Abstract
Li2O2, Li2CO3, and Li2O are three critical compounds in lithium-air and lithium-ion energy storage systems. Extensive measurements have been carried out to study the chemical species and their evolutions at difference stages of the device operation. While x-ray spectroscopy has been demonstrated to be one of the most powerful tools for such purpose, no systematic study on the irradiation effects have been reported. Here we carry out extensive time, position, and irradiation dependent Li K-edge soft x-ray absorption spectroscopy on these compounds with so far the best energy resolution. The ultra-high resolution in the current study allows the features in the absorption spectra to be well-resolved. The spectral lineshape thus serves as the fingerprints of these compounds, enabling the tracking of their evolution under x-ray irradiation. We found that both Li2O2 and Li2CO3 evidently evolve towards Li2O under the soft x-ray irradiation with Li2CO3 exhibiting a surprisingly higher sensitivity to x-rays than Li2O2. On the other hand, Li2O remains the most stable compound despite experiencing substantial irradiation dose. We thus conclude that high resolution soft x-ray spectroscopy could unambiguously fingerprint different chemical species, but special cautions on irradiation effects would be needed in performing the experiments and interpreting the data properly.
Introduction
At the heart of modern sustainable energy applications are the high performance energy storage systems. The demand of revolutionizing the current energy carriers, e.g. fossil fuel, has become ever more pressing especially in the utilization of intermittent renewable energy sources [1] and the realization of electric vehicles (EVs) [2]. Among all current electrochemical energy storage systems, Li-ion batteries have been extensively used in the portable electronic markets ever since their first commercialization by Sony in 1991 [3], and they are expected to remain the dominance in the near future if the safety and performance can be improved to match the requirements of Evs [4]–[6]. In the meantime, scientific attention has been gradually shifted to the next generation energy storage media beyond Li-ion, which could potentially provide much higher specific energy and energy density. One such example is the Li-air battery systems with theoretically several times higher specific energy than that of the Li-ion systems [7]–[14].
However, for both Li-ion and Li-air electrochemical systems, the critical understandings on the fundamental mechanism of battery operations remain incomplete and this severely hinders the rational and speedy improvements on the performance of these energy storage devices. Li-ion battery operates under non-equilibrium states far above the thermodynamic stability of electrolyte, or sometimes even electrodes [6]. In reality, a battery always operates in an alternative way by forming a passivating layer on the surface of the electrodes. This passivating layer, typically around 20–50 nm thick and is known as the solid-electrolyte interphase (SEI), protects electrolyte from further reduction/oxidation. Almost all Li-ion batteries on the market rely on such SEI mechanism for retaining their stability. For example, in the commercially available LiCoO2/Graphite batteries, the graphite based anode turns to reduce the electrolyte, leading to serious stability issues that have frustrated the practical application for decades until the proper electrolyte compounds were found [15]. It is now known that one component of the electrolyte, ethylene carbonate, decomposes and forms a passivating SEI layer on the surface of the carbon anode. This SEI layer, although consumes electrolyte for its formation, does provide the kinetic stability for protecting the electrolyte from further decomposition. SEI often contains Li2O, Li2O2, Li2CO3, fluorides and other organic compounds. However, the dual-functionality and controlling [16], [17] of SEI in batteries remain elusive and a hot topic in Li-ion battery researches [4], [18]–[24].
The principle of Li-air batteries is the catalytic reactions between lithium and oxygen, which is distinct from the insertion/extraction mechanism in the Li-ion batteries. The reversible oxidation of lithium at the anode and reduction of oxygen at the cathode could potentially yield an ultra-high energy capacity. However, the battery module suffers poor cycling performance, high-rate output, as well as stability issues [14], which require extensive further studies on the chemical nature during the battery operations. Besides the much discussed Li2O2 and Li2O as the critical species in the lithium-oxygen reaction [8], [11], [25]–[27], very recent reports [28]–[31] have suggested the presence of Li2CO3 in the discharge products if carbonate based electrolytes are used. At this time, the details of the lithium-oxygen reactions involved in Li-air batteries remains unclear.
Because of the importance of Li2O, Li2O2, and Li2CO3 in both Li-ion SEI and Li-air battery systems, there have been extensive efforts in studying the chemical compositions and the phase evolution of these compounds. In particular, electron energy loss spectroscopy (EELS) [32], hard X-ray non-resonant inelastic X-ray scattering (NIXS) [33]–[35] and soft X-ray absorption spectroscopy (XAS) [25], [36], [37] are used to probe the unoccupied electronic states that are sensitive to the local environment of lithium ions. Among these techniques, soft X-ray XAS typically offers the best experimental resolution with its inherent elemental, chemical and bonding sensitivity by tuning the x-rays from synchrotron sources across the absorption edges. Indeed, high-resolution soft x-ray absorption studies have captured a lot of research interests in fingerprinting these lithium compounds [25], [37]. On the other hand, due to the intrinsic nature of soft x-rays on both penetration depth and high cross-section of interacting with electrons, it often encounters more serious irradiation damage effects than hard x-rays. Specifically, the secondary electrons generated from soft x-ray photon-excitation process could strongly interact with the molecular bonding on surface, leading to undesired species. Such effects have been broadly observed in the organic molecular systems in XAS [38], [39], as well as X-ray photoelectron spectroscopy (XPS) [40]. Although the Li compounds studied in this work are considered ionic systems that are typically stable under x-rays, it is important to address the possible soft x-ray irradiation effects in these compounds in order to obtain accurate XAS spectra for understanding the nature of the battery systems. Not much attention has been paid to this essential topic, and an experimental report is still missing.
In this work, we present a systematic study with position, time and irradiation dependent Li K-edge soft x-ray XAS spectroscopy on Li2O, Li2O2, and Li2CO3 compounds. The experiments were done at beamline 4.0.3 (the milli-eV energy resolution beamline, MERLIN) of the Advanced Light Source (ALS), Lawrence Berkeley National Laboratory (LBNL). The ultra-high resolution enables the features in the XAS spectra to be well-resolved. More importantly, the spectra of both Li2O2 and Li2CO3 evolve unambiguously towards Li2O under soft x-ray irradiation, with Li2CO3 exhibiting a surprisingly higher sensitivity to x-rays than Li2O2. This work provides the clear experimental evidences of decomposition of Li2O2 and Li2CO3 into the final product of Li2O through soft x-ray irradiation.
Experimental
The Li K-edge XAS measurements were conducted at the elliptically polarizing undulator (EPU) beamline 4.0.3 (MERLIN) [41] of the Advanced Light Source (ALS), Lawrence Berkeley National Laboratory (LBNL). The MERLIN beamline was newly constructed with a 1.9 m long, 90 mm period quasi-periodic EPU and spherical grating monochromator. It delivers the photon beam with energies ranging from 10 eV to ∼150 eV onto the sample and with better than 0.01 eV (10 meV) energy resolution, the photon flux is about 1011 photons per second. The complimentary O K-edge XAS spectra were measured at the undulator beamline 8.0.1 [42], where the intense photon beam from a spherical grating monochromator gives an energy resolution better than 0.2 eV at 500–550 eV. We note that, for the Li K-edge XAS, the ultra-high energy resolution offers a unique opportunity to experimentally determine the core-hole life time, because most of the broadening of spectral features is from the thermal effect and the intrinsic core-hole lifetime.
Chemicals in powder form with highest possible purity were purchased from Sigma-Aldrich. The powder samples were pressed into pellets (for Li K-edge measurement) or onto a conducting carbon tape (for O K-edge measurement) in the N2 glove box before loaded into the ultra-high vacuum chamber with base pressure better than 5×10−10 Torr. XAS experiments were performed at room temperature and spectra were recorded in bulk-sensitive total fluorescence yield mode (TFY) using a photodiode detector. All the data shown were normalized to the photon flux measured by the photocurrent of an upstream gold mesh. The probing depth is around tens of nanometers for Li-K and on the order of hundreds of nanometers for O-K.
Results and Discussion
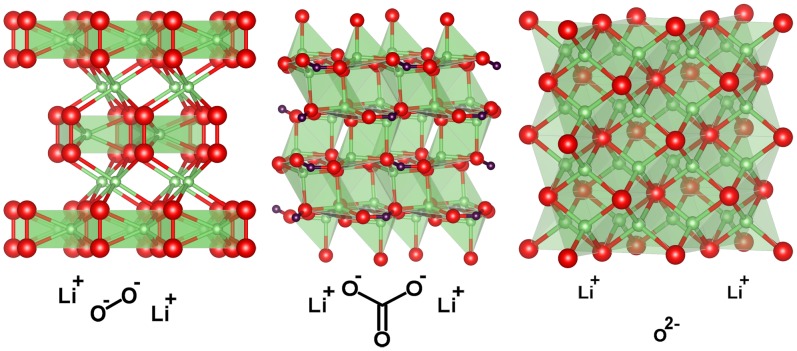
As shown in figure 1, Li2O2 [43], [44] crystallizes in a P63/mmc hexagonal space group where the peroxide anions are arranged in an alternating ABAB stacking. There are two distinct Li+ sites: one is in the same layer as the peroxide anions and the other one is in between the peroxide layers. The crystal structure of Li2CO3 [45] (monoclinic, space group C2/c) is composed of nearly planar CO3 2− anions and Li+ cations tetrahedrally coordinated to oxygen atoms. This crystal structure consists of staggered Li2CO3 units. Li2O adopts a cubic crystal structure (space group Fm-3m), wherein lithium is coordinated to four oxygen anions and each O2− ion is surrounded by eight Li+ ions. These three inorganic compounds are ionic insulators with strong Li-O bonds, and naively, one would not expect to observe pronounced x-ray irradiation damage.
Figure 1. Crystal structure of Li2O2, Li2CO3, and Li2O.
Red (largest) spheres represent oxygen atoms, green (smaller) spheres represent lithium atoms and purple (smallest) spheres are carbon atoms. Li-O polygons are drawn for better view.
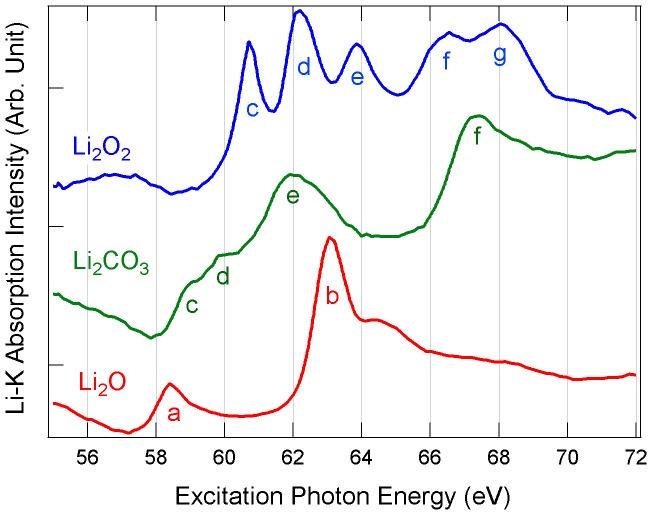
Li K-edge XAS spectra collected from the fresh spots on three lithium compounds, Li2O2, Li2CO3, and Li2O, are shown in Figure 2. First of all, the distinctively different absorption features clearly demonstrate that Li K-edge XAS can be used to unambiguously fingerprint those chemical species in lithium batteries, as previously reported [25], [37]. Secondly, it is important to note that the peak width is not limited by the beamline instrumental resolution, but mostly by the lithium 1s core-hole lifetime and thermal broadening [36], [46]. Since the Li 1s core-hole is not well screened and has strong interaction with the excited 2p electron, the excitonic effect can play an important role in the XAS spectra. In general, the excitonic effect would create sharp absorption features at lower energy and reduce the intensity at higher energy [47], as especially shown by the sharp features denoted a, b (red) in Li2O and c-g (blue) in Li2O2 in Fig. 2 [34], [46].
Figure 2. Li K-edge XAS spectra of Li2O2, Li2CO3, and Li2O.
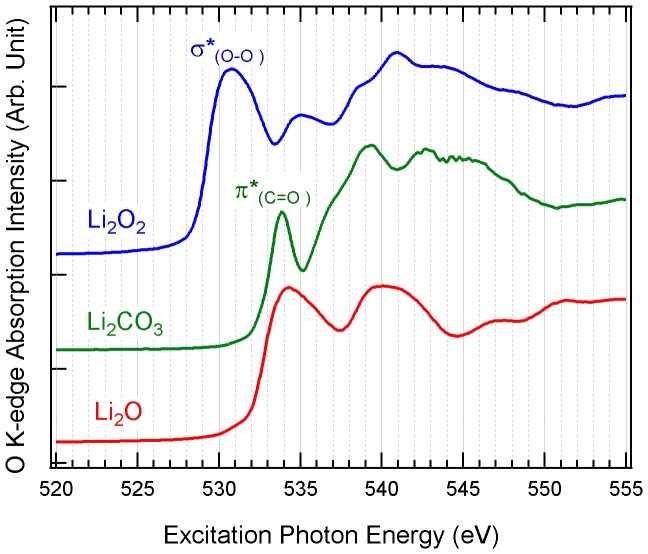
Figure 3 shows the O K-edge XAS spectra of Li2O2, Li2CO3 and Li2O. One significant difference between O and Li K-edge XAS spectra is that the O 1s core hole is now well-screened compared with the shallow Li 1s core hole. Thus O-K spectra are less affected by excitonic effect and better reflect the partial density of states of unoccupied O 2p orbitals. All three lithium compounds display rather different absorption spectra due to variations in the local oxygen environment. As discussed previously, the first absorption feature on the XAS spectrum of Li2O2 is from electron transition to the orbital with largely σ* (O-O) character [48], while the sharp absorption peak of Li2CO3 can be assigned to be transition to π* (C = O) orbital [49]. It is worthy to note that the leading edge of Li2O2 XAS spectrum is about 3.5eV lower than that of Li2CO3 and Li2O, suggesting that the conduction band minimum of Li2O2 is lower than that of Li2CO3 and Li2O due to its O-O bond. Therefore, the O K-edge XAS spectra can also be used to fingerprint the chemical species and be complimentary to the Li K-edge XAS [48], [49].
Figure 3. O K-edge XAS spectra of Li2O2, Li2CO3, and Li2O.
The central results of this work on irradiation effects of Li2O2 and Li2CO3 are presented in Fig. 4 and Fig. 5, respectively. Figure 4 shows the irradiation dependent Li K-edge XAS spectra of Li2O2, in comparison with Li2O. The Li2O2 XAS spectra (from top to bottom) were recorded from spot A on the sample by repeating the photon energy scan every ten minutes. In order to maximize the irradiation effect, after the 10th spectrum, the sample was left exposed to x-rays for one hour, and then the measurement resumed. It is evident that the XAS spectrum of Li2O2 evolves with respect to the X-ray radiation exposure. All the absorption features of the pristine Li2O2 sample, marked by blue dashed lines (c-g), become weaker with irradiation, and most of them are diminished except for feature c. In the meantime, new absorption peaks labeled a and b (red lines) are enhanced with increased irradiation dose. These enhanced features are in good agreement with the ones in Li2O (Fig. 2). Therefore, it is evident that Li2O2 gradually decomposes to Li2O under soft x-ray irradiation.
Figure 4. Soft x-ray irradiation effect on Li2O2 revealed by Li K-edge XAS spectra.
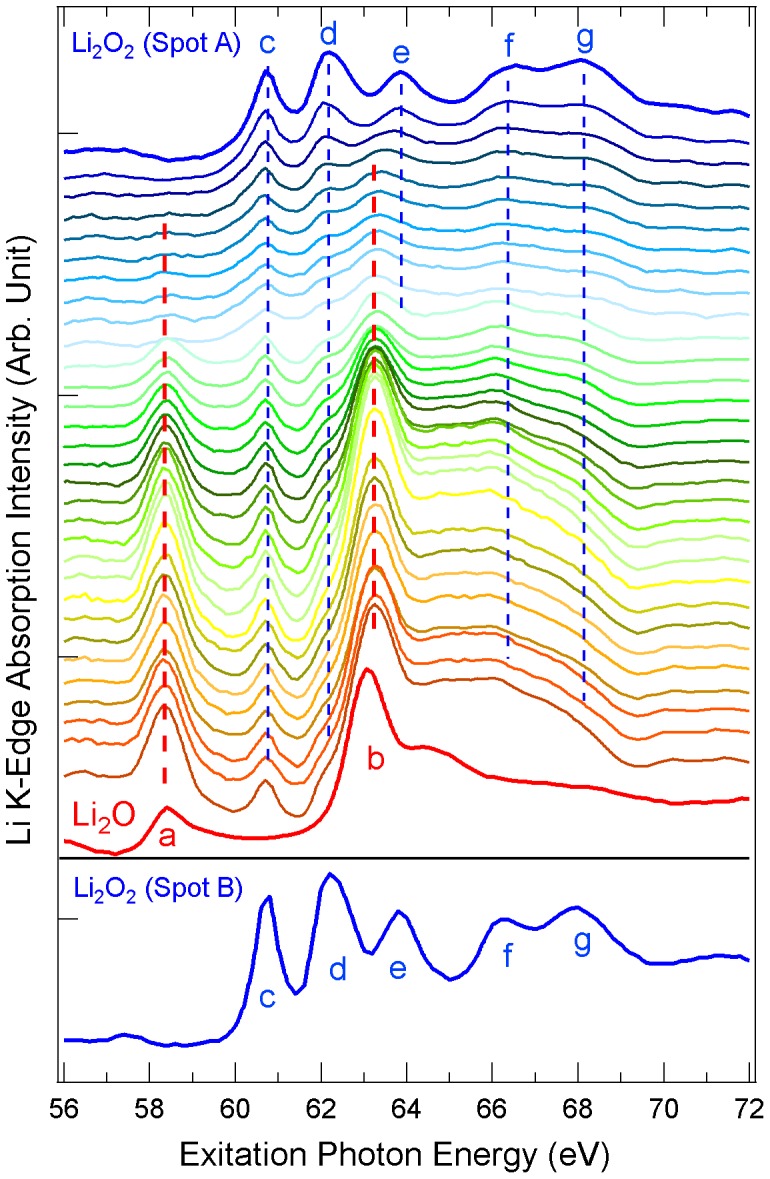
(top) The XAS spectrum of Li2O2 evolves towards that of the Li2O upon increasing the radiation exposure. The top spectrum is the first one collected from spot A. From top to bottom, the first ten spectra were collected every ten minutes, with an hour of x-ray exposure at the same spot to maximize the dosage, then the measurements resumed with again ten minute each spectrum. The bottom red spectrum is collected on Li2O for comparison. (bottom) Li2O2 Li K-edge XAS spectrum measured from a new spot B on the same sample stored in the ultra-high vacuum chamber for one week.
Figure 5. Soft x-ray irradiation effect on Li2CO3 revealed by Li K-edge XAS spectra.
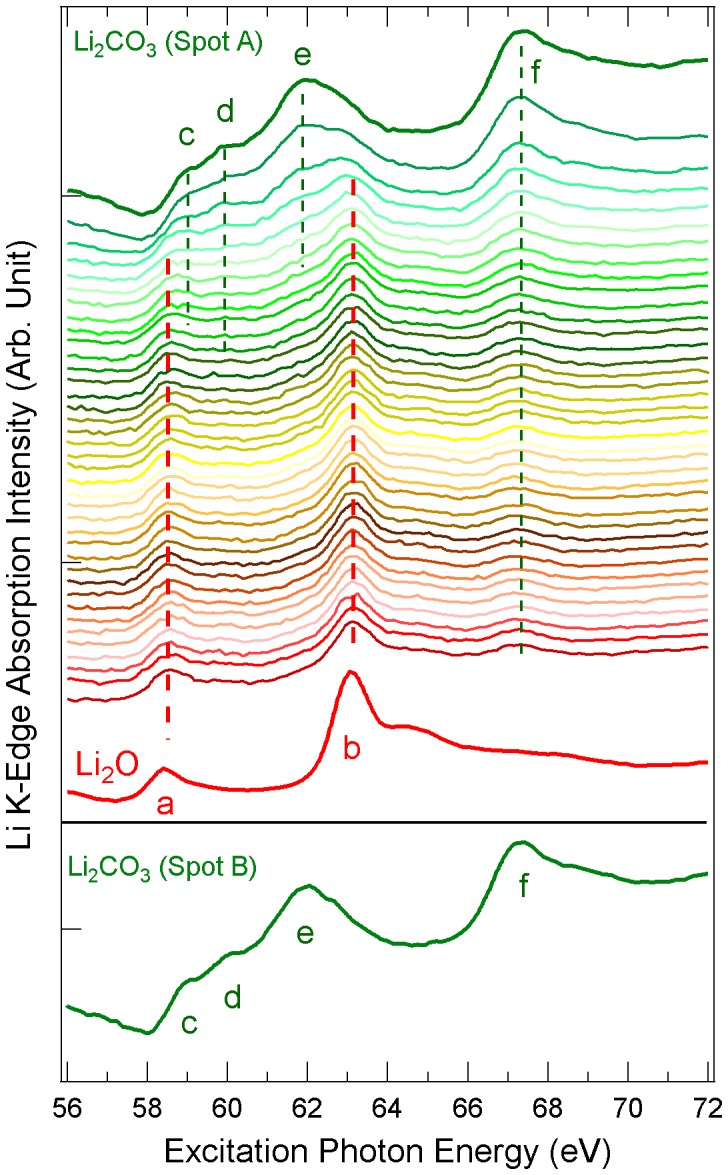
The XAS lineshape of Li2CO3 (from top to bottom) evolve towards that of Li2O (red) after exposed to the soft x-rays. Each spectrum was taken with 10 minute x-ray exposure. The bottom panel shows the Li2CO3 Li K-edge XAS spectrum collected from a new spot B on the same sample.
To make sure that the change of spectral lineshape is from the exposure to soft x-rays instead of surface degradation in ultra-high vacuum, we took the XAS spectrum from a different spot B on the same Li2O2 sample that had been stored in the vacuum chamber for one week. As shown in figure 4 (bottom), the spectrum resembles the top one measured on the fresh surface. The similarity indicates that the sample surface is stable in high-vacuum, and the observed decomposition of Li2O2 is indeed induced by the X-ray irradiation.
Figure 5 shows the Li K-edge XAS spectra of Li2CO3 collected under the same condition and time scale as that for Li2O2. To our surprise, the XAS of Li2CO3 changes quickly with exposure to soft x-rays. The features from Li2CO3 (green c-f) quickly fade out in 30 minutes and the Li2O features (red a and b) can already be seen in the second spectrum (less than 20 minutes of exposure). The Li2O features dominate the overall lineshape from the fourth spectrum. We would like to point out that 10 to 60 minutes of exposure to soft x-rays with a photon flux on the order of 1010–1011 photons per second is typical for soft x-ray experiments, during which, the Li2CO3 may be decomposed and produce Li2O as suggested by the data in Figure 5.
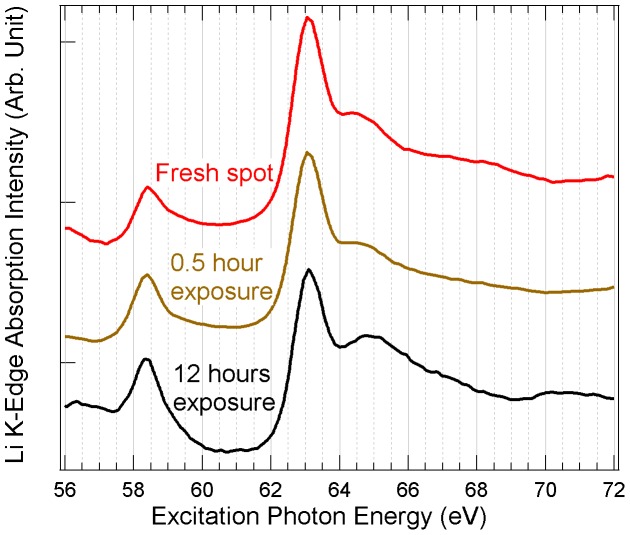
Finally, we confirm that Li2O is the only stable phase under soft x-ray irradiation. Figure 6 shows the Li K-edge XAS of Li2O over the course of 12-hour x-ray exposure, and yet the spectra remain nearly identical. Based on our experimental results, the irradiation induced decomposition of Li2O2 and Li2CO3 can be expressed as 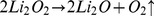 and
and 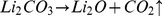 .
.
Figure 6. Li K-edge XAS spectra of Li2O.
The spectra were collected on fresh surface, the same spot with 0.5 hour soft x-ray exposure, and 12 hours of soft x-ray exposure.
Although the radiation damage effects are broadly investigated in organic systems, such as biomolecules and polymers [39], [50], [51], inorganic compounds are often considered resistant to soft x-ray irradiation damage [38]. For the irradiation effect of Li2O2 and Li2CO3 reported in this work, the decomposition may stem from several factors. Overall, both systems are ionic compounds dominated by insulating nature. The lack of enough conduction electrons prevents the quick restoration from irradiated states through electrical neutrality. In addition to the many scenarios based on x-ray excitations of core-hole, secondary electrons, electron-hole pair, and broken surface bonds, it is also well known that some energy losses from x-ray photons to phonons lead to local heat. Interestingly, the X-ray induced decomposition reaction has the same product as the heat induced reaction, in which both Li2O2 and Li2CO3 would decomposed to Li2O at temperature range of 200–450°C and 730–1270°C, respectively. Actually, Li2O2 is a strong oxidant that could be reduced to Li2O under various circumstances, but our results show that Li2O2 is only slowly reduced to Li2O under soft x-rays. On the other hand, Li2CO3 is more stable under ambient condition, yet it exhibits a higher degree of susceptibility to x-ray irradiation.
Conclusions
In summary, we have performed a detailed study on the irradiation effects of Li2O2, Li2CO3 and Li2O. High resolution XAS spectra, capable of revealing distinct spectral features associated with different chemical species, allow us to track the chemical evolution from x-ray irradiation effects. We found both Li2O2 and Li2CO3 show clear evidence of decomposition with soft x-ray exposure with Li2CO3 exhibiting a surprisingly higher degree of sensitivity than the Li2O2. For both systems, the final product of decomposition is Li2O, which is rather stable against soft x-ray irradiation. The data presented in this work demonstrates the potential of soft x-ray XAS for studying the chemical nature and reaction pathway of the lithium compounds; however, it also suggests that experiments and data analysis should be performed with careful considerations of irradiation effects.
Funding Statement
The Advanced Light Source at Lawrence Berkeley National Laboratory was supported by the U. S. Department of Energy (http://energy.gov) under Contract No. DE-AC02-05CH11231. This work was also supported by National Natural Science Foundation of China (http://www.nsfc.gov.cn) No.51125004 and No.10974120, and the National Basic Research Program of China (http://www.973.gov.cn) No. 2013CB922303. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Dunn B, Kamath H, Tarascon J-M (2011) Electrical energy storage for the grid: A battery of choices. Science 334: 928–935. [DOI] [PubMed] [Google Scholar]
- 2. Armand M, Tarascon JM (2008) Building better batteries. Nature 451: 652–657. [DOI] [PubMed] [Google Scholar]
- 3. Nagaura T, Tozawa K (1990) Lithium ion rechargeable battery. Progress in Batteries & Solar Cells 9: 209. [Google Scholar]
- 4.Goodenough JB, Abruña HD, Buchanan MV (2007) Basic research needs for electrical energy storage. Report of the basic energy sciences workshop for electrical energy storage.
- 5.Huggins RA (2008) Advanced batteries: Materials science aspects. New York: Springer.
- 6.Nazri G-A, Pistoia G (2003) Lithium batteries: science and technology. New York: Springer. 716 p.
- 7. Lee J-S, Tai Kim S, Cao R, Choi N-S, Liu M, et al. (2011) Metal–air batteries with high energy density: Li–Air versus Zn–Air. Advanced Energy Materials 1: 34–50. [Google Scholar]
- 8. Débart A, Paterson AJ, Bao J, Bruce PG (2008) α-MnO2 nanowires: A catalyst for the O2 electrode in rechargeable lithium batteries. Angewandte Chemie (International Edition) 47: 4521–4524. [DOI] [PubMed] [Google Scholar]
- 9. He P, Wang Y, Zhou H (2010) A Li-air fuel cell with recycle aqueous electrolyte for improved stability. Electrochemistry Communications 12: 1686–1689. [Google Scholar]
- 10. Wang Y, Zhou H (2010) A lithium-air battery with a potential to continuously reduce O2 from air for delivering energy. Journal of Power Sources 195: 358–361. [Google Scholar]
- 11. Ogasawara T, Débart A, Holzapfel M, Novák P, Bruce PG (2006) Rechargeable Li2O2 electrode for lithium batteries. Journal of the American Chemical Society 128: 1390–1393. [DOI] [PubMed] [Google Scholar]
- 12. Kumar B, Kumar J, Leese R, Fellner JP, Rodrigues SJ, et al. (2010) A solid-state, rechargeable, long cycle life lithium–air battery. Journal of The Electrochemical Society 157: A50–A54. [Google Scholar]
- 13. Abraham KM, Jiang Z (1996) A polymer electrolyte-based rechargeable lithium/oxygen battery. Journal of The Electrochemical Society 143: 1–5. [Google Scholar]
- 14. Bruce PG, Freunberger SA, Hardwick LJ, Tarascon J-M (2012) Li-O2 and Li-S batteries with high energy storage. Nat Mater 11: 19–29. [DOI] [PubMed] [Google Scholar]
- 15. Fong R, von Sacken U, Dahn JR (1990) Studies of lithium intercalation into carbons using nonaqueous electrochemical cells. Journal of The Electrochemical Society 137: 2009–2013. [Google Scholar]
- 16. Jung YS, Cavanagh AS, Gedvilas L, Widjonarko NE, Scott ID, et al. (2012) Improved functionality of lithium-ion batteries enabled by atomic layer deposition on the porous microstructure of polymer separators and coating electrodes. Advanced Energy Materials 2: 1022–1027. [Google Scholar]
- 17. Abe K, Ushigoe Y, Yoshitake H, Yoshio M (2006) Functional electrolytes: Novel type additives for cathode materials, providing high cycleability performance. Journal of Power Sources 153: 328–335. [Google Scholar]
- 18.Balbuena PB, Wang Y (2004) Lithium-Ion Batteries Solid-Electrolyte Interphase: Imperial College Press.
- 19. Xu K (2004) Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chemical Reviews 104: 4303–4418. [DOI] [PubMed] [Google Scholar]
- 20. El Ouatani L, Dedryvère R, Siret C, Biensan P, Gonbeau D (2009) Effect of vinylene carbonate additive in li-ion batteries: Comparison of LiCoO2/C, LiFePO4/C, and LiCoO2/Li4Ti5O12 systems. Journal of The Electrochemical Society 156: A468–A477. [Google Scholar]
- 21. Goodenough JB, Kim Y (2009) Challenges for rechargeable Li batteries. Chemistry of Materials 22: 587–603. [Google Scholar]
- 22. Cho IH, Kim S-S, Shin SC, Choi N-S (2010) Effect of SEI on capacity losses of spinel lithium manganese oxide/graphite batteries stored at 60°C. Electrochemical and Solid-State Letters 13: A168–A172. [Google Scholar]
- 23. Harris SJ, Timmons A, Baker DR, Monroe C (2010) Direct in situ measurements of Li transport in Li-ion battery negative electrodes. Chemical Physics Letters 485: 265–274. [Google Scholar]
- 24. Ochida M, Domi Y, Doi T, Tsubouchi S, Nakagawa H, et al. (2012) Influence of manganese dissolution on the degradation of surface films on edge plane graphite negative-electrodes in lithium-ion batteries. Journal of The Electrochemical Society 159: A961–A966. [Google Scholar]
- 25. Lu Y-C, Kwabi DG, Yao KPC, Harding JR, Zhou J, et al. (2011) The discharge rate capability of rechargeable Li-O2 batteries. Energy & Environmental Science 4: 2999–3007. [Google Scholar]
- 26. Mitchell RR, Gallant BM, Thompson CV, Shao-Horn Y (2011) All-carbon-nanofiber electrodes for high-energy rechargeable Li-O2 batteries. Energy & Environmental Science 4: 2952–2958. [Google Scholar]
- 27. Peng Z, Freunberger SA, Hardwick LJ, Chen Y, Giordani V, et al. (2011) Oxygen reactions in a non-aqueous Li+ electrolyte. Angewandte Chemie (International Edition in English) 50: 6351–6355. [DOI] [PubMed] [Google Scholar]
- 28. Freunberger SA, Chen YH, Peng ZQ, Griffin JM, Hardwick LJ, et al. (2011) Reactions in the rechargeable lithium-O2 battery with alkyl carbonate electrolytes. Journal of the American Chemical Society 133: 8040–8047. [DOI] [PubMed] [Google Scholar]
- 29. McCloskey BD, Bethune DS, Shelby RM, Girishkumar G, Luntz AC (2011) Solvents’ critical role in nonaqueous lithium-oxygen battery electrochemistry. Journal of Physical Chemistry Letters 2: 1161–1166. [DOI] [PubMed] [Google Scholar]
- 30. Xiao J, Hu JZ, Wang DY, Hu DH, Xu W, et al. (2011) Investigation of the rechargeability of Li-O2 batteries in non-aqueous electrolyte. Journal of Power Sources 196: 5674–5678. [Google Scholar]
- 31. Xu W, Viswanathan VV, Wang D, Towne SA, Xiao J, et al. (2011) Investigation on the charging process of Li2O2-based air electrodes in Li-O2 batteries with organic carbonate electrolytes. Journal of Power Sources 196: 3894–3899. [Google Scholar]
- 32. Wang F, Graetz J, Moreno MS, Ma C, Wu L, et al. (2011) Chemical distribution and bonding of lithium in intercalated graphite: Identification with optimized electron energy loss spectroscopy. ACS Nano 5: 1190–1197. [DOI] [PubMed] [Google Scholar]
- 33. Chan MKY, Shirley EL, Karan NK, Balasubramanian M, Ren Y, et al. (2011) Structure of lithium peroxide. The Journal of Physical Chemistry Letters 2: 2483–2486. [Google Scholar]
- 34. Fister TT, Schmidt M, Fenter P, Johnson CS, Slater MD, et al. (2011) Electronic structure of lithium battery interphase compounds: Comparison between inelastic x-ray scattering measurements and theory. The Journal of Chemical Physics 135: 224513. [DOI] [PubMed] [Google Scholar]
- 35. Karan NK, Balasubramanian M, Fister TT, Burrell AK, Du P (2012) Bulk-sensitive characterization of the discharged products in Li–O2 batteries by nonresonant inelastic x-ray scattering. The Journal of Physical Chemistry C 116: 18132–18138. [Google Scholar]
- 36. Tsuji J, Nakamatsu H, Mukoyama T, Kojima K, Ikeda S, et al. (2002) Lithium K-edge XANES spectra for lithium compounds. X-Ray Spectrometry 31: 319–326. [Google Scholar]
- 37. Li Y, Wang J, Li X, Geng D, Banis MN, et al. (2012) Discharge product morphology and increased charge performance of lithium-oxygen batteries with graphene nanosheet electrodes: the effect of sulphur doping. Journal of Materials Chemistry 22: 20170–20174. [Google Scholar]
- 38. Cazaux J (1997) A physical approach to the radiation damage mechanisms induced by X-rays in X-ray microscopy and related techniques. Journal of Microscopy 188: 106–124. [Google Scholar]
- 39. Johnson PS, Cook PL, Liu X, Yang W, Bai Y, et al. (2011) Universal mechanism for breaking amide bonds by ionizing radiation. The Journal of Chemical Physics 135: 044702. [DOI] [PubMed] [Google Scholar]
- 40. Kim YS, Bostwick A, Rotenberg E, Ross PN, Hong SC, et al. (2010) The study of oxygen molecules on Pt (111) surface with high resolution x-ray photoemission spectroscopy. The Journal of Chemical Physics 133: 034501. [DOI] [PubMed] [Google Scholar]
- 41. Reininger R, Bozek J, Chuang Y-D, Howells M, Kelez N, et al. (2007) MERLIN – A meV Resolution Beamline at the ALS. AIP Conference Proceedings 879: 509–512. [Google Scholar]
- 42. Jia JJ, Callcott TA, Yurkas J, Ellis AW, Himpsel FJ, et al. (1995) First experimental results from IBM/TENN/TULANE/LLNL/LBL undulator beamline at the advanced light source. Review of Scientific Instruments 66: 1394–1397. [Google Scholar]
- 43. Föppl H (1957) Die Kristallstrukturen der Alkaliperoxyde. Zeitschrift für anorganische und allgemeine Chemie 291: 12–50. [Google Scholar]
- 44. Cota LG, de la Mora P (2005) On the structure of lithium peroxide, Li2O2 . Acta Crystallographica Section B 61: 133–136. [DOI] [PubMed] [Google Scholar]
- 45. Effenberger H, Zemann J (1979) Verfeinerung der Kristallstruktur des Lithiumkarbonates, Li2CO3 . Zeitschrift für Kristallographie - Crystalline Materials 150: 133–138. [Google Scholar]
- 46. Olovsson W, Tanaka I, Mizoguchi T, Puschnig P, Ambrosch-Draxl C (2009) All-electron Bethe-Salpeter calculations for shallow-core x-ray absorption near-edge structures. Physical Review B 79: 041102. [Google Scholar]
- 47. Shirley EL (1998) Ab initio inclusion of electron-hole attraction: Application to x-ray absorption and resonant inelastic x-ray scattering. Physical Review Letters 80: 794–797. [Google Scholar]
- 48. Rühl E, Hitchcock AP (1991) Oxygen K-shell excitation spectroscopy of hydrogen peroxide. Chemical Physics 154: 323–329. [Google Scholar]
- 49. Augustsson A, Herstedt M, Guo JH, Edstrom K, Zhuang GV, et al. (2004) Solid electrolyte interphase on graphite Li-ion battery anodes studied by soft x-ray spectroscopy. Physical Chemistry Chemical Physics 6: 4185–4189. [Google Scholar]
- 50. Cook PL, Johnson PS, Liu X, Chin A-L, Himpsel FJ (2009) Radiation damage in biomimetic dye molecules for solar cells. The Journal of Chemical Physics 131: 214702. [DOI] [PubMed] [Google Scholar]
- 51. Coffey T, Urquhart SG, Ade H (2002) Characterization of the effects of soft x-ray irradiation on polymers. Journal of Electron Spectroscopy and Related Phenomena 122: 65–78. [Google Scholar]