subjects #6, #9, #10, #13, #14 and #15). Age of onset varied from 2 to 74 years (median: 17.5; mean: 21.7). The most common presenting symptom was muscle weakness (n = 14, 58.3%) followed by myalgia, cramps and/or exercise intolerance (n = 8, 33.3%).
Table 1.
Clinical features of symptomatic carriers ofDMDmutations
| Patient | Age of onset | Phenotype | Presenting symptoms | Age at most recent exam | Clinical symptoms at most recent exam | CK levels (age) | Affected relative |
|---|---|---|---|---|---|---|---|
| 1 |
2 |
DMD-like |
Global developmental delay, weakness |
13 |
Severe mental retardation, severe weakness, wheelchair-bound at 13, normal echocardiogram |
22055 (13) |
no |
| 2 |
4 |
DMD-like |
Weakness, calf pseudohypertrophy |
14 |
Severe weakness, wheelchair-bound at 10, normal echocardiogram |
12000 (4) |
no |
| 3 |
2 |
D/BMD-like |
Frequent falls, seizures |
5 |
Mild weakness in lower limbs, normal echocardiogram |
n/p |
no |
| 4 |
12 |
Severe BMD-like |
Weakness |
29 |
Severe weakness and atrophy |
n/p |
DMD |
| 5 |
4 |
Severe BMD-like |
Exercise intolerance, weakness, poor school performance |
12 |
Incomplete Gower's sign, herculean appearance, calf pseudohypertrophy, mild mental retardation |
7678-333 (8–12) |
no |
| 6 |
7 |
Severe BMD-like |
Asymmetric weakness |
11 |
Asymmetric weakness, more severe in shoulder than in pelvic girdle, normal echocardiogram |
n/p |
no |
| 7 |
20 |
Severe BMD-like |
Weakness |
24 |
Severe weakness of upper and lower limbs, need a wheelchair for long walks, normal echocardiogram |
1700 (24) |
no |
| 8 |
4 |
Severe BMD-like |
Frequent falls, weakness |
26 |
Severe weakness, walks with mayor difficulties, calf pseudohypertrophy, normal echocardiogram |
5331 (22) |
no |
| 9 |
28 |
Mild BMD-like |
Asymmetric weakness |
32 |
Moderate unilateral weakness of right limbs, normal echocardiogram |
1500 (32) |
DMD |
| 10 |
41 |
Mild BMD-like |
Weakness, calf pseudohypertrophy |
42 |
Mild weakness, asymmetric in shoulder, asymmetric atrophy of posterior leg compartment |
6654 (42) |
DMD |
| 11 |
18 |
Mild BMD-like |
Myalgia/cramping, calf pseuhypertrophy |
49 |
Moderate muscle weakness, normal echocardiogram |
1527 (49) |
DMD |
| 12 |
30 |
Mild BMD-like |
Myalgia, weakness |
38 |
Moderate muscle weakness, normal echocardiogram |
1288 (30) |
DMD |
| 13 |
31 |
Mild BMD-like |
Finding elevated CK prompted a neurological examination revealing mild weakness |
51 |
Moderate asymmetric weakness |
2857 (31) |
DMD |
| 14 |
13 |
Mild BMD-like |
Detecting weakness in her mother prompted neurological examination finding mild weakness |
33 |
Moderate asymmetric weakness, calf pseuhypertrophy |
10530-6950 (13–33) |
DMD |
| 15 |
39 |
Mild BMD-like |
Frequent falls, myalgia/cramping, weakness |
51 |
Moderate asymmetric weakness, more affected in lower limbs, mild non-specific echocardiographic changes and bundle branch block |
1770-590 (43–51) |
DMD |
| 16 |
66 |
DCM |
Dilated cardiomyopathy with severe ventricular dysfunction |
69 |
Dilated cardiomyopathy with no muscle weakness |
n/p |
DMD |
| 17 |
74 |
DCM |
Dilated cardiomyopathy |
77 |
Dilated cardiomyopathy with no muscle weakness |
n/p |
DMD |
| 18 |
7 |
Behavioural issues |
Abnormal behaviour, elevated CK levels |
12 |
Behavioural abnormalities without mental retardation, no muscle weakness, normal echocardiogram |
3000 (7) |
BMD |
| 19 |
4 |
MR |
Delayed speech development, abnormal behaviour, elevated CK levels |
8 |
Mild mental retardation, no muscle weakness |
1137 (4) |
no |
| 20 |
4 |
MR |
Learning difficulties, elevated CK levels |
18 |
Mild mental retardation, no muscle weakness |
1193 (14) |
no |
| 21 |
31 |
Myalgia |
Precordial pain, myalgia |
36 |
Regular myalgia, no muscle weakness, normal echocardiogram |
897 (36) |
DMD |
| 22 |
33 |
Myalgia |
Myalgia |
34 |
Regular myalgia, no muscle weakness |
450 (34) |
DMD |
| 23 |
17 |
Myalgia |
Exercise intolerance |
19 |
Regular myalgia, no muscle weakness |
n/p |
no |
| 24 | 30 | Myalgia | Myalgia | 32 | Regular myalgia, no muscle weakness | n/p | no |
Patient #3, D/BMD-like, was too young to be assigned to either DMD-like or severe BMD-like phenotypes. DCM: dilated cardiomyopathy, MR: mental retardation.
Cognitive abnormalities were found, in isolation or together with muscle weakness, in 5 cases (20.8%). These abnormalities ranged from behavioural issues to severe global developmental delay. Subject #1, who presented a severe DMD-like phenotype, showed severe cognitive impairment with comprehensive and expressive language almost absent. Subjects #5, #19 and #20 presented with learning difficulties or poor academic performance showing mild mental retardation in a WISC-IV test, while subject #18 presented poor social and communication skills but no mental retardation. In subjects #18, #19 and #20, cognitive abnormalities were the only clinical symptoms. In these subjects, other aetiologies of mental retardation such as metabolic diseases, brain malformations, chromosomal disorders or fragile X syndrome were considered. These were ruled out by hormone and metabolic profiling in blood and urine (subjects #18, #19 and #20), neuroimaging studies (subjects #19 and #20), karyotype analysis (subjects #18 and #19) and FMR1 molecular analysis (subject #19). Subject #18 presented a BMD affected brother while in subjects #19 and #20, in absence of previous family history of neuromuscular disease, elevation of CK levels or a muscle biopsy showing abnormal dystrophin expression prompted DMD molecular studies. We could not exclude that the behavioural abnormalities of subject #18 were aetiologically independent of the dystrophinopathy. However, her BMD affected brother presented mild muscle symptoms and mental retardation with autistic behaviour indicating that the behavioural issues could be related to the dystrophinopathy.
Echocardiographic studies were performed in 14 cases and abnormalities were detected in three (21.4%). Severe cardiac dysfunction caused by dilated cardiomyopathy (DCM) was found in two cases (subjects #16 and #17) that did not present accompanying muscle symptoms. Onset of symptoms in these two patients was 66 and 74 years. Subject #15 showed mild non-specific echocardiographic abnormalities and a bundle branch block on EKG but no signs of cardiac failure.
Muscle biopsy pathological findings
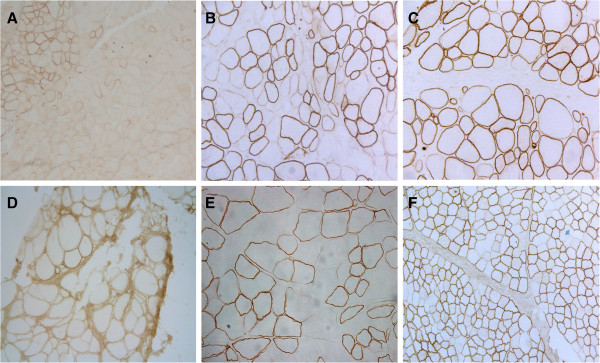
Muscle biopsy was performed in 17 cases. Results are summarized in Table 2 and Figure 1. Abnormal pathological features were found in all analyzed cases, and included variation in fibre size, increased numbers of internal nuclei, muscle fibre necrosis and regeneration, and variable degree of endomysial fibro-fatty tissue proliferation. Non-specific myopathic changes were detected in four patients, whereas mild to severe dystrophic changes were found in the remaining thirteen. Immunohistochemical analysis revealed a mosaic pattern of dystrophin-positive and reduced or absent dystrophin fibres in 9 cases (53%). In 4 cases only isolated dystrophin-negative fibres were observed. Normal dystrophin staining was found in a single patient (#18) who presented behavioural issues as the only symptom. Generalized absence of dystrophin expression was observed in two patients (subjects #1 and #3), both of whom suffered from muscle weakness from early childhood.
Table 2.
Summary of genetic findings and muscle biopsy features in symptomatic carriers ofDMDmutations
| Patient | Phenotype | DMDmutation | Muscle biopsy features | Dystrophin immunostaining | Blood DNA XCI | Muscle DNA XCI |
Most inactive X-chr. |
Origin ofDMDmutation | Familial skewed XCI |
|---|---|---|---|---|---|---|---|---|---|
| 1 |
DMD-like |
Deletion exons 1–44 (c.(?_-244)_6438 + ?del, frameshift) |
Severe dystrophic pattern |
Generalized absence |
93:7 |
n/p |
maternal |
paternal |
yes |
| 2 |
DMD-like |
Subexonic deletion/insertion exon 17 (c.2095delinsTC, frameshift) |
End-stage muscular dystrophy |
n/p |
100:0 |
51:49 |
paternal |
n/p |
no |
| 3 |
D/BMD-like |
Stop exon 8 (c.724C > T, p.Gln242X) |
Severe dystrophic pattern |
Generalized absence |
100:0 |
61:39 |
paternal |
n/p |
yes |
| 4 |
Severe BMD-like |
Splice site exon 27 (c.3786 + 1G > A, predicted frameshift) |
Moderate dystrophic pattern |
Mosaic pattern |
94:6 |
81:19 |
paternal |
maternal |
yes |
| 5 |
Severe BMD-like |
Deletion exon 68 (c.9808-?_9974 + ?del, frameshift) |
Moderate dystrophic pattern |
Mosaic pattern |
69:31 |
n/p |
maternal |
n/p |
yes |
| 6 |
Severe BMD-like |
Stop exon 41 (c.5893C > T, p.Gln1965X) |
Severe dystrophic pattern |
Reduction/absence in isolated fibres |
74:26 |
52:48 |
n/i |
n/p |
yes |
| 7 |
Severe BMD-like |
Deletion exons 5–7 (c.265-?_649 + ?del, frameshift) |
Severe dystrophic pattern |
Mosaic pattern |
81:19 |
87:12 |
n/p |
n/p |
n/p |
| 8 |
Severe BMD-like |
Deletion exon 44 (c.6291-?_6438 + ?del, frameshift) |
Moderate dystrophic |
Mosaic pattern with predominance of negative fibres |
100:0 |
n/p |
n/p |
n/p |
n/p |
| 9 |
Mild BMD-like |
Deletion exons 43–45 (c.6118-?_6614 + ?del, frameshift) |
Mild dystrophic pattern |
Mosaic pattern |
n/i |
n/p |
n/p |
n/p |
n/p |
| 10 |
Mild BMD-like |
Deletion exons 45–50 (c.6439-?_7309 + ?del, frameshift) |
Moderate dystrophic pattern. |
Mosaic pattern |
71:29 |
n/p |
n/i |
maternal |
no |
| 11 |
Mild BMD-like |
Deletion exons 53–54 (c.7661-?_8027 + ?del, frameshift) |
Mild dystrophic pattern |
Reduction/absence in isolated fibres |
81:19 |
n/p |
n/i |
maternal |
no |
| 12 |
Mild BMD-like |
Duplication exons 50–55 (c.7201-?_8217 + ?dup, predicted in-frame) |
n/p |
n/p |
n/i |
n/p |
n/p |
maternal |
n/p |
| 13 |
Mild BMD-like |
Splice site exon 48 (c.6913-1G > A, frameshift) |
Mild dystrophic pattern |
Mosaic pattern |
81:19 |
53:47 |
maternal |
maternal |
yes |
| 14 |
Mild BMD-like |
Splice site exon 48 (c.6913-1G > A, frameshift) |
n/p |
n/p |
72:28 |
n/p |
paternal |
maternal |
yes |
| 15 |
Mild BMD-like |
Deletion exons 48–50 (c.6913-?_7309 + ?del, frameshift) |
Mild dystrophic pattern |
Mosaic pattern |
52:48 |
40:60 |
n/p |
n/p |
n/p |
| 16 |
DCM |
Deletion exon 44 (c.6291-?_6438 + ?del, frameshift) |
n/p |
n/p |
99:1 |
n/p |
n/i |
n/p |
Yes |
| 17 |
DCM |
Deletion exons 46–52 (c.6615-?_7660 + ?del, frameshift) |
n/p |
n/p |
63:37 |
n/p |
n/p |
n/p |
n/p |
| 18 |
Behavioural issues |
Duplication exons 13–27 (c.1483-?_3786 + ?dup, predicted in-frame) |
Myopathic changes |
Normal |
99:1 |
97:3 |
paternal |
maternal |
Yes |
| 19 |
MR |
Subexonic deletion exon 46 (c.6638delT, frameshift) |
Myopathic changes |
Absence in isolated fibres |
88:12 |
69:31 |
paternal |
maternal |
yes |
| 20 |
MR |
Deletion exons 46–55 (c.6615-?_8217 + ?del, frameshift) |
n/p |
n/p |
100:0 |
n/p |
paternal |
maternal |
n/i |
| 21 |
Myalgia |
Duplication exons 38–43 (c.5326-?_6290 + ?dup, predicted frameshift) |
n/p |
n/p |
74:26 |
n/p |
paternal |
maternal |
yes |
| 22 |
Myalgia |
Deletion exons 10–43 (c.961-?_6290 + ?del, frameshift) |
n/p |
n/p |
51:49 |
n/p |
n/p |
n/p |
n/p |
| 23 |
Myalgia |
Deletion exon 7 (c.531-?_649 + ?del, frameshift) |
Myopathic changes |
Reduction/absence in isolated fibres |
78:22 |
n/p |
n/p |
n/p |
n/p |
| 24 | Myalgia | Deletion exons 3–13 (c.94-?_1602 + ?del, in-frame) | Myopathic changes | Mosaic pattern | 50:50 | n/p | n/p | n/p | n/p |
X-chromosome inactivation (XCI): skewed patterns >80:20 are in bold. n/p: not performed, n/i = non-informative AR alleles.
Figure 1.
Dystrophin immunostaining in muscle biopsy sections from representative symptomatic carriers.A) D/BMD-like subject #3, B) severe BMD-like subject #4, C) severe BMD-like subject #6, D) severe BMD-like subject #8, E) mild BMD-like subject #13, F) subject #19 presenting mild mental retardation but no muscle weakness. No association was found between dystrophin abnormalities and clinical phenotype.
Related probands and DMD mutation spectrum
Among the 24 symptomatic carriers 13 had a previous family history of dystrophinopathy affected males (54.2%) while the 11 remaining cases were isolated symptomatic carriers (45.8%). The vast majority of cases with previous family history had DMD affected relatives (12/13, 92.3%). Only subject #18, who presented behavioural issues as the only clinical symptom, had a BMD affected brother. Most cases with no previous family history presented mutations predominantly associated to the DMD phenotype according to the Leiden Open Variation Database (LOVD, http://www.dmd.nl).
Twenty-two different DMD mutations were identified, all in heterozygous state. These are listed in Table 2 and included: 13 exonic deletions (59%), 3 exonic duplications (14%) and 6 point mutations (27%). Point mutations consisted of two nonsense mutations, two small deletions/insertions and two splicing mutations. Subjects #2, #4, #18 and #19 presented novel mutations according to LOVD. Most patients carried predicted frameshift or nonsense mutations (87.5%, 21/24). In-frame mutations included: duplication 13–27 (subject #18), not previously described, duplication 50–55 (subject #12) and, deletion 3–13 (subject #24). The latter two mutations were both associated mainly with DMD phenotype according to LOVD.
Splice site mutations were analyzed at muscle cDNA level or by predictive software in order to identify pre-mRNA splicing defects. The c.6913-1G > A mutation, identified in patients #13 and #14, a mother and daughter, destroyed the putative acceptor splice site of exon 48. At the muscle cDNA level the mutation was observed to provoke a single base deletion due to the creation of a new acceptor site at position c.6913. The c.3786 + 1G > A mutation identified in subject #4 destroyed the putative donor splice site of exon 27. Although this mutation was not analyzed at the muscle cDNA level, the presence of a cryptic donor splice site located at position c.3786 + 53 possibly provoked the retention of an intronic fragment of 52 bp.
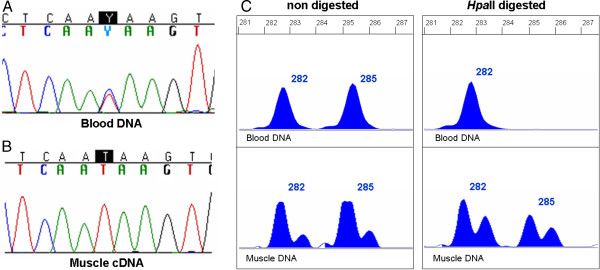
In four cases (#3, #6, #13 and #19) mutation analysis was performed both at genomic and muscle cDNA level. In subjects #6, #13 and #19, both wild-type and mutated transcripts were detected in muscle cDNA. In subject #3, only the mutated transcript carrying a nonsense mutation was detected, while at genomic level, the mutation was in heterozygous state (Figure 2).
Figure 2.
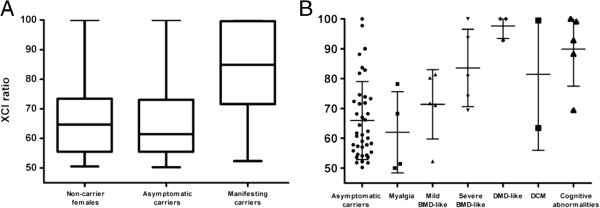
X-chromosome inactivation patterns in blood. A) Distribution of XCI patterns among non-carrier females (n = 41), asymptomatic carriers (n = 40) and symptomatic carriers excluding patients presenting only myalgia (n = 18). For each group, the five parameters are the lowest observation, lower quartile, median, upper quartile, and highest observation. B) Distribution of XCI patterns among symptomatic carriers according to clinical phenotype. Individual XCI data, mean and standard deviation are shown. The extent of XCI skewing in blood tended to increase with increasing severity of muscle symptoms.
X-chromosome inactivation studies (XCI)
XCI analysis was performed in lymphocyte DNA of the 24 symptomatic carriers, 40 non-symptomatic carriers and 41 non-carrier females. Results are summarized in Table 2 and Figure 3. XCI studies were informative in 22 symptomatic carriers. Among these, the mean XCI ratio was 80:20 ± 16.8 and a skewed XCI pattern was found in 12 cases (54.6%). If we do not consider patients presenting myalgia as a true symptomatic carriers as previously published criteria, [10,12], skewed XCI among symptomatic carriers (n = 18) increased to 66.7% with a mean XCI ratio of 84:16 ± 14.9. Asymptomatic carriers and non-carrier females presented similar distribution of XCI ratios: among asymptomatic carriers, 17.5% presented skewed XCI with a mean ratio of 66:34 ± 13.1, while among non-carrier females skewed XCI was found in 12.2% with a mean ratio of 66:34 ± 11.9. Differences in the XCI ratio and proportion of skewed cases between symptomatic carriers and asymptomatic carriers or non-carrier females were statistically significant (p values <0.001 by paired t-test and Fisher's Exact Test).
Figure 3.
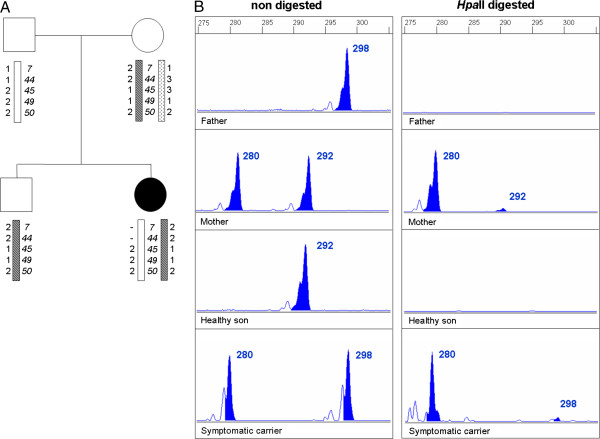
Inheritance of DMD mutation, parental origin of most inactivated X-chromosome and familial skewed XCI in family of subject #1.A) Family pedigree. Haplotype analysis of Xp21 locus indicates that the mutation is located at the paternal X-chromosome. B) Genemapper traces of non-digested and HpaII digested AR alleles in blood. Methylated status of the AR revealed that both mother and daughter present highly skewed XCI. Maternal X-chromosome is preferentially inactivated in affected daughter indicating that the paternal X-chromosome carrying the DMD mutated allele is active.
The extent of XCI skewing in blood tended to increase in line with the severity of symptoms among phenotype groups presenting skeletal muscle involvement (Figure 3B). While all patients with myalgia showed random XCI (mean ratio: 62:38), skewed XCI was present in 50% of cases presenting a mild BMD-like phenotype (mean ratio: 71:29), in 60% of patients with severe BMD-like phenotype (mean ratio: 84:16) and in all DMD-like cases (including subject #3, mean ratio: 98:2). However, these differences only reached statistical significance between DMD-like and myalgia groups. Skewed XCI was present in 80% of cases showing cognitive abnormalities (mean ratio 90:10). One of the two patients who presented a DCM showed a random pattern and the other showed a highly skewed pattern (mean ratio 81:19).
XCI analysis was also performed in muscle DNA in nine symptomatic carriers (Table 2). Similar XCI ratios between blood and muscle were found only in two cases, while the remaining seven exhibited significant differences. From the seven cases showing skewed XCI in blood only three presented a skewed pattern in muscle (43%). In all cases the most active X-chromosome was the same in blood and muscle.
Parental origin of most inactivated X-chromosome and inheritance of DMD mutation
The parental origin of X chromosomes was determined by comparing AR alleles in the patient with those of her parents, while inheritance of DMD mutation was established either by carrier status of patient’s mother and/or by segregation analysis of Xp21 locus using polymorphic microsatellites (Table 2 and Figure 4). In most cases, most inactivated X chromosome was from paternal origin (8/11, 73%) while DMD mutation was inherited from the mother (10/11, 91%). In 7 of 8 cases, mutant DMD allele segregated with the most unmethylated AR allele (active X-chromosome). However, two of them did not reach a skewed XCI pattern (>80:20) in blood.
Figure 4.
Preferential expression of the DMD mutated allele correlates with blood XCI but not with muscle XCI in subject #3.A) Automated sequence analysis of DMD gene exon 8 showing the nonsense mutation c.724C > T/p.Gln242X in heterozygous state. B) At muscle cDNA level, only the mutated transcript was detected. C) Genemapper traces of non-digested and HpaII digested AR alleles in blood and muscle. Blood DNA presented a skewed XCI pattern of 100:0 while muscle DNA exhibited a random pattern of 61:39.
Familial skewed X-chromosome inactivation and analysis of the XIST promoter
XCI studies in at least one first degree female relative (mother, daughter or sister) were performed in 15 of the 23 different families with symptomatic carriers (Figure 4). Evidence of familial skewed XCI was found in eleven of fourteen families with informative AR alleles (78.6%). Surprisingly, mothers of three symptomatic carriers showing random XCI in blood (subjects #5, #6 and #21) exhibited skewed XCI. The promoter region of XIST non-coding gene was screened in all symptomatic carriers but no mutations were detected.
Discussion
We report the largest series of DMD symptomatic carriers, consisting of 24 cases covering a wide spectrum of clinical phenotypes. The main mechanism found to provoke symptoms in these patients was skewed XCI favouring expression of the DMD-mutated allele. We provide data consistent with the hypothesis that the extent of XCI skewing is related to phenotype severity and that skewed XCI patterns are in most cases familial inherited. We also describe what is, to our knowledge, the first report of DMD female carriers presenting cognitive impairment but no muscle weakness, broadening the clinical phenotypes associated with dystrophinopathy.
In line with previous reports [33], isolated symptomatic carriers were relatively frequent, suggesting that dystrophinopathy should always be considered in females suffering from a myopathy of unknown cause, even in the absence of an X-linked family history. In these cases dystrophin immunostaining on muscle sections remains the best diagnostic tool even though an exon copy number screening technique (such as MLPA) can be considered prior to muscle biopsy. Muscle biopsies showed variable pathological features in all studied cases. Furthermore, dystrophin abnormalities were found in all but one case. In accordance with previous publications [6,20,34], we did not find a correlation between dystrophin expression and clinical phenotype, although the most affected subjects showed generalized dystrophin absence (Figure 1).
Our findings confirm that symptomatic carriers present a wide clinical variability ranging from nearly asymptomatic forms to disabling DMD-like phenotypes. The progression of muscle weakness among symptomatic carriers correlates with onset of symptoms. Onset during the first decade was associated in all cases with rapid progression leading to severe phenotypes (DMD-like or severe BMD-like), while later onset, from the third decade onwards, was associated with mild progression. However, patients presenting onset of symptoms during the second decade exhibited variable severity. Subject #7 presented onset of weakness at 20 years with rapid progression leading to wheelchair support five years later, whereas subject #11 who manifested the first symptoms at 18 years is still able to walk at the age of 49 years. In accordance with previous observations [10,12,33], muscle weakness was asymmetric in 40% of our patients. Asymmetry may be caused by somatic mosaicism [35] or reflect different XCI patterns between different muscle groups and tissues. In keeping with the later hypothesis, most of our cases presenting muscle weakness and random XCI in blood showed asymmetric distribution of weakness (4/5). Five patients presented symptoms without skeletal muscle involvement: two subjects presented isolated dilated cardiomyopathy while three showed cognitive abnormalities.
Cognitive abnormalities, observed in five patients in isolation or together with muscle weakness, ranged from behavioural issues to severe global developmental delay. Risk of cognitive deficits among DMD patients is thought to be the result of the cumulative loss of dystrophin isoforms in the central nervous system during development [36]. According to this hypothesis, mutations in three subjects (#5, #19 and #20) were predicted to destroy most dystrophin isoforms. Nevertheless, in the other two subjects (subjects #1 and #18) only long isoforms were predicted to be affected. It is of particular note that four of the five cases presenting cognitive deficits showed highly skewed XCI patterns in blood independently of muscle symptoms. This could indicate that the central nervous system is more affected than muscle by reduced dystrophin expression due to biased XCI.
Skewed XCI favouring expression of the DMD-mutated X chromosome has been proposed as the main mechanism accounting for symptoms in symptomatic carriers without chromosomal rearrangements. However, results concerning the use of XCI as a prognostic marker are controversial. While some studies found that the great majority of symptomatic cases exhibited skewed profiles [20-22] others found that XCI is not a reliable measure to predict whether carrier female will develop symptoms [6,10,24]. Most women in the normal population present a random XCI pattern in peripheral blood. However, a skewed pattern is present in 8.8% of females, increasing up to 14.2% in adult women [29]. We found a distribution of XCI ratios in blood among control groups (asymptomatic carriers and non-carrier females) similar to those described in the normal adult population [29]. Regarding our group of symptomatic carriers, excluding those manifesting only myalgia, the number of skewed cases was 49.2% higher (mean XCI ratio 18% higher) than in asymptomatic carriers. In cases presenting skeletal muscle involvement, the extent of XCI skewing in blood tended to increase in line with the severity of symptoms (Figure 3B). However, assessing XCI through the AR methylation assay seems to have a poor prognostic value, since some subjects showing similar XCI patterns exhibited different phenotype severity. Furthermore, four subjects (#16, #18, #19 and #20) with highly skewed patterns presented no skeletal muscle involvement. When XCI analysis was performed on muscle DNA we observed no correlation between the extent of XCI skewing and clinical severity or proportion of dystrophin-negative fibres. As previously described [6,23], in most cases XCI profile in muscle differed significantly from that found in blood. This could reflect a biochemical and genetic normalization process in skeletal muscle [25,37,38], or tissue-specific differences in XCI or in the methylation status of the AR gene. In keeping with the latter hypothesis, in subject #3 the preferential expression of the DMD mutated allele correlated with the XCI ratio in blood but not with that in muscle (Figure 2). This case suggests that the methylation status of the AR gene in muscle may not reflect in all cases that of the DMD gene.
Soltanzadeh et al. [10] found that DMD deletions or duplications were significantly more associated with skewed XCI compared with point mutations suggesting a correlation between XCI and mutation class. Analysing our data, we found that skewed XCI was more frequent in patients with point mutations (5/7, 71.4%) compared with those carrying deletions or duplications (7/15, 46.7%). However, these differences do not reach statistical significance in a Fisher’s Exact Test suggesting that the DMD mutation class is not involved in the development of X inactivation skewness.
We found evidence of familial XCI skewing in 78.6% of analyzed families suggesting that in many cases this may be under genetic control. X inactivation is a complex process restricted to early embryogenesis in which an X chromosome is silenced at random depending on the stable expression of the cis-acting XIST gene [30]. Skewed X inactivation may be caused primarily by preferential silencing of one specific X chromosome due to genetic factors, or by chance due to the very limited number of precursor cells present at the moment of inactivation [39]. In X chromosome defects, skewing can also be secondary during development due to post-inactivation selection. Selection tends to preserve gene dosage: in balanced X:autosome translocations the normal X is generally inactivated while in X deletions the inactivated chromosome is the deleted X [39]. Mutations in the XIST gene promoter have been reported to cause primary non-random X-chromosome inactivation [31,32]. We analyzed the XIST promoter in all patients but no changes were detected. Other loci on the X chromosome have been associated with familial skewing of X inactivation, suggesting that factors other than XIST may control the primary choice of X chromosome or be secondarily involved in cell survival [40,41].
We consider that it could be clinically important to identify the genetic factors that alter random X inactivation in heterozygous females expressing X-linked recessive traits such as dystrophinopathy.
The reduced sample size and lack of systematic case collection limit the generalizability of the results.
Conclusion
Our results demonstrate that the extent of XCI skewing is related to phenotype severity in symptomatic female carriers of dystrophinopathy. The methylation status of the AR gene in muscle may not reflect in all cases the methylation status of the DMD gene, conferring a poor prognostic value to the AR methylation assay for the assessment of XCI. Furthermore, skewed XCI is in many cases familial inherited.
Abbreviations
DMD: Duchenne muscular dystrophy; BMD: Becker muscular dystrophy; XLCM: X-linked cardiomyopathy; XCI: X-chromosome inactivation; AR: Androgen receptor; MLPA: Multiple ligation-dependent probe amplification; DCM: Dilated cardiomyopathy; MR: Mental retardation; CK: Creatine kinase.
Competing interests
All the authors stated that they have no interests which might be perceived as posing a conflict or bias.
Authors’ contribution
JJM and PG designed the research, analyzed data and wrote the paper. JJM, MJR, EV and LGQ performed DMD molecular analysis in all the samples. AN, CP, MM, PSA, LGM, FM, MRQ, MR, JDM, JP, JC and MO performed the clinical characterization of the patients. CJM, ER, CJ, FM, AHL, EG and MO performed the pathological and immunohistochemical analyses of muscle biopsies. MB gave intellectual support. MO collaborated in the data analysis and in the writing of the paper. JJM and PG were primarily responsible for this work. All the authors read and approved the final manuscript.
Contributor Information
Jonàs Juan-Mateu, Email: jjuanm@santpau.cat.
Maria José Rodríguez, Email: mrodriguezf@santpau.cat.
Andrés Nascimento, Email: anascimento@hsjdbcn.org.
Cecilia Jiménez-Mallebrera, Email: cjimenezm@fsjd.org.
Lidia González-Quereda, Email: lgonzaleq@santpau.cat.
Eloy Rivas, Email: eloy.rivas.sspa@juntadeandalucia.es.
Carmen Paradas, Email: carmenparadas@yahoo.com.
Marcos Madruga, Email: mapolgra@yahoo.es.
Pedro Sánchez-Ayaso, Email: sanchezayaso@gmail.com.
Cristina Jou, Email: cjou@hsjdbcn.org.
Laura González-Mera, Email: 36747lgm@comb.cat.
Francina Munell, Email: francina.munell@gmail.com.
Manuel Roig-Quilis, Email: manroig@gmail.com.
Maria Rabasa, Email: mrabasa.hflr@salud.madrid.org.
Aurelio Hernández-Lain, Email: aurelioneu@yahoo.es.
Jorge Díaz-Manera, Email: JDiazM@santpau.cat.
Eduard Gallardo, Email: egallardo@santpau.cat.
Jordi Pascual, Email: JPascual@parcdesalutmar.cat.
Edgard Verdura, Email: everdura@santpau.cat.
Jaume Colomer, Email: colomer@hsjdbcn.org.
Montserrat Baiget, Email: mbaiget@santpau.cat.
Montse Olivé, Email: 25169mop@comb.cat.
Pia Gallano, Email: pgallano@santpau.cat.
Acknowledgments
The authors thank all patients and their family members for their participation in this study. We thank Carolyn Newey for her kind language assistance. This study was supported by grants from Fondo de Investigación Sanitaria (PI08/0347) and CIBER de Enfermedades Raras (U-705) initiatives of Instituto de Salud Carlos III.
References
- Muntoni F, Torelli S, Ferlini A. Dystrophin and mutations: one gene, several proteins, multiple phenotypes. Lancet Neurol. 2003 Dec;2(12):731–740. doi: 10.1016/S1474-4422(03)00585-4. [DOI] [PubMed] [Google Scholar]
- Cotton S, Voudouris NJ, Greenwood KM. Intelligence and Duchenne muscular dystrophy: full-scale, verbal, and performance intelligence quotients. Dev Med Child Neurol. 2001 Jul;43(7):497–501. doi: 10.1017/S0012162201000913. [DOI] [PubMed] [Google Scholar]
- Aartsma-Rus A, Van Deutekom JC, Fokkema IF, Van Ommen GJ, Den Dunnen JT. Entries in the Leiden Duchenne muscular dystrophy mutation database: an overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve. 2006 Aug;34(2):135–144. doi: 10.1002/mus.20586. [DOI] [PubMed] [Google Scholar]
- Tuffery-Giraud S, Beroud C, Leturcq F, Yaou RB, Hamroun D, Michel-Calemard L, Moizard MP, Bernard R, Cossee M, Boisseau P, Blayau M, Creveaux I, Guiochon-Mantel A, de Martinville B, Philippe C, Monnier N, Bieth E, Khau Van Kien P, Desmet FO, Humbertclaude V, Kaplan JC, Chelly J, Claustres M. Hum Mutat. 2009. pp. 934–945. [DOI] [PubMed]
- Monaco AP, Bertelson CJ, Liechti-Gallati S, Moser H, Kunkel LM. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988 Jan;2(1):90–95. doi: 10.1016/0888-7543(88)90113-9. [DOI] [PubMed] [Google Scholar]
- Bushby KM, Goodship JA, Nicholson LV, Johnson MA, Haggerty ID, Gardner-Medwin D. Variability in clinical, genetic and protein abnormalities in manifesting carriers of Duchenne and Becker muscular dystrophy. Neuromuscul Disord. 1993 Jan;3(1):57–64. doi: 10.1016/0960-8966(93)90042-I. [DOI] [PubMed] [Google Scholar]
- Norman A, Harper P. A survey of manifesting carriers of Duchenne and Becker muscular dystrophy in Wales. Clin Genet. 1989 Jul;36(1):31–37. doi: 10.1111/j.1399-0004.1989.tb03363.x. [DOI] [PubMed] [Google Scholar]
- Seemann N, Selby K, McAdam L, Biggar D, Kolski H, Goobie S, Yoon G, Campbell C. Canadian Pediatric Neuromuscular Group. Symptomatic dystrophinopathies in female children. . Neuromuscul Disord. 2011 Mar;21(3):172–177. doi: 10.1016/j.nmd.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Rajakulendran S, Kuntzer T, Dunand M, Yau SC, Ashton EJ, Storey H, McCauley J, Abbs S, Thonney F, Leturcq F, Lobrinus JA, Yousry T, Farmer S, Holton JL, Hanna MG. Marked hemiatrophy in carriers of Duchenne muscular dystrophy. Arch Neurol. 2010 Apr;67(4):497–500. doi: 10.1001/archneurol.2010.58. [DOI] [PubMed] [Google Scholar]
- Soltanzadeh P, Friez MJ, Dunn D, von Niederhausern A, Gurvich OL, Swoboda KJ, Sampson JB, Pestronk A, Connolly AM, Florence JM, Finkel RS, Bonnemann CG, Medne L, Mendell JR, Mathews KD, Wong BL, Sussman MD, Zonana J, Kovak K, Gospe SM Jr, Gappmaier E, Taylor LE, Howard MT, Weiss RB, Flanigan KM. Clinical and genetic characterization of manifesting carriers of DMD mutations. Neuromuscul Disord. 2010 Aug;20(8):499–504. doi: 10.1016/j.nmd.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka M. Clinically manifesting carriers in Duchenne muscular dystrophy. Clin Genet. 1981 Jul;20(1):6–12. doi: 10.1111/j.1399-0004.1981.tb01799.x. [DOI] [PubMed] [Google Scholar]
- Hoogerwaard EM, Bakker E, Ippel PF, Oosterwijk JC, Majoor-Krakauer DF, Leschot NJ, Van Essen AJ, Brunner HG, van der Wouw PA, Wilde AA, de Visser M. Signs and symptoms of Duchenne muscular dystrophy and Becker muscular dystrophy among carriers in The Netherlands: a cohort study. Lancet. 1999 Jun 19;353(9170):2116–2119. doi: 10.1016/S0140-6736(98)10028-4. [DOI] [PubMed] [Google Scholar]
- Boyd Y, Buckle V, Holt S, Munro E, Hunter D, Craig I. Muscular dystrophy in girls with X;autosome translocations. J Med Genet. 1986 Dec;23(6):484–490. doi: 10.1136/jmg.23.6.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K, Minami N, Hayashi Y, Nishino I, Nonaka I, Tanabe Y, Takanashi J, Kohno Y. Homozygous female Becker muscular dystrophy. Am J Med Genet A. 2009 May;149A(5):1052–1055. doi: 10.1002/ajmg.a.32808. [DOI] [PubMed] [Google Scholar]
- Chelly J, Marlhens F, Le Marec B, Jeanpierre M, Lambert M, Hamard G, Dutrillaux B, Kaplan JC. De novo DNA microdeletion in a girl with Turner syndrome and Duchenne muscular dystrophy. Hum Genet. 1986 Oct;74(2):193–196. doi: 10.1007/BF00282093. [DOI] [PubMed] [Google Scholar]
- Satre V, Monnier N, Devillard F, Amblard F, Lunardi J. Prenatal diagnosis of DMD in a female foetus affected by Turner syndrome. Prenat Diagn. 2004 Nov;24(11):913–917. doi: 10.1002/pd.1031. [DOI] [PubMed] [Google Scholar]
- Baiget M, Tizzano E, Volpini V, del Rio E, Perez-Vidal T, Gallano P. DMD carrier detection in a female with mosaic Turner's syndrome. J Med Genet. 1991 Mar;28(3):209–210. doi: 10.1136/jmg.28.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan F, Janas J, Toth-Fejel S, Johnson DB, Wolford JK, Popovich BW. Uniparental disomy of the entire X chromosome in a female with Duchenne muscular dystrophy. Am J Hum Genet. 1997 Jan;60(1):160–165. [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Tran VK, Hoan NT, Zhang Z, Goji K, Yagi M, Takeshima Y, Saiki K, Nhan NT, Matsuo M. Co-occurrence of mutations in both dystrophin- and androgen-receptor genes is a novel cause of female Duchenne muscular dystrophy. Hum Genet. 2006 Jun;119(5):516–519. doi: 10.1007/s00439-006-0159-4. [DOI] [PubMed] [Google Scholar]
- Azofeifa J, Voit T, Hubner C, Cremer M. X-chromosome methylation in manifesting and healthy carriers of dystrophinopathies: concordance of activation ratios among first degree female relatives and skewed inactivation as cause of the affected phenotypes. Hum Genet. 1995 Aug;96(2):167–176. doi: 10.1007/BF00207374. [DOI] [PubMed] [Google Scholar]
- Yoshioka M, Yorifuji T, Mituyoshi I. Skewed X inactivation in manifesting carriers of Duchenne muscular dystrophy. Clin Genet. 1998 Feb;53(2):102–107. doi: 10.1111/j.1399-0004.1998.tb02655.x. [DOI] [PubMed] [Google Scholar]
- Pegoraro E, Schimke RN, Arahata K, Hayashi Y, Stern H, Marks H, Glasberg MR, Carroll JE, Taber JW, Wessel HB. Detection of new paternal dystrophin gene mutations in isolated cases of dystrophinopathy in females. Am J Hum Genet. 1994 Jun;54(6):989–1003. [PMC free article] [PubMed] [Google Scholar]
- Matthews PM, Benjamin D, Van Bakel I, Squier MV, Nicholson LV, Sewry C, Barnes PR, Hopkin J, Brown R, Hilton-Jones D. Muscle X-inactivation patterns and dystrophin expression in Duchenne muscular dystrophy carriers. Neuromuscul Disord. 1995 May;5(3):209–220. doi: 10.1016/0960-8966(94)00057-G. [DOI] [PubMed] [Google Scholar]
- Sumita DR, Vainzof M, Campiotto S, Cerqueira AM, Canovas M, Otto PA, Passos-Bueno MR, Zatz M. Absence of correlation between skewed X inactivation in blood and serum creatine-kinase levels in Duchenne/Becker female carriers. Am J Med Genet. 1998 Dec 4;80(4):356–361. doi: 10.1002/(SICI)1096-8628(19981204)80:4<356::AID-AJMG10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Juan-Mateu J, Paradas C, Olive M, Verdura E, Rivas E, Gonzalez-Quereda L, Rodriguez MJ, Baiget M, Gallano P. Isolated cardiomyopathy caused by a DMD nonsense mutation in somatic mosaicism: genetic normalization in skeletal muscle. Clin Genet. 2011. [DOI] [PubMed]
- Flanigan KM, von Niederhausern A, Dunn DM, Alder J, Mendell JR, Weiss RB. Rapid direct sequence analysis of the dystrophin gene. Am J Hum Genet. 2003 Apr;72(4):931–939. doi: 10.1086/374176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deburgrave N, Daoud F, Llense S, Barbot JC, Recan D, Peccate C, Burghes AH, Beroud C, Garcia L, Kaplan JC, Chelly J, Leturcq F. Protein- and mRNA-based phenotype-genotype correlations in DMD/BMD with point mutations and molecular basis for BMD with nonsense and frameshift mutations in the DMD gene. Hum Mutat. 2007 Feb;28(2):183–195. doi: 10.1002/humu.20422. [DOI] [PubMed] [Google Scholar]
- Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet. 1992 Dec;51(6):1229–1239. [PMC free article] [PubMed] [Google Scholar]
- Amos-Landgraf JM, Cottle A, Plenge RM, Friez M, Schwartz CE, Longshore J, Willard HF. X chromosome-inactivation patterns of 1,005 phenotypically unaffected females. Am J Hum Genet. 2006 Sep;79(3):493–499. doi: 10.1086/507565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny GD, Kay GF, Sheardown SA, Rastan S, Brockdorff N. Requirement for Xist in X chromosome inactivation. Nature. 1996 Jan 11;379(6561):131–137. doi: 10.1038/379131a0. [DOI] [PubMed] [Google Scholar]
- Plenge RM, Hendrich BD, Schwartz C, Arena JF, Naumova A, Sapienza C, Winter RM, Willard HF. A promoter mutation in the XIST gene in two unrelated families with skewed X-chromosome inactivation. Nat Genet. 1997 Nov;17(3):353–356. doi: 10.1038/ng1197-353. [DOI] [PubMed] [Google Scholar]
- Tomkins DJ, McDonald HL, Farrell SA, Brown CJ. Lack of expression of XIST from a small ring X chromosome containing the XIST locus in a girl with short stature, facial dysmorphism and developmental delay. Eur J Hum Genet. 2002 Jan;10(1):44–51. doi: 10.1038/sj.ejhg.5200757. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Arahata K, Minetti C, Bonilla E, Rowland LP. Dystrophinopathy in isolated cases of myopathy in females. Neurology. 1992 May;42(5):967–975. doi: 10.1212/WNL.42.5.967. [DOI] [PubMed] [Google Scholar]
- Hoogerwaard EM, Ginjaar IB, Bakker E, de Visser M. Dystrophin analysis in carriers of Duchenne and Becker muscular dystrophy. Neurology. 2005 Dec 27;65(12):1984–1986. doi: 10.1212/01.wnl.0000188909.89849.59. [DOI] [PubMed] [Google Scholar]
- van Essen AJ, Mulder IM, van der Vlies P, van der Hout AH, Buys CH, Hofstra RM, den Dunnen JT. Detection of point mutation in dystrophin gene reveals somatic and germline mosaicism in the mother of a patient with Duchenne muscular dystrophy. Am J Med Genet A. 2003 Apr 30;118A(3):296–298. doi: 10.1002/ajmg.a.10056. [DOI] [PubMed] [Google Scholar]
- Taylor PJ, Betts GA, Maroulis S, Gilissen C, Pedersen RL, Mowat DR, Johnston HM, Buckley MF. Dystrophin gene mutation location and the risk of cognitive impairment in Duchenne muscular dystrophy. PLoS One. 2010 Jan 20;5(1):e8803. doi: 10.1371/journal.pone.0008803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegoraro E, Schimke RN, Garcia C, Stern H, Cadaldini M, Angelini C, Barbosa E, Carroll J, Marks WA, Neville HE, Marks H, Appleton S, Toriello H, Wessel HB, Donnelly J, Bernes SM, Taber JW, Weiss L, Hoffman EP. Genetic and biochemical normalization in female carriers of Duchenne muscular dystrophy: evidence for failure of dystrophin production in dystrophin-competent myonuclei. Neurology. 1995 Apr;45(4):677–690. doi: 10.1212/WNL.45.4.677. [DOI] [PubMed] [Google Scholar]
- Kesari A, Neel R, Wagoner L, Harmon B, Spurney C, Hoffman EP. Somatic mosaicism for Duchenne dystrophy: evidence for genetic normalization mitigating muscle symptoms. Am J Med Genet A. 2009 Jul;149A(7):1499–1503. doi: 10.1002/ajmg.a.32891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CJ, Robinson WP. The causes and consequences of random and non-random X chromosome inactivation in humans. Clin Genet. 2000 Nov;58(5):353–363. doi: 10.1034/j.1399-0004.2000.580504.x. [DOI] [PubMed] [Google Scholar]
- Pegoraro E, Whitaker J, Mowery-Rushton P, Surti U, Lanasa M, Hoffman EP. Familial skewed X inactivation: a molecular trait associated with high spontaneous-abortion rate maps to Xq28. Am J Hum Genet. 1997 Jul;61(1):160–170. doi: 10.1086/513901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cau M, Addis M, Congiu R, Meloni C, Cao A, Santaniello S, Loi M, Emma F, Zuffardi O, Ciccone R, Sole G, Melis MA. A locus for familial skewed X chromosome inactivation maps to chromosome Xq25 in a family with a female manifesting Lowe syndrome. J Hum Genet. 2006;51(11):1030–1036. doi: 10.1007/s10038-006-0049-6. [DOI] [PubMed] [Google Scholar]