Abstract
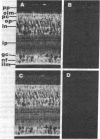
The developmental profile and cellular localization of carbonic anhydrase C (carbonate dehydratase; carbonate hydro-lyase, EC 4.2.1.1) in the neural retina of chicken embryos and adults were investigated by immunochemical and immunohistochemical methods. Carbonic anhydrase C is present in the retina by the 3rd day of embryonic development. In the undifferentiated retina, it is detectable in virtually all the cells; however, as cell specialization progresses, its level declines rapidly in the emerging neurons and increases in Müller glia cells. An exception is certain amacrine neurons that contain carbonic anhydrase C to about the 16th day of development. In the adult retina, the enzyme is confined exclusively to Müller cells (the only gliocytes in the retina). Their identification was confirmed by immunostaining for glutamine synthase, an established Müller cell "marker." The presence in the mature retina of both these enzymes in Müller cells indicates that retinal gliocytes combine functional features that, in the brain, are segregated in astrocytes and oligodendrocytes. In the embryonic retina, carbonic anhydrase C and glutamine synthase differ markedly in their developmental profiles, cellular distribution, and susceptibility to regulation by cortisol and by cell interactions. Such differences make these two enzymes an attractive "marker team" for studying developmental mechanisms in embryonic retina and specific functions of Müller cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacharjee J. Developmental changes of carbonic anhydrase in the retina of the mouse: a histochemical study. Histochem J. 1976 Jan;8(1):63–70. doi: 10.1007/BF01004006. [DOI] [PubMed] [Google Scholar]
- GIACOBINI E. A cytochemical study of the localization of carbonic anhydrase in the nervous system. J Neurochem. 1962 Mar-Apr;9:169–177. doi: 10.1111/j.1471-4159.1962.tb11859.x. [DOI] [PubMed] [Google Scholar]
- Ghandour M. S., Langley O. K., Vincendon G., Gombos G. Double labeling immunohistochemical technique provides evidence of the specificity of glial cell markers. J Histochem Cytochem. 1979 Dec;27(12):1634–1637. doi: 10.1177/27.12.118210. [DOI] [PubMed] [Google Scholar]
- Hamberger A. C., Chiang G. H., Nylén E. S., Scheff S. W., Cotman C. W. Glutamate as a CNS transmitter. I. Evaluation of glucose and glutamine as precursors for the synthesis of preferentially released glutamate. Brain Res. 1979 Jun 8;168(3):513–530. doi: 10.1016/0006-8993(79)90306-8. [DOI] [PubMed] [Google Scholar]
- Kahn A. J. An autoradiographic analysis of the time of appearance of neurons in the developing chick neural retina. Dev Biol. 1974 May;38(1):30–40. doi: 10.1016/0012-1606(74)90256-5. [DOI] [PubMed] [Google Scholar]
- Linser P. J., Moscona A. A. Induction of glutamine synthetase in embryonic neural retina: its suppression by the gliatoxic agent alpha-aminoadipic acid. Brain Res. 1981 Jan;227(1):103–119. doi: 10.1016/0165-3806(81)90097-3. [DOI] [PubMed] [Google Scholar]
- Linser P., Moscona A. A. Induction of glutamine synthetase in embryonic neural retina: localization in Müller fibers and dependence on cell interactions. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6476–6480. doi: 10.1073/pnas.76.12.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELLER K., GLEES P. THE DIFFERENTIATION OF NEUROGLIA-MUELLER-CELLS IN THE RETINA OF CHICK. Z Zellforsch Mikrosk Anat. 1965 May 6;66(3):321–332. doi: 10.1007/BF00334715. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967 Oct;47(4):595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Maren T. H. The rates of movement of Na+, Cl-, and HCO-3 from plasma to posterior chamber: effect of acetazolamide and relation to the treatment of glaucoma. Invest Ophthalmol. 1976 May;15(5):356–364. [PubMed] [Google Scholar]
- Moscona A. A., Degenstein L. Normal development and precocious induction of glutamine synthetase in the neural retina of the quail embryo. Dev Neurosci. 1981;4(3):211–219. doi: 10.1159/000112758. [DOI] [PubMed] [Google Scholar]
- Moscona M., Moscona A. A. The development of inducibility for glutamine synthetase in embryonic neural retina: inhibition by BrdU. Differentiation. 1979;13(3):165–172. doi: 10.1111/j.1432-0436.1979.tb01579.x. [DOI] [PubMed] [Google Scholar]
- Musser G. L., Rosen S. Localization of carbonic anhydrase activity in the vertebrate retina. Exp Eye Res. 1973 Jan 1;15(1):105–119. doi: 10.1016/0014-4835(73)90195-4. [DOI] [PubMed] [Google Scholar]
- Norenberg M. D. Distribution of glutamine synthetase in the rat central nervous system. J Histochem Cytochem. 1979 Mar;27(3):756–762. doi: 10.1177/27.3.39099. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Osborne W. R., Tashian R. E. An improved method for the purification of carbonic anhydrase isozymes by affinity chromatography. Anal Biochem. 1975 Mar;64(1):297–303. doi: 10.1016/0003-2697(75)90434-0. [DOI] [PubMed] [Google Scholar]
- Riepe R. E., Norenberg M. D. Glutamine synthetase in the developing rat retina: an immunohistochemical study. Exp Eye Res. 1978 Oct;27(4):435–444. doi: 10.1016/0014-4835(78)90022-2. [DOI] [PubMed] [Google Scholar]
- Saad A. D., Soh B. M., Moscona A. A. Modulation of cortisol receptors in embryonic retina cells by changes in cell-cell contacts: correlations with induction of glutamine synthetase. Biochem Biophys Res Commun. 1981 Feb 12;98(3):701–708. doi: 10.1016/0006-291x(81)91170-0. [DOI] [PubMed] [Google Scholar]
- Sarthy P. V., Lam D. M. Biochemical studies of isolated glial (Müller) cells from the turtle retina. J Cell Biol. 1978 Sep;78(3):675–684. doi: 10.1083/jcb.78.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart R. M., Rosenberg R. N. Physiology of glia: glial-neuronal interactions. Int Rev Neurobiol. 1979;21:275–309. doi: 10.1016/s0074-7742(08)60641-8. [DOI] [PubMed] [Google Scholar]