Abstract
An antibacterial protein (about 12 kDa) was isolated from human amniotic fluid through dialysis, ultrafiltration and C18 reversed-phase HPLC steps. Automated Edman degradation showed that the N-terminal sequence of the antibacterial protein was NH2-Ile-Gln-Arg-Thr-Pro-Lys-Ile-Gln-Val-Tyr-Ser-Arg-His-Pro-Ala-Glu-Asn-Gly-. The N-terminal sequence of the antibacterial protein was found to be identical to that of β2-microglobulin, a component of MHC class I molecules, which are present on all nucleated cells. Matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS) revealed that the molecular mass of the antibacterial protein was 11,631 Da. This antibacterial protein, β2M, possessed potent antibacterial activity against pathogenic bacteria. Specially, antibacterial activity was observed in potassium buffer, and potassium ion was found to be critical for the antibacterial activity. Interestingly, the antibacterial action of β2M was associated with dissipation of the transmembrane potential, but the protein did not cause damage to the membrane that would result in SYTOX green uptake. In addition, stimulation of WISH amniotic epithelial cells with the bacterial endotoxin lipopolysaccharide (LPS) induced dose-dependent upregulation of β2M mRNA expression. These results suggest that β2M contributes to a self-defense response when amniotic cells are exposed to pathogens.
Introduction
In humans, antimicrobial peptides and proteins are the first line of defense against bacteria, fungi and enveloped viruses [1], [2]. Amniotic fluid was recently reported to have antimicrobial properties [3], [4], and several antimicrobial peptides and proteins, including human neutrophil peptides 1, 2 and 3 [5], lysozyme [6], bactericidal/permeability-increasing protein (BPI) [7], LL-37 [6], calprotectin (MRP8/14) [8] and ubiquitin [9], have since been found in amniotic components. β2-microglobulin, which was isolated from human amniotic fluid (HAF) in the present study, is the noncovalently bound light chain of major histocompatibility (MHC) class I, an 11.6 kDa nonglycosylated protein found on the surface of all nucleated cells [10]. MHC class I molecules play an important role in alerting the immune system to the presence of virally infected cells. β2-microglobulin is an interesting and underutilized metabolite that can be used to assess renal function, particularly in kidney-transplant recipients and in patients suspected of having renal tubulo-interstitial disease [11]. It can also serve as a nonspecific but relatively sensitive marker for various neoplastic, inflammatory and infectious conditions [12]. Early hopes that β2-microglobulin would serve as the basis for a serum test for malignancy have not been fulfilled, but this molecule does appear to have prognostic value for patients with lymphoproliferative diseases, particularly multiple myeloma. In addition, recent studies have suggested that β2-microglobulin can be used as a prognostic marker in patients infected with human immunodeficiency virus (HIV) [13]. Still, the normal physiological functions of β2-microglobulin in vivo have not yet been elucidated. The findings presented here suggest that β2-microglobulin serves as an antibacterial agent in HAF.
In this report, we describe the isolation and characterization of an antibacterial protein, β2M, from HAF. This protein exhibited potent antibacterial activity against pathogenic microbial strains, and its sequence was identical to that of β2-microglobulin.
Results and Discussion
Purification of β2M protein
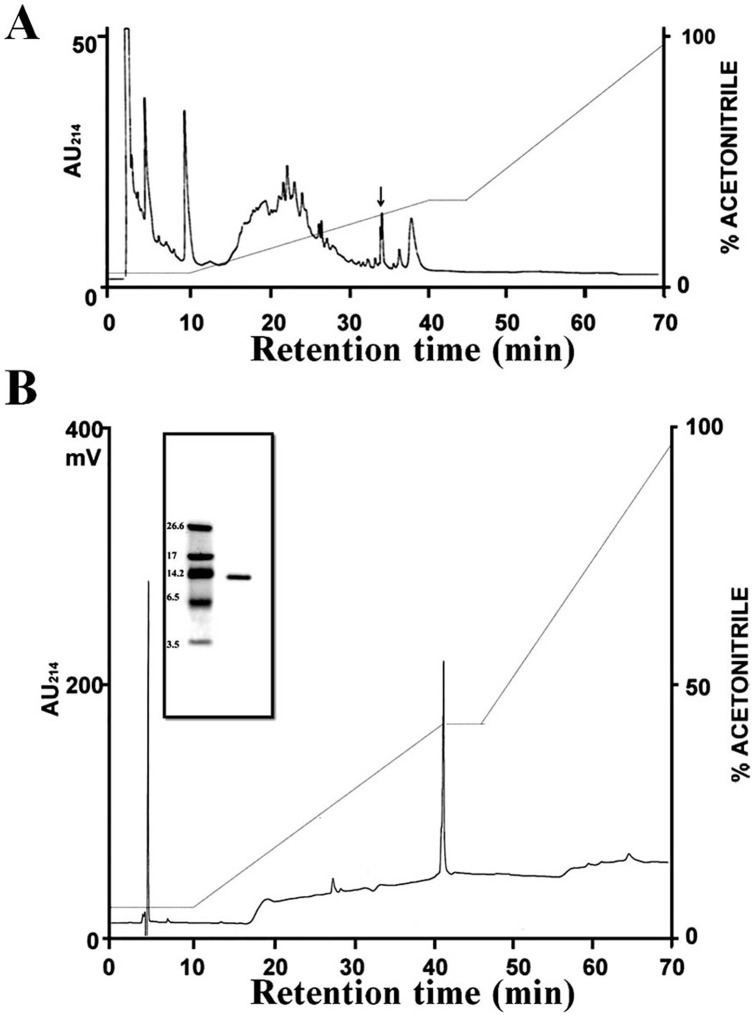
Following dialysis and ultrafiltration to remove urine and salts, HAF samples were applied to a RP-HPLC equipped with a C18 column (Fig. 1A). This dialysis process removes salts and small molecules coming from urine but not larger proteins such as β2M which has a mass of 11600. Thus the beta2M may come from amniotic fluid but could be contaminated with β2M from urine. Because the peak shown in Fig. 1A was found to have potent antibacterial activity against Listeria monocytogenes, this fraction was further purified using RP-HPLC with a delayed gradient program (Fig. 1B). The resultant purified protein was homogeneous, and its molecular weight was determined to be about 12 kDa using tricine SDS-PAGE (inset in Fig. 1B). Sequencing revealed that the N-terminal amino acid sequence of β2M is NH2-Ile-Gln-Arg-Thr-Pro-Lys-Ile-Gln-Val-Tyr-Ser-Arg-His-Pro-Ala-Glu-Asn-Gly-, which was 100% identical to that of β2-microglobulin [14]. Moreover, matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS) showed that β2M has a molecular mass of 11,631 Da, which is identical to the relative molecular weight calculated from the protein sequence of the peptide (11,631 Da).
Figure 1. RP-HPLC profiles and Tricine SDS-PAGE analysis of fractions >10 kDa.
Following dialysis and ultrafiltration steps, a representative HAF sample was injected into a HPLC system equipped with a Vydac C18 column (A). The indicated fraction (arrow) was subjected to a second RP-HPLC (B) and eluted using a gradient of acetonitrile in 0.1% TFA. The purified protein was analyzed by Tricine SDS-PAGE.
Antibacterial activity of β2M against antibiotic-susceptible and -resistant bacteria
The antibacterial activity of the purified protein was then assayed against L. monocytogenes and Escherichia coli, and was found to potently inhibit the growth of both organisms (Fig. 2). In addition, amino acid sequencing revealed this protein to be identical to β2-microglobulin, and its molecular mass indicated it to be the mature form of the protein. Although increases in the β2-microglobulin concentration in HAF and in adult and fetal serum have been observed under abnormal conditions, the molecular basis of these increases is still not known, nor is the physiological function of β2-microglobulin in humans. This study is thus the first to report on the antibacterial properties of β2-microglobulin and its expression in amniotic cells in response to pathogenic stimulation.
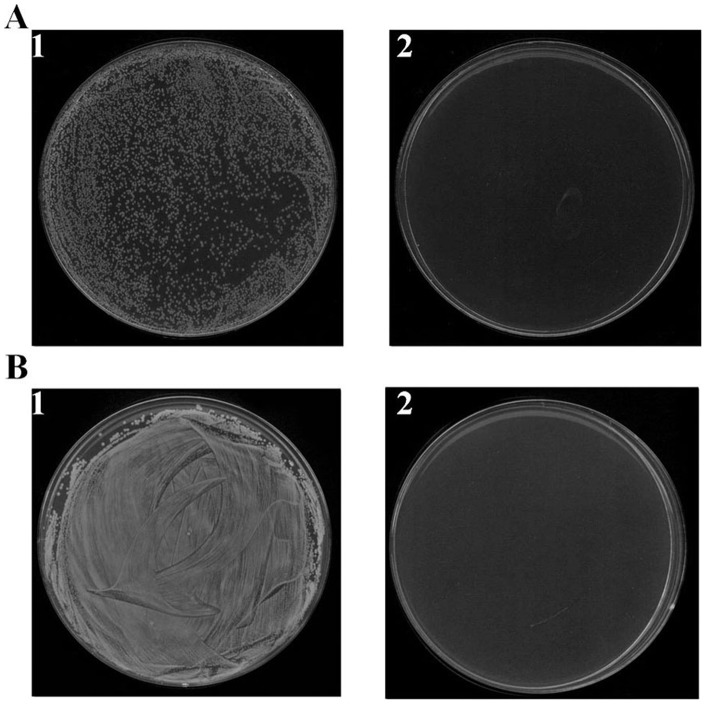
Figure 2. Antibacterial activity of purified β2M against L. monocytogenes (A) and E. coli (B).
Shown are representative cultures grown in the absence (1) and presence (2) of β2M (2.5 μg).
Determination of the minimum inhibitory concentrations (MICs) revealed that β2M more effectively inhibits the growth of antibiotic-susceptible strains (American Type Culture Collection (ACTC) and Korean Collection for Type Cultures (KCTC) strains) than resistant strains (Culture Collection of Antibiotics Resistance Microbes, CCARM strains) (Table 1). To assess the influence of ion salts on the growth-inhibiting activity of β2M, antibacterial assays were performed in four buffers, potassium or sodium phosphate (10 mM, pH 7.2), HEPES (10 mM, pH 7.2), and potassium phosphate containing 150 mM NaCl (10 mM, pH 7.2). Under these conditions, β2M showed potent antibacterial activity against antibiotic-susceptible bacteria, but not against antibiotic-resistant bacteria. Moreover, β2M significantly inhibited growth only in potassium phosphate buffer, indicating that potassium ion salts are essential to its antibacterial activity. Although the solute concentrations in HAF varies, depending on the stage of pregnancy and among individuals, they are reportedly about 130 mEq/L sodium, 4 mEq/L potassium, 110 mEq/L chloride, 3.4 mEq/L calcium and 3 mEq/L phosphorous [15]. Based on these values and the results of the present study, the antibacterial activity of β2M we observed in vitro would also be observed in natural HAF. Notably, β2M did not inhibit antibiotic-resistant strains. These strains often express a multidrug-resistance (MDR) efflux pump/transporter in their plasma membrane, which alters the membrane potential when activated. Our findings indicate that exposure to β2M in potassium ion salt led to alteration of the bacterial membrane potential, which is consistent with the presence of the MDR efflux pump/transporter.
Table 1. Minimal inhibitory concentrations of β2M against antibiotic-susceptible and resistant pathogens.
| Strains | MICs (µM) | |||
| Buffer Ia | Buffer IIb | Buffer IIIc | Buffer IVd | |
| S. aureus ATCC25923 | 2.4 | >2.4 | >2.4 | >2.4 |
| L. monocytogenes ATCC19115 | 0.3 | >2.4 | >2.4 | >2.4 |
| S. epidermidis KCTC1917 | 0.6 | >2.4 | >2.4 | >2.4 |
| E. coli ATCC25922 | 0.3 | >2.4 | >2.4 | >2.4 |
| P. vulgaris KCTC2433 | 0.3 | >2.4 | >2.4 | >2.4 |
| S. typhimurium KCTC1926 | >2.4 | >2.4 | >2.4 | >2.4 |
| S. aureus CCARM3114 | >10 | - | - | - |
| S.aureus CCARM3126 | >10 | - | - | - |
| E. coli CCARM1229 | >10 | - | - | - |
| E. coli CARM1238 | >10 | - | - | - |
Minimum inhibitory concentration in 10 mM potassium phosphate, pH 7.2.
Minimum inhibitory concentration in 10 mM HEPES, pH 7.2.
Minimum inhibitory concentration in 10 mM sodium phosphate, pH 7.2.
Minimum inhibitory concentration in 10 mM potassium phosphate containing 150 mM NaCl, pH 7.2.
ATCC strains were obtained from the American Type Culture Collection. KCTC strains were from the Korean Collection for Type Cultures. CCARM strains were from Seoul Women's University.
Mechanism of β2M antibacterial activity
To investigate the mechanism by which β2M inhibits the growth of bacterial cells, we used the membrane potential-sensitive fluorescent probe DiSC3-5 to assess the ability of β2M to induce membrane depolarization in antibiotic-susceptible (ATCC 25922) and -resistant E. coli (CCARM 1229). β2M-induced changes in membrane permeability leading to dissipation of the transmembrane potential were monitored by measuring increases in fluorescent emission caused by release of the membrane potential-sensitive dye DiSC3-5. As shown in Fig. 3A, the magnitude of the β2M-induced depolarization differed significantly between the two strains: whereas depolarization of E. coli CCARM 1229 was minimal, even at a β2M concentration of 1.2 μM, maximum depolarization of E. coli ATCC 1229 was detected at 0.3 μM β2M, which was the MIC for β2M with both strains. These results do not confirm that β2M interacts with or binds to the cell membrane, but suggest that potassium ion is a unique factor involved in the antibacterial activity of β2M. To determine whether β2M influences membrane permeability, the influx of SYTOX green into the cytosol of bacterial cells was measured after adding β2M to the cells at 1, 5 or 10 times the MIC. When the cell membrane is disrupted or permeabilized by an exogenous agent, SYTOX green dye enters and binds to intracellular nucleic acids, resulting in an increase in the fluorescence [16]. Therefore, β2M-induced increases in SYTOX fluorescence, reflecting the binding of the dye to intracellular DNA, were monitored (excitation wavelength: 485 nm, emission wavelength: 520 nm). For example, melittin, a membranolytic peptide, caused a significant influx of SYTOX green. On the other hand, β2M did not induce an influx of dye into the cells, even at 10 times the MIC (Fig. 3B). Thus, although the antibacterial action of β2M is mediated through dissipation of the membrane potential, β2M does not appear to damage the bacterial cell membrane.
Figure 3. Effect of β2M on bacterial membrane potential.
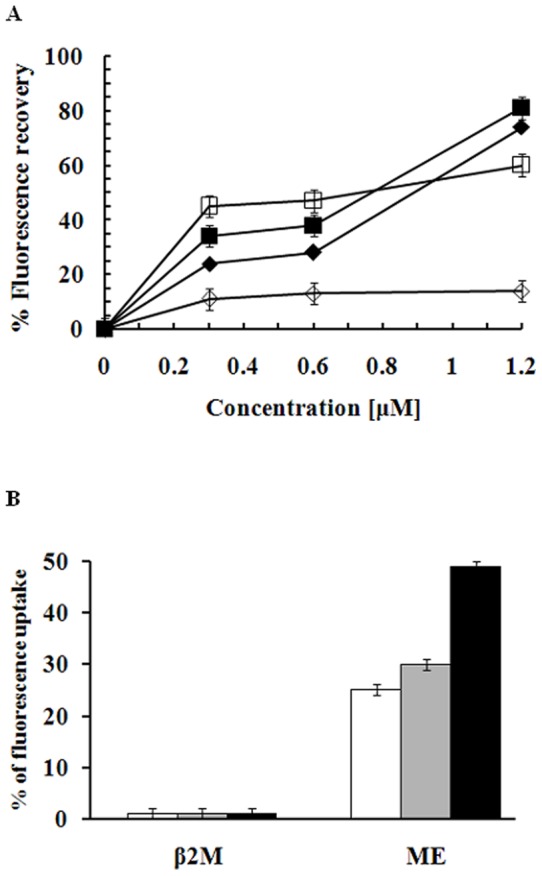
Bacteria (E. coli ATCC25922, E. coli CCARM1229) grown to mid-logarithmic phase were pre-equilibrated for 60 min with disC3-5, a voltage-sensitive fluorescent dye. β2M was then added to cell suspensions at various concentrations, and changes in fluorescence were recorded. Black, E. coli ATCC25922; white, E. coli CCARM1229; diamonds, β2M; squares, melittin (A). The influx of SYTOX green into E. coli ATCC25922 cells after addition of β2M and melittin was monitored at an excitation wavelength of 485 nm and an emission wavelength of 520 nm. The maximum increase in uptake was determined using 1% triton X-100 (B).
Induced β2M mRNA expression in WISH amniotic epithelial cells
To determine whether the level of β2M expression increases when WISH amniotic epithelial cells are exposed to pathogens, we used RT-PCR to assess its mRNA expression in cells stimulated with bacterial endotoxin LPS. Quantitative analysis following exposure to LPS (0, 10, 50, 100, 200, and 500 ng/ml) revealed a remarkable dose-dependent increase in β2M mRNA. By contrast, the housekeeping gene GAPDH was constitutively expressed at the same level, irrespective of the LPS concentration (Fig. 4A). Correspondingly, LPS also elicited concentration-dependent increases in the secretion of β2M protein (Fig. 4B). These findings further confirm that β2M contributes to the defense against pathogens.
Figure 4. LPS-induced expression and secretion of β2M mRNA (A) and protein (B) in cultured WISH cells.

WISH amniotic epithelial cells were cultured at a density of 2×105 cells/well for 24 h and then stimulated with LPS (0, 10, 50, 100, 200, 500 ng/ml). GAPDH served as a control in (A). Levels of secreted β2M were monitored using an ELISA, based on the 450 nm fluorescence (B).
In conclusion, the results of the present study suggest that the antibacterial activity of β2-microglobulin in HAF occurs via dissipation of the membrane potential of bacterial pathogens, and that potassium is an important factor in that reaction. In addition, β2-microglobulin appears to be upregulated in amniotic cells during bacterial infection.
Materials and Methods
Human amniotic fluid
This study was approved by the institutional ethics committee, and all pregnant women provided written informed consent before treatment. Samples of amniotic fluid were obtained from 10 pregnant women in their third trimester. The samples were immediately centrifuged, and the supernatants were sterilized by filtration through a disposable membrane filter (pore size = 0.2 µm, Millipore), after which they were divided into aliquots and stored at −80°C until needed.
Purification and mass spectrometry of antibacterial proteins
Samples of HAF were dialyzed against 10 mM ammonium acetate buffer (pH 6.0) to remove urine and salts, after which the dialysates were subjected to ultrafiltration using a membrane with a 10,000 Da molecular weight cut-off. Aliquots of the ultrafiltrates were applied to a RP-C18 column (5 μm, 300 Å, 4.6×250 mm, Vydac, Hesperia, CA) and separated using a 10–60% acetonitrile gradient for 50 min at a flow rate of 1 ml/min. The effluent was monitored by measuring the absorbance at 214 nm, and the peak fractions were assayed for antibacterial activity. The indicated fraction (arrow) was showed most antibacterial activity and only the peak was subjected to a second RP-HPLC. To obtain highly purified homogenous proteins, peak fraction was reanalyzed using a delayed program, an RP-C18 column (5 μm, 300 Å, 2.1×150 mm, Vydac, Hesperia, CA) and a slower flow rate (0.2 ml/min). The purity of the purified peptide was assessed using 16.5% Tricine SDS-PAGE and analytical reversed-phase HPLC [9].
The amino-terminal amino acid sequence of the purified protein was analyzed using automated Edman degradation on a pulse liquid automatic sequencer (Applied Biosystems Inc., model 473A) in the Sequence Centre at the Korea Basic Science Institute (Seoul, Korea). Matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS) was carried out in the linear mode using a Voyager DE RP instrument (Perseptive Biosystems, Framingham, MA) as described by Pouvreau et al. [17].
Microbial strains
Streptococcus aureus (ATCC 259231), Listeria monocytogenes (ATCC 19115) and Escherichia coli (ATCC 25922) were obtained from the American Type Culture Collection. Staphylococcus epidermidis (KCTC 1917), Proteus vulgaris (KCTC 2434) and Salmonella typhimurium (KCTC 1926) were from the Korean Collection for Type Cultures. Staph. aureus CCARM 3114, Staph. aureus CCARM 3126, E. coli CCARM 1229 and E. coli CCARM 1238 were distributed from the Culture Collection of Antibiotic-Resistant Microbes at the Seoul Women's University, Korea.
Antibacterial activity
The bacteria were grown to mid-logarithmic phase in medium containing (g/l) 10 bactotryptone, 5 yeast extract and 10 NaCl (pH 7.0). The isolated protein was diluted stepwise in 1% bactopeptone medium. The tested organism (final bacterial suspension: 5×103 CFU/ml) suspended in growth medium (100 µl) was then mixed with 100 µl of test peptide solution in a microtiter plate well. Each test solution was examined in triplicate. Microbial growth was determined based on the increase in optical density at 620 nm after incubation for 10 h at 37°C [13].
Protein quantification and N-terminal sequencing
The protein concentration was determined using the BCA protein assay method [18] with bovine serum albumin as the standard. All protein assays were conducted in triplicate. After homogenous β2M was subjected to Tricine-SDS-PAGE, the protein was electroblotted (200 mA for 2 h) onto a polyvinylidene fluoride (PVDF) membrane with a pore size of 0.2 μM. The β2M band used for sequencing was cut out of the membrane and air-dried, after which the N-terminal amino acid sequence was determined based on Edman degradation [19] on an amino acid sequencer (Applied Biosystems Inc., Model 473A). Finally, the sequence was compared with sequences in the NCBI database using BLAST.
Transmembrane potential depolarization
Membrane depolarization in E. coli ATCC 25922 and CCARM 1229 cells was assessed using DiSC3-5 as described previously [20]. Each treatment protocol was replicated 3 times during the experiment.
SYTOX green uptake assay
E. coli cells were washed and suspended (2×107 cells/ml) in PBS, after which SYTOX green (Molecular probes) was added to a final concentration of 1 μM, and the cells were incubated at 37°C for 15 min with agitation in the dark [21]. Thereafter, β2M and melittin were added, and the increase in fluorescence was monitored at an excitation wavelength of 485 nm and an emission wavelength of 520 nm. Each treatment group was assayed in triplicate.
Expression of β2M in amniotic cells
WISH cells, which are a human amniotic epithelial cell line, were obtained from the Korean Cell Line Bank (Seoul, Korea) and grown at 37°C in DMEM supplemented with 10% FBS and 100 U/ml penicillin/streptomycin. The cells were then plated at 2×105 cells/well in a 12-well culture plate in the same media. To evaluate production of β2M in response to bacterial stimulation, lipopolysaccharide (LPS) from E. coli 255:B5 was added, and the cells were incubated for 24 h [22]. The cells were then washed twice with PBS, and the total RNA was extracted using TRI reagent for later use as a template in RT-PCR. The cDNA products were amplified using the primer pairs, 5′- GGATCCATCCAGCGTACTCCAAAGATTCA-3′ and 5′-CTCGAGTTACATGTCTCGATCCCACTTA-3′ for β2M and 5′-CCATCAACGACCCCTTCATTGAC-3′ and 5′-GGATGACCTTGCCCACAGCCTTG-3′ for glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Finally, the PCR-amplified products were electrophoresed on 1% agarose gel [23].
To estimate production of β2M protein, amnion epithelial WISH cells were cultured at 2×105 cells/well for 24 h and then stimulated with LPS from E. coli 255:B5 (0, 10, 50, 100, 200, 500 ng/ml). Thereafter, the increase in β2M was monitored using an ELISA, based on changes in 450 nm fluorescence. Each treatment group was assayed in triplicate.
Funding Statement
This work was supported by the National Research Foundation of Korea grant funded by the Korea government (MEST) (No. 2011-0017532). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Yang D, Chertov O, Oppenheim JJ (2001) Participation of mammalian defensins and cathelicidins in anti-microbial immunity: receptors and activities of human defensins and cathelicidins (LL-37). J Leukoc Biol 69: 691–697. [PubMed] [Google Scholar]
- 2. Zanetti M, Gennaro R, Romeo D (1995) Cathelicidins: a novel protein family with a common preregion and a variable C-terminal antimicrobial domain. FEBS Lett 374: 1–5. [DOI] [PubMed] [Google Scholar]
- 3. Miller J, Michel J, Bercovici B, Arqaman M, Sacks T (1976) Studies on the antimicrobial activity of amniotic fluid. Am J Obstet Gynecol 125: 212–214. [DOI] [PubMed] [Google Scholar]
- 4. Galask RP, Snyder IS (1970) Antimicrobial factors in amniotic fluid. Am J Obstet Gynecol 106: 59–65. [DOI] [PubMed] [Google Scholar]
- 5. Spitznagel JK (1990) Antibiotic proteins of human neutrophils. J Clin Invest 86: 1381–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoshio H, Tollin M, Gudmundsson GH, Lagercrantz H, Jornvall H, et al. (2003) Antimicrobial polypeptides of human vernix caseosa and amniotic fluid: Implications for newborn innate defense. Pediatr Res 53: 211–216. [DOI] [PubMed] [Google Scholar]
- 7. Elsbach P, Weiss J (1993) The bactericidal/permeability-increasing protein, a potent element in host-defense against gram-negative bacteria and lipopolysaccharide. Immunobiology 187: 417–429. [DOI] [PubMed] [Google Scholar]
- 8. Lehrer RI (1993) Holocrine secretion of calprotin: a neutrophil-mediated defense against Candida albicans? J Lab Clin Med 121: 193–194. [PubMed] [Google Scholar]
- 9. Kim J-Y, Lee SY, Park S-C, Shin SY, Choi SJ, et al. (2007) Purification and antimicrobial activity studies of the N-terminal fragment of ubiquitin from human amniotic fluid. Biochim Biophys Acta 774: 1221–1226. [DOI] [PubMed] [Google Scholar]
- 10. Bjorkman PJ, Saper MA, Samraoui B, Bennett WS, Strominger JL, et al. (1987) Structure of the human class 1 histocompativility antigen, HLA-A2. Nature 329: 506–512. [DOI] [PubMed] [Google Scholar]
- 11. Cooper EH, Plesner T (1980) Beta-2-microglobulin review: its relevance in clinical oncology. Med Pediatr Oncol 23: 247–249. [DOI] [PubMed] [Google Scholar]
- 12. Xie J, Yi Q (2003) β2-Microglobulin as a potential initiator of inflammatory responses. Trends Immunol 24: 228–229. [DOI] [PubMed] [Google Scholar]
- 13. Park SC, Kim MH, Hossain MA, Shin SY, Kim Y, et al. (2008) Amphipathic α-helical peptide, HP (2–20), and its analogues derived from Helicobacter pylori: Pore formation mechanism in various lipid compositions. Biochim Biophys Acta 1778: 229–241. [DOI] [PubMed] [Google Scholar]
- 14. Valueva TA, Revina TA, Mosolov VV, Mentele R (2000) Primary structure of potato Kunitz-type serine proteinase inhibitor. Biol Chem Hoppe-Seyler 381: 1215–1221. [DOI] [PubMed] [Google Scholar]
- 15. Oliveira FR, Barros EG, Magalhães JA (2002) Biochemical profile of amniotic fluid for the assessment of fetal and renal development. Braz J Med Biol Res 35: 215–522. [DOI] [PubMed] [Google Scholar]
- 16. Roth BL, Poot M, Yue ST, Millaard PJ (1997) Bacterial viability and antibiotic susceptibility testing with SYTOX green nucleic acid stain. App Environ Microbiol 63: 2421–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pouvreau L, Gruppen H, Piersma SR, van den Broek LAM, van Koningsveld GA, et al. (2001) Relative abundance and inhibitory distribution of protease inhibitors in potato juice from cv. Elkana. J Agric Food Chem 49: 2864–2874. [DOI] [PubMed] [Google Scholar]
- 18. Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, et al. (1985) Measurement of Protein Using Bicinchoninic Acid. Anal Biochem 150: 76–85. [DOI] [PubMed] [Google Scholar]
- 19. Niall HD (1973) Automated Edman degradation: the protein sequenator. Methods Enzymol 27: 942–1010. [DOI] [PubMed] [Google Scholar]
- 20. Papo N, Oren Z, Pag U, Sahi HG, Shai Y (2002) The consequence of sequence alteration of an amphipathic α-Helical antimicrobial peptide and its diastereomers. J Biol Chem 37: 33913–33921. [DOI] [PubMed] [Google Scholar]
- 21. Mangoni ML, Papo N, Barra D, Simmaco M, Bozzi A, et al. (2004) Effects of the antimicrobial peptide temporin L on cell morphology, membrane permeability and viability of Escherichia coli . Biochem J 380: 859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim HS, Cho JH, Park HW, Yoon H, Kim MS, et al. (2002) Endotoxin-neutralizing antimicrobial proteins of the human placenta. J Immunol 168: 2356–2364. [DOI] [PubMed] [Google Scholar]
- 23. Resenfeld Y, Papo N, Shai Y (2006) Endotoxin (lipopolysaccharide) neutralization by innate immunity host-defense peptides. J Biol Chem 281: 1636–1643. [DOI] [PubMed] [Google Scholar]