Abstract
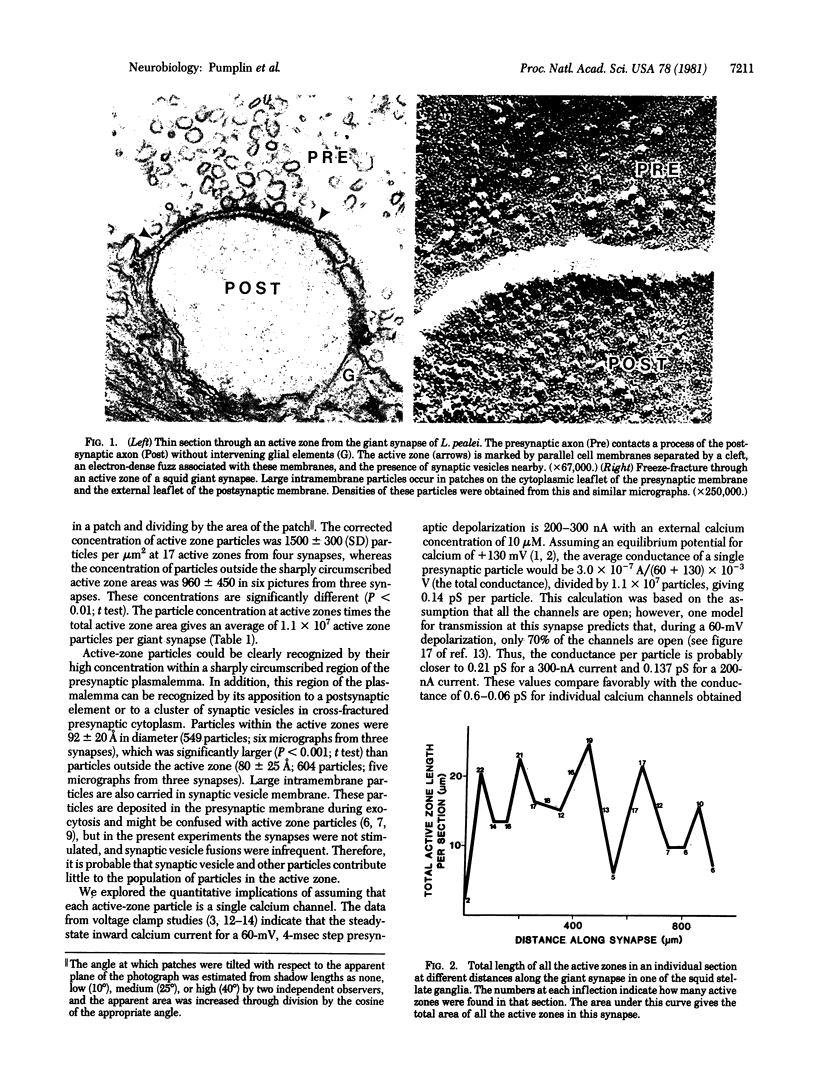
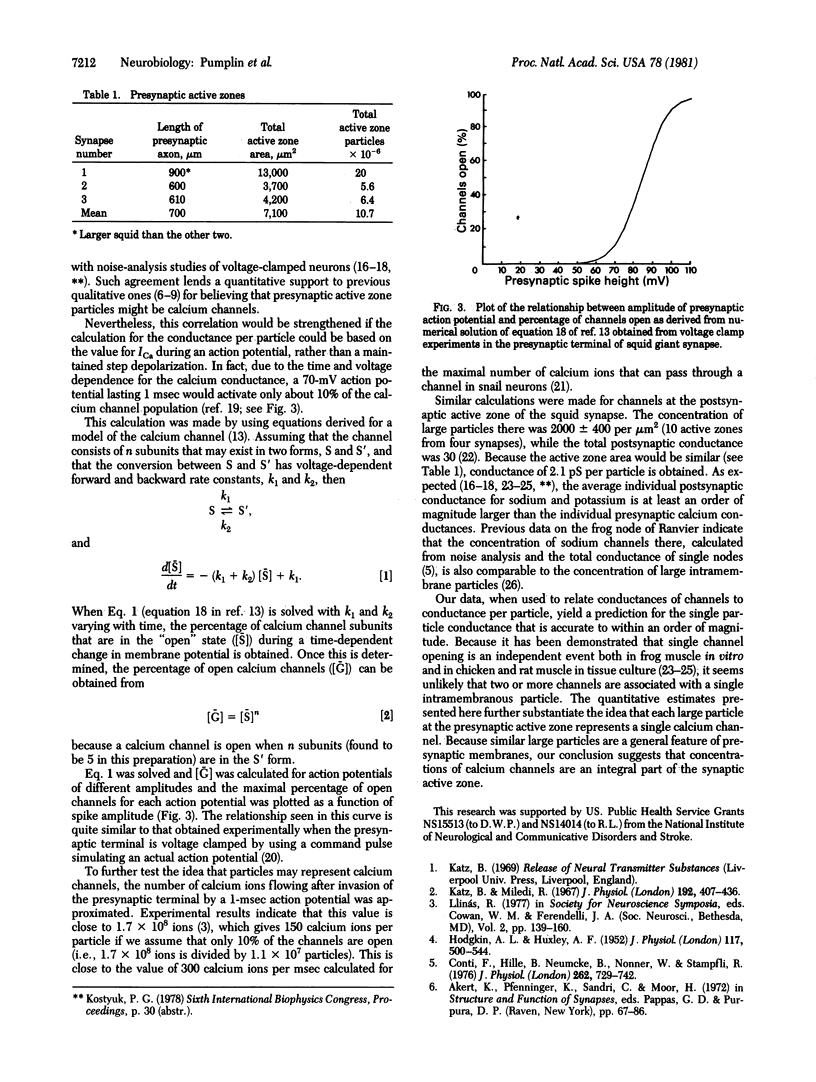
The number of large intramembrane particles associated with sites of synaptic vesicle release at the squid giant synapse was determined and compared to the average maximal presynaptic calcium current in order to derive an estimate of the conductance each particle would have if it were a calcium channel. This value, 0.21 pS, compares favorably with conductances of calcium channels in other preparations, substantiating the idea that the large intramembrane particles, which are concentrated at "active zones," represent calcium channels.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Fishman H. M., Lee K. S., Moore L. E., Brown A. M. The units of calcium conduction in Helix neurones. Nature. 1978 Jul 27;274(5669):379–382. doi: 10.1038/274379a0. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Akaike N., Lee K. S. The calcium conductance of neurons. Ann N Y Acad Sci. 1978 Apr 28;307:330–344. doi: 10.1111/j.1749-6632.1978.tb41960.x. [DOI] [PubMed] [Google Scholar]
- Conti F., Hille B., Neumcke B., Nonner W., Stämpfli R. Conductance of the sodium channel in myelinated nerve fibres with modified sodium inactivation. J Physiol. 1976 Nov;262(3):729–742. doi: 10.1113/jphysiol.1976.sp011617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupland R. E. Determining sizes and distribution of sizes of spherical bodies such as chromaffin granules in tissue sections. Nature. 1968 Jan 27;217(5126):384–388. doi: 10.1038/217384a0. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Dennis M. J., Jan Y., Jan L., Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979 May;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Landis D. M. Functional changes in frog neuromuscular junctions studied with freeze-fracture. J Neurocytol. 1974 Mar;3(1):109–131. doi: 10.1007/BF01111936. [DOI] [PubMed] [Google Scholar]
- Jackson M. B., Lecar H. Single postsynaptic channel currents in tissue cultured muscle. Nature. 1979 Dec 20;282(5741):863–864. doi: 10.1038/282863a0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Joyner R. W., Nicholson C. Equilibrium potential for the postsynaptic response in the squid giant synapse. J Gen Physiol. 1974 Nov;64(5):519–535. doi: 10.1085/jgp.64.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents and their relation to synaptic transmission: voltage clamp study in squid giant synapse and theoretical model for the calcium gate. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2918–2922. doi: 10.1073/pnas.73.8.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents in squid giant synapse. Biophys J. 1981 Mar;33(3):289–321. doi: 10.1016/S0006-3495(81)84898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse. Biophys J. 1981 Mar;33(3):323–351. doi: 10.1016/S0006-3495(81)84899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Nelson D. J., Sachs F. Single ionic channels observed in tissue-cultured muscle. Nature. 1979 Dec 20;282(5741):861–863. doi: 10.1038/282861a0. [DOI] [PubMed] [Google Scholar]
- Pumplin D. W., Reese T. S. Membrane ultrastructure of the giant synapse of the squid Loligo pealei. Neuroscience. 1978;3(8):685–696. doi: 10.1016/0306-4522(78)90065-9. [DOI] [PubMed] [Google Scholar]
- Rosenbluth J. Intramembranous particle distribution at the node of Ranvier and adjacent axolemma in myelinated axons of the frog brain. J Neurocytol. 1976 Dec;5(6):731–745. doi: 10.1007/BF01181584. [DOI] [PubMed] [Google Scholar]




