Abstract
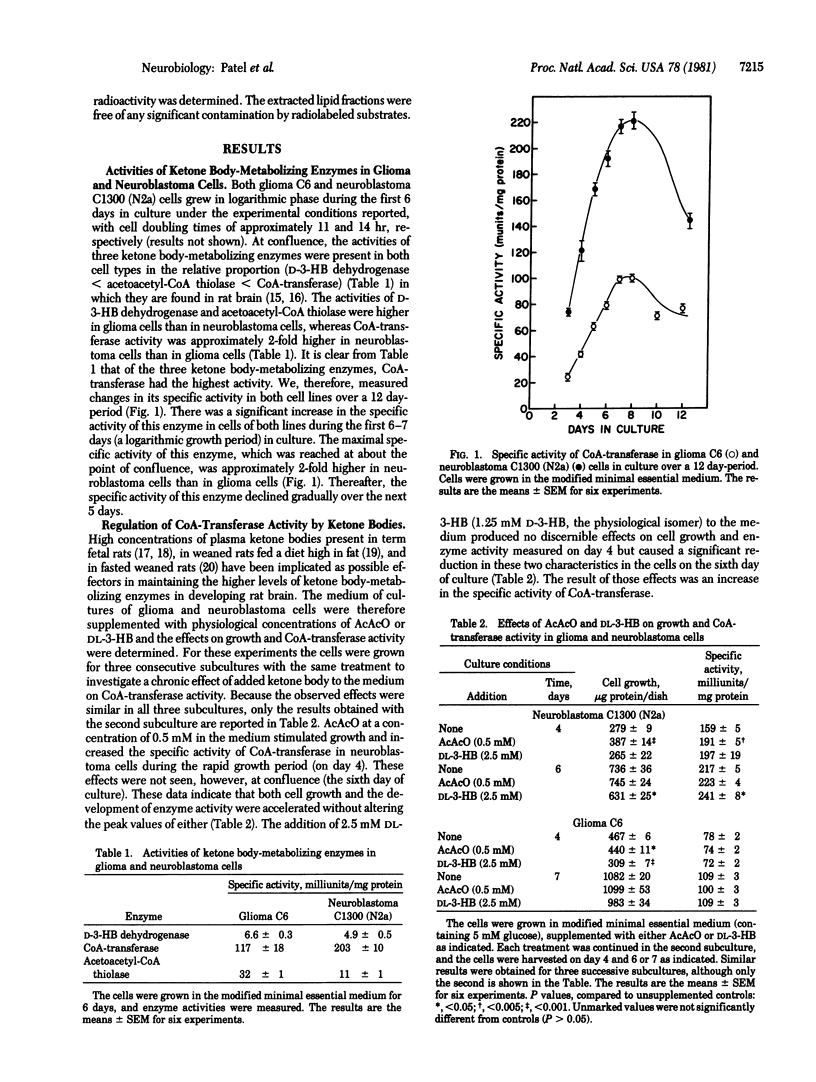
We have examined the metabolism of ketone bodies in neuroblastoma C1300 and glioma C6 cells, two established lines of neural origin. The three ketone body-metabolizing enzymes are present in cells of both lines in the relative proportions normally found in brain (D-3-hydroxybutyrate dehydrogenase less than acetoacetyl-CoA thiolase less than 3-ketoacid CoA-transferase), the activities of the first two are higher in glioma cells than in neuroblastoma, and that of the third is 2-fold higher in neuroblastoma cells than in glioma cells. The specific activity of 3-ketoacid CoA-transferase (EC 2.8.3.5) in both cell lines increased as the cultures achieved confluence, then decreased. Ketone bodies and especially acetoacetate are preferred substrates for synthesis of neural lipids in cells of both lines. The incorporation of glucose carbon into lipids is significantly reduced in cells of both lines in the presence of ketone bodies. Addition of acetoacetate but not DL-3-hydroxybutyrate to the culture medium resulted in a significant increase in the activity of 3-ketoacid CoA-transferase and also in the rate of acetoacetate oxidation in neuroblastoma cells but not glioma cells. These findings indicate that specific differences exist in the capacity of these two cell lines to metabolize ketone bodies and also that substrate-level regulation of the ketone body-metabolizing pathway exists. These two lines therefore provide a potentially useful system in which the mechanisms of regulation of these enzymes may be examined.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckley B. M., Williamson D. H. Acetoacetate and brain lipogenesis: developmental pattern of acetoacetyl-coenzyme A synthetase in the soluble fraction of rat brain. Biochem J. 1973 Mar;132(3):653–656. doi: 10.1042/bj1320653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer J. E., Heath D. F. The estimation of rates of utilization of glucose and ketone bodies in the brain of the suckling rat using compartmental analysis of isotopic data. Biochem J. 1974 Sep;142(3):527–544. doi: 10.1042/bj1420527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierks-Ventling C. Prenatal induction of ketone-body enzymes in the rat. Biol Neonate. 1971;19(4):426–433. doi: 10.1159/000240435. [DOI] [PubMed] [Google Scholar]
- Fitzgerald G. G., Kaufman E. E., Sokoloff L., Shein H. M. D(-)-beta-hydroxybutyrate dehydrogenase activity in cloned cell lines of glial and neuronal origin. J Neurochem. 1974 Jun;22(6):1163–1165. doi: 10.1111/j.1471-4159.1974.tb04355.x. [DOI] [PubMed] [Google Scholar]
- Fredericks M., Ramsey R. B. 3-Oxo acid coenzyme A transferase activity in brain and tumors of the nervous system. J Neurochem. 1978 Dec;31(6):1529–1531. doi: 10.1111/j.1471-4159.1978.tb06581.x. [DOI] [PubMed] [Google Scholar]
- Hawkins R. A., Williamson D. H., Krebs H. A. Ketone-body utilization by adult and suckling rat brain in vivo. Biochem J. 1971 Mar;122(1):13–18. doi: 10.1042/bj1220013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Middleton B. The acetoacetyl-coenzyme A thiolases of rat brain and their relative activities during postnatal development. Biochem J. 1973 Apr;132(4):731–737. doi: 10.1042/bj1320731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M. A., Krebs H. A., Williamson D. H. Activities of enzymes of ketone-body utilization in brain and other tissues of suckling rats. Biochem J. 1971 Jan;121(1):49–53. doi: 10.1042/bj1210049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passonneau J. V., Crites S. K. Regulation of glycogen metabolism in astrocytoma and neuroblastoma cells in culture. J Biol Chem. 1976 Apr 10;251(7):2015–2022. [PubMed] [Google Scholar]
- Patel M. S. Influence of neonatal hypothyroidism on the development of ketone-body-metabolizing enzymes in rat brain. Biochem J. 1979 Oct 15;184(1):169–172. doi: 10.1042/bj1840169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M. S., Owen O. E. Development and regulation of lipid synthesis from ketone bodies by rat brain. J Neurochem. 1977 Jan;28(1):109–114. doi: 10.1111/j.1471-4159.1977.tb07715.x. [DOI] [PubMed] [Google Scholar]
- Patel M. S., Owen O. E. Lipogenesis from ketone bodies in rat brain. Evidence for conversion of acetoacetate into acetyl-coenzyme A in the cytosol. Biochem J. 1976 Jun 15;156(3):603–607. doi: 10.1042/bj1560603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasure D., Lichtman C., Eastman S., Lieb M., Abramsky O., Silberberg D. Acetoacetate and D-(-)-beta-hydroxybutyrate as precursors for sterol synthesis by calf oligodendrocytes in suspension culture: extramitochondrial pathway for acetoacetate metabolism. J Neurochem. 1979 May;32(5):1447–1450. doi: 10.1111/j.1471-4159.1979.tb11083.x. [DOI] [PubMed] [Google Scholar]
- Pull I McIlwain H. 3-Hydroxybutyrate dehydrogenase of rat brain on dietary change and during maturation. J Neurochem. 1971 Jun;18(6):1163–1165. doi: 10.1111/j.1471-4159.1971.tb12046.x. [DOI] [PubMed] [Google Scholar]
- Robinson A. M., Williamson D. H. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev. 1980 Jan;60(1):143–187. doi: 10.1152/physrev.1980.60.1.143. [DOI] [PubMed] [Google Scholar]
- Sherman T. G., Wilson J. E. Effect of prenatally-induced and postnatally-maintained ketosis on beta-hydroxybutyrate dehydrogenase and hexokinase levels in the developing rat brain. J Neurochem. 1978 Mar;30(3):639–641. doi: 10.1111/j.1471-4159.1978.tb07820.x. [DOI] [PubMed] [Google Scholar]
- Smith A. L., Satterthwaite H. S., Sokoloff L. Induction of brain D(--)-beta-hydroxybytrate dehydrogenase activity by fasting. Science. 1969 Jan 3;163(3862):79–81. doi: 10.1126/science.163.3862.79. [DOI] [PubMed] [Google Scholar]
- Thaler M. M. Effects of starvation on normal development of -hydroxybutyrate dehydrogenase activity in foetal and newborn rat brain. Nat New Biol. 1972 Apr 5;236(66):140–141. doi: 10.1038/newbio236140a0. [DOI] [PubMed] [Google Scholar]
- Volpe J. J., Marasa J. C. Regulation of palmitic acid synthesis in cultured glial cells: effects of glucocorticoid on fatty acid synthetase, acetyl-CoA carboxylase, fatty acid and sterol synthesis. J Neurochem. 1976 Oct;27(4):841–845. doi: 10.1111/j.1471-4159.1976.tb05144.x. [DOI] [PubMed] [Google Scholar]
- Webber R. J., Edmond J. Utilization of L(+)-3-hydroxybutyrate, D(-)-3-hydroxybutyrate, acetoacetate, and glucose for respiration and lipid synthesis in the 18-day-old rat. J Biol Chem. 1977 Aug 10;252(15):5222–5226. [PubMed] [Google Scholar]
- Williamson D. H., Bates M. W., Page M. A., Krebs H. A. Activities of enzymes involved in acetoacetate utilization in adult mammalian tissues. Biochem J. 1971 Jan;121(1):41–47. doi: 10.1042/bj1210041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh Y. Y., Streuli V. L., Zee P. Ketone bodies serve as important precursors of brain lipids in the developing rat. Lipids. 1977 Nov;12(11):957–964. doi: 10.1007/BF02533318. [DOI] [PubMed] [Google Scholar]



