Abstract
Changes in the respiratory rate and the contribution of the cytochrome (Cyt) c oxidase and alternative oxidase (COX and AOX, respectively) were investigated in soybean (Glycine max L. cv Stevens) root seedlings using the 18O-discrimination method. In 4-d-old roots respiration proceeded almost entirely via COX, but by d 17 more than 50% of the flux occurred via AOX. During this period the capacity of COX, the theoretical yield of ATP synthesis, and the root relative growth rate all decreased substantially. In extracts from whole roots of different ages, the ubiquinone pool was maintained at 50% to 60% reduction, whereas pyruvate content fluctuated without a consistent trend. In whole-root immunoblots, AOX protein was largely in the reduced, active form at 7 and 17 d but was partially oxidized at 4 d. In isolated mitochondria, Cyt pathway and succinate dehydrogenase capacities and COX I protein abundance decreased with root age, whereas both AOX capacity and protein abundance remained unchanged. The amount of mitochondrial protein on a dry-mass basis did not vary significantly with root age. It is concluded that decreases in whole-root respiration during growth of soybean seedlings can be largely explained by decreases in maximal rates of electron transport via COX. Flux via AOX is increased so that the ubiquinone pool is maintained in a moderately reduced state.
The rate of plant respiration is linked to the rate of metabolism and growth due to requirements for ATP, reductant, and carbon skeletons during cell maintenance, division, and expansion (Hunt and Loomis, 1979; Lambers et al., 1983). For example, respiration rates are often lower in species with intrinsically slower growth rates (Poorter et al., 1991). Moreover, respiration is rapid in tissues with high energy demands, such as thermogenic floral spadices (Meeuse, 1975), and in rapidly growing tissues, such as the elongation zone of roots (Lambers et al., 1996). Plant respiration can also increase rapidly in response to both biotic and abiotic stress (for a recent review, see Lambers et al., 1996). Conversely, decreases in respiratory rate often occur as plant tissues age (Azcon-Bieto et al., 1983; McDonnell and Farrar, 1993; Atkin and Cummins, 1994; Winkler et al., 1994). Various factors may be responsible for these changes, including substrate availability, enzyme activation, specific protein degradation or de novo protein synthesis, and alterations in mitochondrial numbers.
The extent to which such changes in respiration rate alter the rate of oxidative phosphorylation also depends on the partitioning of electron flux between the Cyt and the alternative pathways of electron transport. The Cyt pathway (terminating at COX) couples the reduction of O2 to water with the translocation of protons across the inner mitochondrial membrane, thereby building a proton-motive force that drives ATP synthesis. The alternative pathway branches directly from Q and reduces O2 to water without further proton translocation. This pathway appears to consist of a single-subunit cyanide-resistant quinol oxidase, AOX. Electron flow via AOX in plants can allow carbon flux through the TCA cycle when ADP is limiting, thereby providing carbon skeletons for other cellular processes (Lambers and Steingröver, 1978). This pathway may also protect against harmful reactive O2 generation when the Q pool is highly reduced (Purvis and Shewfelt, 1993; Wagner and Krab, 1995), allow respiration to proceed in the presence of nitric oxide (Millar and Day, 1996), and help avoid the production of fermentation products when pyruvate accumulates (Vanlerberghe et al., 1995).
Partitioning between COX and AOX can be dramatically affected by factors that influence the AOX activation state (Hoefnagel et al., 1995; Ribas-Carbo et al., 1995a, 1997). AOX exists as a dimer in plants, and sulfhydryl linkages between paired subunits must be reduced for maximal AOX activity (Umbach and Siedow, 1993). A variety of 2-oxo acids, notably pyruvate, have been shown to specifically and reversibly stimulate AOX activity at micromolar concentrations (Millar et al., 1993, 1996). These activators apparently increase the Vmax of AOX and may prevent inhibition by oxidized Q (Hoefnagel et al., 1997). Because of these regulatory features, the previous use of inhibitor titrations to estimate the partitioning between respiratory pathways in vitro and in vivo has been largely discredited (Millar et al., 1995; Day et al., 1996). A noninvasive technique using differences in discrimination against 18O has now been developed to measure partitioning between the Cyt and alternative pathways in whole-plant tissues and isolated mitochondria (Guy et al., 1992; Robinson et al., 1992; Ribas-Carbo et al., 1995a).
In this report ontogenetic changes in whole-root respiration and the partitioning between COX and AOX are investigated using the 18O-discrimination technique. Respiratory changes of whole roots were measured in conjunction with the redox poise of the Q pool and of the AOX protein in whole-root extracts. These were then compared with the kinetics of Q-oxidizing and -reducing pathways and the abundance of terminal oxidase proteins in isolated mitochondria. This information is used to identify factors influencing root respiration during growth and differentiation.
MATERIALS AND METHODS
Reagents
Percoll was purchased from Pharmacia and Folin and Ciocalteau's reagents were purchased from BDH Chemicals (Melbourne, Australia). All other reagents were purchased from Sigma.
Plant Culture and Organelle Isolation
Roots were harvested from soybean (Glycine max L. cv Stevens) seedlings propagated in trays of vermiculite in a growth cabinet at 28/25°C with a 16-h light/8-h dark cycle. At d 4 the cotyledons and hypocotyls were greening and the root system (approximately 150 mg fresh mass/seedling) consisted of a single taproot without branches. At d 7 cotyledons were green and beginning to open, and the primary leaf was expanding. The primary root (approximately 300 mg fresh mass/seedling) had developed branches at the base in a classic taproot structure. At d 17 cotyledons were fully open and slightly yellowing, primary leaves were fully expanded, and the first trifoliate leaf was expanding. The root system (approximately 600 mg fresh mass/seedling) was a network of first- and second-order branches. Published methods were used to isolate mitochondria from roots of 4-, 7-, and 17-d-old seedlings (Day et al., 1985).
Mitochondrial Assays
O2 consumption was measured at 25°C using an electrode (Rank Brothers, Cambridge, UK). A standard reaction medium (0.3 m Suc, 10 mm TES (N-tris[hydroxymethyl]methyl-2-aminoethane sulfonic acid) buffer, [pH 7.2], 5 mm KH2PO4, 10 mm NaCl, 2 mm MgSO4, 0.1% [w/v] BSA) was assumed to contain an air-saturated O2 concentration of 240 μm. Assays containing succinate also included 0.1 mm ATP to activate succinate dehydrogenase. The redox state of Q was measured voltametrically with glassy carbon and platinum electrodes according to the method of Moore et al. (1988). COX activity was measured as Cyt c-dependent O2 consumption sensitive to 0.5 mm KCN in the presence of 0.05% (w/v) Triton X-100 and 5 mm ascorbate. Whole tissues were frozen in liquid N2, ground to a fine powder with sand, and resuspended in mitochondria-grinding buffer (Day et al., 1985) supplemented with 0.05% (w/v) Triton X-100, and the filtrate was used for COX assays. Significant ascorbate-dependent O2 consumption was observed in whole-tissue extracts in the absence of added Cyt c, but this activity was not affected by 0.5 mm KCN. This value was subtracted from that in the presence of Cyt c to provide the rate of COX activity. In isolated mitochondria endogenous ascorbate-dependent O2 consumption was negligible. Protein content was determined by the method of Lowry et al. (1951). NAD-ME activities were assayed as NADH production at 340 nm, according to the method of Day et al. (1984), in a reaction medium consisting of 2 mm NAD+, 2 mm MnCl2, 4 mm DTT, 0.02% (v/v) Triton TX-100, 1 μm antimycin A, 50 μm n-propyl gallate, and 50 mm Mes/1,3-bis[Tris(hydroxymethyl)-methylamino]propane, pH 6.5.
Whole-Tissue Pyruvate Content
Root samples (1–1.5 g) were snap frozen in liquid N2, ground to a powder, thoroughly mixed with 6 mL of 6% perchloric acid, and kept in ice for 10 min. Extracts were then centrifuged for 5 min at 9500g and the supernatant filtered and neutralized with KOH. After removal of precipitate, aliquots were assayed for lactate dehydrogenase (10 units)-dependent NADH oxidation at 340 nm in a solution of 0.1 mm NADH, 50 mm Hepes, pH 7.5.
On-Line δ18O Measurements on Intact Tissues
Whole root systems (5–10 g fresh weight) were placed in a water-jacketed, 50-mL, adjustable-volume (but note that the volume was constant throughout the experiment) closed cuvette maintained under darkness at 25°C, either directly after harvest or after prior treatment with inhibitors. A constant supply of gaseous HCN was formed in the chamber from a 1 m swab of KCN; SHAM was administered by soaking tissue in 20 mm SHAM for 20 min and lightly patting dry before placing in the cuvette. Treatment with both inhibitors together decreased O2 consumption to 10% to 15% of the control values. The cuvette was connected through a two-way valve (Valco, Sydney, Austalia) to a 125-μL sample loop and to a 5-mL syringe to facilitate gas mixing before sampling. In one position, the two-way valve connected the cuvette and the sample loop; in the other position, the valve connected the sample loop to a pressure-regulated He (ultra-high purity) carrier gas line. The time between withdrawal of successive samples from the reaction chamber was 270 s.
The sample gas stream flowed through a water/CO2 trap to the GC column of a NA 1500 elemental analyzer (Strumentazione, Milan, Italy) and through an open split to an Isochrom-EA mass spectrometer (Micromass UK, Ltd., Manchester, UK). O2 and N2 were separated by a 2-m × 6-mm o.d., 4-mm i.d. molecular sieve MS 5-A column (Alltech, Sydney, Australia) maintained at 40°C. The O2 and N2 were detected using a thermal conductivity detector and integrated using OS/2.2.1 Isochrom-EA software (Micromass UK, Ltd.). The isotope ratio 18O/16O was measured as the ratio of masses 34 to 32. D values were determined from slopes of ln(R/Ro) versus −ln(f) plots, according to the method of Guy et al. (1989). The reproducibility of measurement of the isotope ratio of O2 in the empty chamber was ± 0.01 to 0.1‰. The cuvette was tested for leaks by filling with He, closing the cuvette, and sampling; no leaks were detected. Leaks were also tested for during an experiment by plotting ln(R/Ro) against −ln(f) and looking at the slope; in the presence of a leak, one would expect to see a flattening of the curve as the contribution of air, and hence 16O, leaking into the cuvette became more significant as the tissue respiration depleted the O2. We did not observe this, and the linear regressions for these fits, from 7 to 12 points per curve, had r2 values of 0.94 to 0.99 even when the O2 concentration was very low toward the end of an experiment.
Analysis of Q-Pool Reduction
The extraction of Q from intact root material was conducted according to a modified version of a procedure described by Wagner and Wagner (1995). Approximately 1 g of whole soybean root systems was immersed in liquid N2 and crushed to a fine powder with a mortar and pestle. Extracts were freeze dried overnight under a vacuum. This step removed the root aqueous phase and decreased the possibility of Q oxidation during organic extraction. Dried samples were vortexed for 3 min in a mixture of 1.5 mL of methanol (containing 0.2 m perchloric acid) and 1.5 mL of petroleum ether (35–50°C boiling point, D = 0.64). After centrifugation at 1000g for 3 min to separate the phases, the upper phase was evaporated to dryness under N2. Additional petroleum ether was added to the lower phase, the earlier steps repeated, and the upper phases combined. The extraction and drying was performed under safe lights (Ilford S902, Kodak GBX-2) in a darkroom to prevent light-dependent formation of semiquinone species. Dried samples were dissolved in 150 to 300 μL of methanol containing 1 mm HCl, purged with N2, and passed through a 0.22-μm filter before injection onto an HPLC column. A 250- × 4.6-mm reverse-phase C18 Alltima column (Alltech, Sydney, Australia) was used with an LKB-Pharmacia system with an isocratic mobile phase of ethanol:methanol (7:3, v/v) under N2 at a flow rate of 1 mL min−1. The effluent from the column was monitored continuously at 275 and 290 nm. Retention times of reduced and oxidized Q9 and Q10 (Sigma) standards were determined for comparison with root extracts. The retention times were 8.5 and 13.9 min for reduced and oxidized Q9, and 10.9 and 18 min for reduced and oxidized Q10. Both Q homologs were reduced to their respective QH2 according to the method of Rich (1978). The extinction coefficients for reduced and oxidized Q homologs at 275 and 290 nm in standard solutions were determined spectrally, according to the method of Crane and Barr (1971), and used to determine the ratio of reduced and oxidized Q in root extracts.
Electrophoresis and Immunological Probing
For purified mitochondria, aliquots containing 40 μg of protein were solubilized in sample buffer (2% [w/v] SDS, 62.5 mm Tris-HCl [pH 6.8], 10% [v/v] glycerol, 0.002% [w/v] bromphenol blue, and 50 mm DTT) and boiled for 1 to 2 min. For whole-tissue extracts, approximately 250 mg fresh weight of soybean roots was snap frozen in liquid N2, glass beads (80 mesh) were added, and the sample was crushed to a fine powder with a mortar and pestle. Samples were then solubilized in 400 μL of standard sample buffer, boiled for 5 min, and centrifuged at 10,000g for 5 min; 20 μL of the supernatant was loaded per lane for SDS-PAGE. For oxidation of samples, 5 mg of diamide (azobis-dimethyl formamide) in 200 μL of 50 mm Tris-HCl, pH 7.0, was added to powdered root samples, and the mixture was allowed to thaw and incubate at room temperature for 5 min before addition of sample buffer. Whole-root extracts were supplemented with 1 mm PMSF, 1 mm paminobenzamidine, and 5 μm trans-epoxysuccinyl-l-leucylamide(4-guanidino)-butane to inhibit proteases.
Proteins were separated by SDS-PAGE as described by Kearns et al. (1992). A modified version of the method of Towbin et al. (1979) was used for immunoblotting. The probes were the AOA monoclonal antibody raised against AOX proteins from Voodoo lily (Sauromatum guttatum; generously supplied by T.E. Elthon, University of Nebraska, Lincoln, and by L. McIntosh, Michigan State University, East Lansing) and a monoclonal antibody raised against human COX subunit I (Molecular Probes, Eugene, OR). Immunoreactive proteins were visualized using a chemiluminescence system (Boehringer Mannheim) for isolated mitochondria blots, and the Super-Signal Ultra chemiluminescence system (Pierce) for whole-tissue extract blots.
RESULTS
Respiration and Growth of Soybean Roots
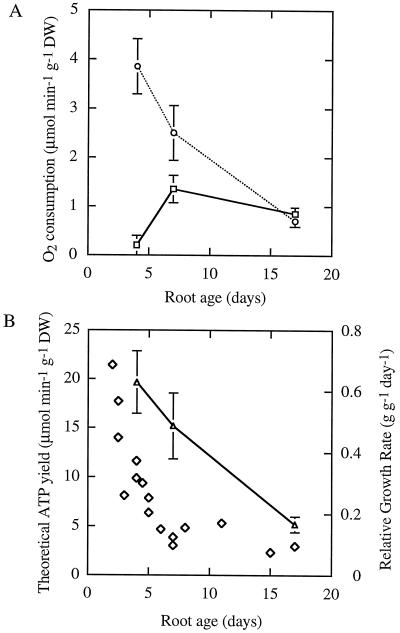
The rate of O2 consumption by whole soybean root systems declined by 60% on a dry-mass basis from d 4 to d 17 of seedling development (Table I). The 18O fractionation of respiration increased from 16.4‰ at d 4 to 20.5‰ at d 17, whereas the 18O fractionation via COX (plus SHAM) or AOX (plus KCN) operating alone remained unchanged at the three root ages tested (Table I). This indicates that a change in electron partitioning away from the Cyt pathway and toward the alternative pathway of mitochondrial O2 consumption occurred during root growth. Calculation of the contribution of each pathway to total respiration, using the D values in Table I, shows that the percentage of respiration via AOX increased from 5% on d 4 to 35% on d 7, and finally reached 55% on d 17. Presentation of this data on a dry-mass basis shows that part of the change in percentage respiration via AOX was caused by a decline in the respiration rate via COX, although AOX activity per se also increased (Fig. 1A). This is consistent with the age-dependent decrease in SHAM-insensitive respiration rate in whole roots (Table I).
Table I.
Respiration rates and 18O-discrimination values of whole soybean root respiration, first in the absence of inhibitors and then in the presence of KCN or SHAM in 4-, 7-, and 17-d-old roots
| Parameter | d 4 | d 7 | d 17 |
|---|---|---|---|
| Respiration rate | μmol O2 min−1 g−1 dry mass | ||
| Control | 4.05 ± 0.40 | 3.8 ± 0.50 | 1.55 ± 0.14 |
| +KCN | 2.70 ± 0.35 | 2.60 ± 0.30 | 1.07 ± 0.09 |
| +SHAM | 3.80 ± 0.35 | 2.40 ± 0.10 | 0.87 ± 0.13 |
| D 18O fractionation | 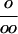 |
||
| Control | 16.4 ± 0.07 | 19.1 ± 0.45 | 20.5 ± 0.40 |
| +KCN | 24.5 ± 1.0 | 24.6 ± 0.85 | 24.2 ± 0.09 |
| +SHAM | 16.0 ± 0.25 | 16.3 ± 0.10 | 16.2 ± 0.03 |
Data are means ± se (n = 3).
Figure 1.
A, O2 consumption via COX (○) and AOX (□) in intact roots from 4- to 17-d-old soybean seedlings based on the 18O- discrimination values of Table I. B, The theoretical ATP yield via oxidative phosphorylation (▵) and the relative growth rate (⋄) of soybean roots of the same ages. DW, Dry weight.
Calculation of the theoretical ATP yield of mitochondrial oxidative phosphorylation showed a nearly 4-fold decline in the rate of ATP synthesis in roots from 4- to 17-d-old seedlings (Fig. 1B) Several assumptions underlie these estimations. We did not know the precise mixture of the substrates oxidized in vivo but assumed that matrix NADH was the main substrate and set an ADP/O2 ratio of 2.5 via COX, and 1 via AOX, based on ADP/O2 values determined for isolated soybean cotyledon mitochondria (Day et al., 1988). Whatever the real values, it is obvious that the ATP yield will decline as the respiration rate declines with age, and Figure 1B serves to illustrate this trend. The relative growth rate of the soybean root system declined nearly 7-fold during development, from 0.67 g g−1 d−1 in 2-d-old seedlings to 0.095 g g−1 d−1 in 17-d-old seedlings (Fig. 1B).
Redox Poise of the Q Pool in Vivo
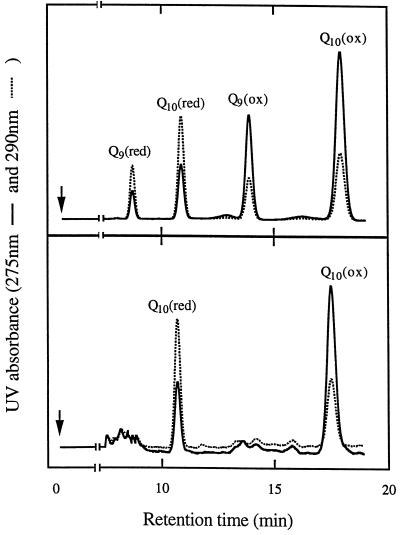
Organic extraction and reverse-phase HPLC separation were used to determine the redox poise of the Q pool in snap-frozen soybean roots. Comparison of root extracts with commercial Q samples revealed that the Q10 homolog was the predominant form of Q present in soybean roots (Fig. 2), in agreement with studies on isolated soybean root mitochondria (Ribas-Carbo et al., 1995b; Millar et al., 1997). The oxidized and reduced forms of Q10 were differentiated by retention time and their extinction coefficients at 275 and 290 nm.
Figure 2.
Typical HPLC separation chromatographs of standard Q homologs (top) and organic extracts of whole soybean roots (bottom).
The Q pools of control extracts from 4-, 7-, and 17-d-old roots were 53% to 62% reduced (Table II). Addition of KCN and SHAM to inhibit respiratory oxidases increased Q-pool reduction to 76% to 79% in extracts from 4- and 7-d-old roots. For reasons unknown, we were unable to avoid oxidation of the extracted Q from 17-d-old roots to which KCN and SHAM had been added. Repeated freeze thawing of root tissue decreased Q-pool reduction to 6 to 10% in roots of all ages, as did heating of roots to 65°C for 5 min (data not shown). We found it very difficult to protect the Q complement of roots from chemical oxidation during sample handling, especially in older roots, with some samples showing only 0% to 2% reduced Q, despite attempts to remove O2 and maintain aqueous phases at an acid pH. These samples were omitted from the results presented. In all other samples, the Qr/Qt ratio was found to be in the range 50% to 65%, which agrees with the data of Wagner and Wagner (1995) and with measurements on isolated mitochondria (see below). We assume, therefore, that these values are reasonable estimations of the status of the Q pool in vivo.
Table II.
Redox poise of extracted Q from soybean roots of various ages (4, 7, or 17 d old), untreated or treated with respiratory inhibitors (1 mm KCN + 20 mm SHAM) or by freeze-thawing
| Treatment | Q
Reduced
|
||
|---|---|---|---|
| 4 d | 7 d | 17 d | |
| % | |||
| Control | 56 ± 3.5 | 53 ± 3.5 | 62 ± 5.5 |
| +KCN +SHAM | 76 ± 1.5 | 79 ± 5.0 | |
| Freeze-thawed | 6 ± 4.5 | 10 ± 4 | 8 ± 3 |
Numbers are means ± se (n = 3–6).
Respiration by Isolated Root Mitochondria from Different-Aged Seedlings
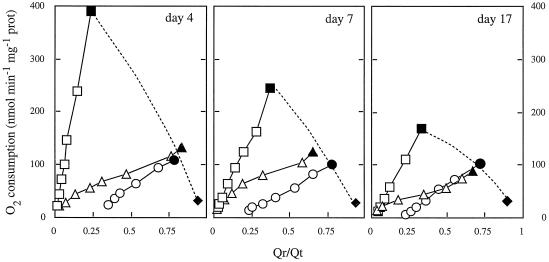
The respiratory characteristics of roots were further investigated by comparing the relationship between Q redox poise (Qr/Qt) and the respiratory rate in mitochondria purified from 4-, 7-, and 17-d-old soybean root systems. Using succinate as a substrate, O2 consumption rates were titrated with the specific succinate dehydrogenase inhibitor malonate (Fig. 3). Cyt pathway rates were measured in the presence and absence of ADP. AOX rates were measured in the presence of myxothiazol, with or without added pyruvate.
Figure 3.
Succinate-dependent O2 consumption rate as a function of Qr/Qt in root mitochondria isolated from 4-, 7-, and 17-d-old soybean seedlings. Data points are in the presence (squares) and in the absence (triangles) of ADP, and in the presence of myxothiazol (5 μm) with (circles) or without (diamonds) added pyruvate (1 mm). Filled symbols denote data in the absence of malonate; unfilled symbols are data from malonate titration of succinate oxidation. prot, Protein.
The control respiration rate (i.e. without myxothiazol) in the presence of ADP declined more than 2-fold on a mitochondrial protein basis with increasing root age, but the Qr/Qt ratio in the absence of malonate (i.e. at maximum O2 uptake rate) remained relatively stable at 0.2 to 0.3 (Fig. 3). When ADP was withheld, O2 consumption was lower at each age of a root, and under these conditions the Qr/Qt ratio in the absence of malonate was 0.7 to 0.8. Malonate-titration kinetics in the absence of myxothiazol reflect flux via the Cyt pathway alone. This is because without added pyruvate AOX did not significantly contribute to respiratory flux below a Qr/Qt ratio of 0.8 to 0.9 (Fig. 3; see also Millar et al., 1997). Data taken from malonate titrations of respiration (in the presence of ADP) versus Qr/Qt in mitochondria from different-aged roots were only able to be fitted on a single kinetic curve by decreasing the apparent Vmax of the Q-oxidizing pathway (not shown). Because electron transport under these conditions is predominantly via COX, the capacity of the Cyt pathway is less at any given Qr/Qt in mitochondria from older roots compared with those from younger roots.
Upon addition of pyruvate, respiration via AOX in the absence of malonate increased 3- to 4-fold at all root ages, to a rate of 100 to 120 nmol O2 min−1 mg−1 protein. Data from malonate titrations of AOX activity in the presence of pyruvate could be fitted to a single sigmoidal curve without modification, regardless of the age of roots, suggesting that the amount of functional AOX on a protein basis remained unchanged in mitochondria isolated from different-aged roots.
The last data point in each curve of Figure 3 (i.e. the value obtained in the absence of malonate, shown as filled symbols) represents the point of intersection between the COX and AOX kinetic curves, and the kinetics of the Q-reducing pathway (in this case, succinate dehydrogenase). A curve fitted through these points represents the activity of succinate dehydrogenase in the face of an increasingly reduced Q pool (Fig. 3, broken lines); these curves are mirror images of Q-oxidizing-pathway kinetics. When these data points (obtained with mitochondria from roots of different ages) were plotted on a separate graph versus root age, they would fit on a single curve only when the apparent Vmax of the Q-reducing pathway was decreased (not shown), suggesting that succinate dehydrogenase activity also decreased with root age.
Steady-state Qr/Qt values reflect the balance between Q-reducing and Q-oxidizing pathways (Van den Bergen et al., 1994). A coordinated decrease in both Q-oxidizing and Q-reducing pathways would, therefore, explain why the steady-state Qr/Qt values in root mitochondria in different metabolic states were constant with increasing root age, despite the large decrease in absolute respiratory rate (Fig. 3). Presumably, a decrease in succinate dehydrogenase activity also contributes to the rather constant endogenous Qr/Qt ratio in intact roots (Fig. 3). Total Q content of the mitochondria did not change significantly over the time period studied (not shown).
COX Activity in Mitochondria and Whole Roots
Measurement of cyanide-sensitive Cyt c-dependent O2 consumption (COX activity) provides a basis for comparison of isolated mitochondria and whole-tissue respiratory measurements. In isolated mitochondria from 4-, 7-, and 17-d-old roots, COX activity declined with age on a protein basis (Table III). Whole-tissue measurements showed a similar decline in Cyt c-dependent O2 consumption with root age on a dry-mass basis. The ratio of the two measurements provides an estimate of the amount of mitochondria in soybean roots in milligrams of mitochondrial protein per gram of root dry mass. This parameter did not change during the period of growth measured.
Table III.
COX activity in whole-tissue extracts and isolated mitochondria from soybean roots of various ages (4, 7, and 17 d old)
| Source | COX
Activity
|
||
|---|---|---|---|
| 4 d | 7 d | 17 d | |
| Whole tissue | μmol O2 min−1 g−1 dry mass | ||
| 26 ± 3 | 19 ± 1 | 11 ± 3 | |
| Purified mitochondria | μmol O2 min−1 mg−1 protein | ||
| 1.25 ± 0.08 | 0.80 ± 0.05 | 0.50 ± 0.04 | |
| Tissue mitochondrial content | mg mitochondrial protein g−1 dry mass | ||
| 22 ± 2 | 24 ± 2 | 22 ± 5 | |
Numbers are means ± se (n = 4).
The data in Table III also allow a comparison between the measured rate of respiration and the capacity of COX in intact roots. Control respiratory rates from Table I were only 14% to 20% of the COX capacity in whole roots from Table III. Furthermore, the rates of succinate-dependent respiration in the presence of ADP in isolated mitochondria (Fig. 3) was only 30% to 38% of the COX capacity in solubilized mitochondrial samples (Table III). This suggests that root mitochondria were not strictly limited in vivo by the capacity of COX, even though this decreased more than 2-fold during the time of the experiment, but rather are limited by the combined effect of decreasing dehydrogenase and COX activities. Respiration in vivo may also be restricted by adenylates and/or substrate supply to the mitochondria.
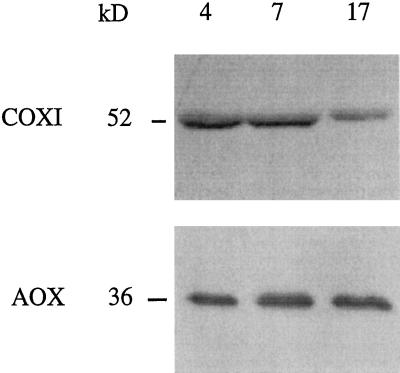
COX and AOX Protein Abundance in Isolated Mitochondria
To determine whether the observed changes in AOX and COX activities were correlated with changes in de novo synthesis or degradation of oxidase proteins, antibodies against both oxidases were reacted with mitochondrial proteins isolated from 4-, 7-, and 17-d-old plants on immunoblots (Fig. 4). A monoclonal antibody specific for AOX cross-reacted with a single 36-kD polypeptide among mitochondrial proteins from all three ages of soybean roots and no significant difference was observed in the intensity of the reactions. A monoclonal antibody specific for COX subunit I reacted with a 52-kD protein in soybean root mitochondria, but in this case the intensity of the reaction decreased noticeably with root age (Fig. 4). This suggests that turnover of key COX subunits may be largely responsible for the decreased total COX activity in mitochondria isolated from older roots (Table III).
Figure 4.
Immunoblots of AOX and COX subunit I proteins in root mitochondria isolated from 4-, 7-, and 17-d-old soybean seedlings. Mitochondrial protein equivalent to a 40-μg BSA standard was loaded in each lane in the presence of DTT (50 mm).
AOX Redox State in Whole Roots
The AOX protein exists in two forms: an inactive, covalently linked dimer and an active, noncovalently linked dimer (Umbach and Siedow, 1993). These two forms can be identified on SDS-PAGE under nonreducing conditions: the reduced dimer is separated into monomers of 32 to 39 kD, whereas the oxidized dimers remain in pairs and migrate with an apparent molecular mass of 70 to 80 kD. The redox status of AOX in isolated mitochondria can be measured in this manner, but this ratio does not necessarily reflect that in vivo because oxidation can occur during the mitochondrial isolation process (Umbach and Siedow, 1997).
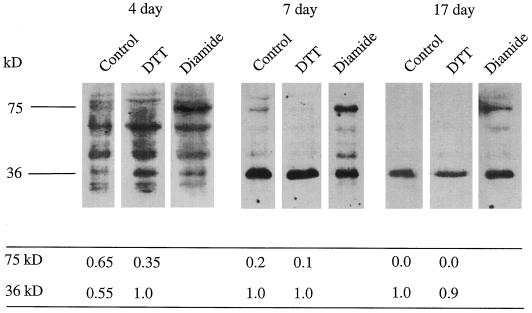
Using recent advances in chemiluminescence technology, the AOX protein can now be immunodetected in whole-tissue extracts, even in nonthermogenic tissues such as soybean, in which AOX is expressed at relatively low levels. In Figure 5, immunoblots of total root protein rapidly extracted under denaturing conditions are shown after separation in the presence and absence of chemical oxidants and reductants. Each blot was probed with the monoclonal anti-AOX antibody. In root extracts from 7- and 17-d-old seedlings, control lanes show that nearly all of the immunoreactive protein is present at an apparent molecular mass of 36 kD. At both ages, the addition of DTT had little effect, whereas incubation of root samples with diamide (a strong oxidant) yielded an immunoreactive protein with an apparent molecular mass of 75 kD. This suggests that most, if not all, of the AOX protein in d-7 and -17 roots was present in the reduced, active form in vivo. Extracts from d-4 roots were more problematic, with high background-to-signal ratios observed in all experiments. This was probably caused by the substantially higher protein content in the young roots, which dilutes the mitochondrial component. However, focusing only on the bands at 75 and 36 kD, which we think is justified because these were the only bands observed in the blots of roots from older seedlings, it can be seen that the addition of DTT markedly increased the intensity of an immunoreactive band at 36 kD (Fig. 5). The relative intensities of the 75- and 36-kD immunoreactive bands, representing oxidized and reduced AOX, respectively, were 0.65 and 0.55 in the control and 0.35 and 1.0 in the DTT-treated lanes, respectively, as determined by image analysis of the x-ray film. We suggest, therefore, albeit tentatively, that AOX was partly oxidized in d-4 roots.
Figure 5.
Immunoblots of AOX in rapidly extracted whole-root samples from 4-, 7-, and 17-d-old soybean seedlings. Samples treated in the presence and absence of DTT (50 mm) and diamide (5 mm) are presented. For each age, lanes are from a single gel, and each lane shown was separated by two empty lanes to avoid cross-contamination of redox chemicals. The intensity of the reaction at 35 and 75 kD was measured using National Institutes of Health imaging software. At a given root age, the 35-kD band in the DTT lanes had the highest density of all bands present in the three lanes, and this was arbitrarily set at 1.0; the densities of the other bands in those lanes are presented as fractions of that highest-density band.
Tissue Pyruvate Content and Mitochondrial NAD-ME Activities
The activity of AOX is reversibly stimulated from within the mitochondrial matrix by short-chained α-keto acids such as pyruvate. In vivo, intramitochondrial pyruvate may be supplied via the transport of cytosolic pyruvate from the cytosol or via the decarboxylation of malate to pyruvate by NAD-ME in the matrix. Pyruvate content in total soybean root tissue increased from d 4 to 7 and then decreased to the former value by d 17 (Table IV). The activity of NAD-ME in isolated mitochondria did not change significantly during this time (Table IV) and was only 20% to 40% of activity measured in soybean cotyledon, potato tuber, and Arum maculatum spadix mitochondria (data not shown).
Table IV.
Pyruvate content of whole-root systems and NAD-ME activities of mitochondria isolated from whole-root systems of soybean seedlings of various ages (4, 7, and 17 d old)
| Parameter | 4 d | 7 d | 17 d |
|---|---|---|---|
| nmol g−1 fresh mass | |||
| Pyruvate content | 48 ± 7 | 87 ± 5 | 47 ± 4 |
| μmol min−1 mg−1 protein | |||
| NAD-ME activity | 85 ± 7 | 67 ± 20 | 69 ± 14 |
Numbers are means ± se (n = 3).
DISCUSSION
Ontogenetic Changes in Total Respiration and Electron Partitioning
This study demonstrates that total respiration, and the contribution of COX to that respiration, decreases with age in whole roots of soybean seedlings. The amount of AOX protein and the capacity of AOX did not change during this period, but electron flux through AOX increased with age and at 17 d AOX activity equaled that of COX (Fig. 1). This increase agrees with previous developmental work on legumes (Azcon-Bieto et al., 1983; Obenland et al., 1990; Wen and Liang, 1993; Lennon et al., 1995) and two Arctic species (Atkin and Cummins, 1994).
The ontogenetic decline in total respiration and COX activity of our soybean roots correlated with a decline in root relative growth rate during aging, as reported by others (Azcön-Bieto et al., 1983; McDonnell and Farrar 1993; Winkler et al., 1994; Lambers et al., 1996). The decline in Cyt pathway activity with age may reflect a decline in the demand for ATP associated with the slower growth rates, as has been suggested previously (Amthor, 1989). Because the amount of COX I protein also declined, there may be a feedback effect of ATP demand on the synthesis of Cyt-chain components. This may not be a general phenomenon, however, because a decline in Cyt-chain capacity was not seen in developing pea leaves (Lennon et al., 1995).
Measurement of Electron Partitioning between Respiratory Pathways
In this study an 18O-discrimination method was used to determine the partitioning of electrons between AOX and COX (Guy et al., 1989). This method overcomes many of the problems associated with the classic inhibitor-titration methods used previously (Atkin et al., 1995; Millar et al., 1995; Day et al., 1996). Our results generally agree with other recent work using this technique, which has shown that 30% to 40% of respiratory flux occurs via AOX in whole soybean roots (Robinson et al., 1992, 1995). We observed consistent D values by the Cyt and alternative pathways measured in soybean roots of various ages (Table I). Our determinations for AOX do not vary much from others reported in the literature. Published values for the Cyt pathway in soybean vary substantially between studies, from 17.2 (Robinson et al., 1995) to 20.3 (Ribas-Carbo et al., 1997); our values fall closer to the former. The reason for the different values may reflect differences in growth and measuring conditions in the different experiments. Clearly, it is necessary to determine these values for each set of experiments, even with the same species.
Control of AOX Activity in Vivo
To explain the observed changes in partitioning between respiratory pathways, we attempted to determine factors known to affect AOX activity in isolated mitochondria and intact roots.
Intramitochondrial pyruvate is one such factor. Measurements of mitochondrial pyruvate content in vivo are extremely difficult, but measurements of whole-tissue pyruvate content have been made in the hope that these might help elucidate the importance of this regulatory factor in vivo. Wagner and Wagner (1995) showed that addition of the uncoupler S13 to a petunia cell culture stimulated AOX activity without increasing Q-pool reduction. They suggested that a concomitant increase from 100 to 166 nmol pyruvate g−1 fresh weight in the cells on addition of the uncoupler may have been responsible for this stimulation. Variation in pyruvate content from 30 to 60 nmol g−1 fresh weight in pea, spinach, and wheat leaves also positively correlates with partitioning to AOX as judged by inhibitor titration (Day and Lambers, 1983). In soybean roots, pyruvate content did vary during development but showed no clear trend that correlated with the in vivo respiratory flux via AOX (Table IV). Because only very small amounts of intramitochondrial pyruvate are needed to activate AOX in soybean (Millar et al., 1996), estimates of whole-tissue pyruvate contents are unlikely to answer the question of whether AOX is fully activated by α-keto acids in vivo. However, the lack of significant change in mitochondrial ME activity (Table IV) indicates that the potential for intramitochondrial pyruvate formation in soybean roots does not change with seedling age. Furthermore, Q redox titrations of mitochondria isolated from 17-d-old roots indicate that pyruvate was required to obtain appreciable AOX activity at 60% to 70% reduced Q (Fig. 3); because this was the value of Q reduction in intact roots (Fig. 2) and because AOX was clearly active in these roots (Fig. 1), it can be concluded that pyruvate levels in vivo were sufficient to activate AOX. Because the same level of pyruvate was observed in 4-d-old roots in which AOX was inactive (Table IV), and Q-reduction levels were also similar (Fig. 2), it is obvious that factors other than whole-tissue pyruvate content were involved.
The redox state of AOX protein is known to affect AOX activity (Umbach and Siedow, 1993), and our results suggest that in roots of very young soybean seedlings AOX oxidation may be one factor contributing to its low activity (Fig. 5). In this context, a study of AOX in developing pea leaves found that although AOX activity increased during the time period studied, the amount of AOX protein in mitochondria remained constant (Lennon et al., 1995). The increase in AOX activity was correlated with a shift in the redox state of AOX protein toward the more reduced state (Lennon et al., 1995).
Electron flow to AOX is also stimulated by the absence of ADP, and it is likely that respiration in the roots under study here was adenylate limited, particularly in the older roots, in which the relative growth rates were relatively low. The observation that the ratio of succinate-dependent respiratory rate (Fig. 3) to COX capacity (Table III) for isolated root mitochondria in the absence of ADP is very similar to the ratio observed between respiratory rate and COX activity of whole tissues (Tables I and III) supports this prediction, as does the relatively high whole-root Q-reduction levels (Fig. 2, compare with Fig. 6).
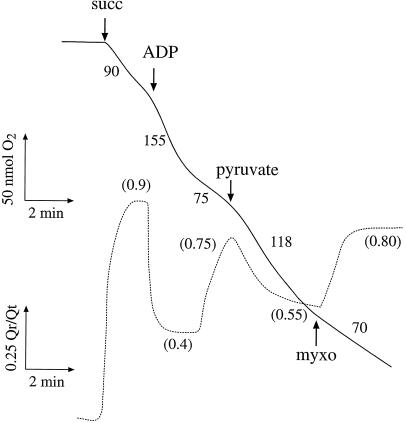
Figure 6.
Succinate-dependent O2 consumption and Q-pool reduction by mitochondria isolated from 17-d-old soybean roots. Ten millimolar succinate (succ), 2 μm myxothiazol (myxo), and 1 mm pyruvate were added as indicated. Numbers on traces indicate nmol O2 min−1 mg−1 protein and the steady-state Qr/Qt ratio (in parentheses).
It is interesting to note that the KCN-insensitive respiration rate measured in intact roots decreased during seedling growth from 7 to 17 d (Table I), whereas AOX capacity in isolated mitochondria did not change (Fig. 3). This discrepancy could be the result of restricted substrate supply to the mitochondria in the oldest roots; this could have resulted from high cytosolic ATP/ADP ratios, which, together with low ADP concentrations (Dry and Wiskich 1982), could also have restricted electron transport-chain activity. However, it must also be kept in mind that structural changes during root development will underlie the changes in respiration seen on a whole-root basis. During root development cortical cell tissue is replaced with vascular cell tissue and the proportion of meristematic to nongrowing tissue in the whole-root system also changes (Esau, 1977). The relative contributions of the meristematic and nongrowing tissues to both in vivo respiration rates and the isolated mitochondria remain unknown, and may influence comparisons of the two.
Maintenance of Q Redox State in Roots
Table II shows that intact roots maintained a relatively constant Q redox poise despite a 3-fold decrease in respiratory rate (Table I) and a large change in partitioning between respiratory pathways (Fig. 1). In isolated mitochondria steady-state Qr/Qt ratios under standard conditions in the absence of malonate also remained relatively constant with root age (Fig. 3). However, although 95% of total cellular Q is located within mitochondria (Swiezewska et al., 1993), the Qr/Qt derived from Q-electrode measurements on isolated mitochondria (Fig. 3) may not accurately represent the redox poise of Q extracted from roots, because 10% to 30% of mitochondrial Q appears to be redox inactive in isolated plant mitochondria (Van den Bergen et al., 1994; Ribas-Carbo et al., 1995b). The residual reduced and oxidized Q in the presence of KCN plus SHAM can be used as estimates of nonreactive Q in vivo. On this basis, 50% to 60% of Q reduction in vivo is equivalent to 0.6 to 0.7 redox active Qr/Qt in isolated mitochondria. However, it is possible that the inactive Q pool found in isolated mitochondria is an artifact caused by damage of a proportion of mitochondria during isolation. In this case, the in vivo percentage Q reduction could be compared directly with Qr/Qt in isolated mitochondria.
Wagner and Wagner (1995) have reported that petunia cell cultures also maintain a Q-pool reduction of approximately 60% for most of the culture cycle, despite large changes in respiratory rate. These authors suggested that electron partitioning between respiratory pathways is varied in vivo to maintain a steady-state Q redox poise, rather than vice versa.
The Role of AOX in Soybean Roots
This study shows that respiratory rates of soybean roots during aging are largely dictated by changes in the activity of Cyt-pathway electron transport (probably mediated by changes in the content of relevant enzymes, as well as by adenylate control) and changes in AOX activation status. The decline in Cyt-chain activity is at least partially offset by increased AOX activity to provide a relatively stable steady-state Q-pool reduction in the mitochondrial inner membrane. Possible changes in succinate dehydrogenase activity may also play a role here. The changes in AOX activity may be important to ensure a smooth transition through developmental changes in ATP demand.
It seems that although ATP demand decreases as roots age, some provision for carbon flux through respiratory pathways is maintained (as proposed by Palmer, 1976), and the role of AOX here may be to ensure that this occurs without overreduction of the Q pool with its associated generation of destructive O2 intermediates (Wagner and Krab, 1995; Millar and Day, 1997). The ability of AOX engagement to modulate the redox state of mitochondrial Q is illustrated in Figure 6. This shows simultaneous recordings of O2 consumption and Q reduction during succinate oxidation by mitochondria isolated from 17-d-old roots. In these mitochondria, AOX protein is largely reduced, but pyruvate must be added to observe AOX activity (Day et al., 1994). Respiration was rapid and the level of Q reduction quite low (Qr/Qt = 0.4) in the presence of ADP; upon depletion of ADP, respiration slowed and Q-reduction level increased to 0.75. Subsequent addition of pyruvate to activate AOX stimulated respiration almost 2-fold and decreased Qr/Qt to 0.55 (Fig. 6). (It is interesting to note that this was the level of Q reduction in intact roots of this age [Fig. 2].) Thus, AOX activation allows increased resting rates of respiration without large increases in Q-reduction levels.
More work is needed to understand the mechanisms behind down-regulation of Cyt-pathway activity during development of plant tissues, but it is interesting that the decline in roots with age is accompanied by decreases in COX protein levels, implying some coarse control of respiratory pathways. COX deficiencies in mammals have also been detected in whole tissues and in isolated mitochondria (Nicoletti et al., 1995). Such deficiencies have been linked to aging and to a variety of inflammatory and degenerative myopathies (Chariot et al., 1996). Establishing a link between this mammalian work and the ontogenetic- and stress-induced changes in plant respiration will be a valuable step in furthering our understanding of the regulation of mitochondrial respiration.
ACKNOWLEDGMENTS
Sue Young and Julie Styles are thanked for technical assistance. David Greber and Agnieszka Dombek contributed to this work through undergraduate research projects. The generous support of Micromass UK, Ltd., to this project is acknowledged.
Abbreviations:
- AOX
alternative oxidase
- COX
Cyt c oxidase
- D
discrimination value for 18O fractionation
- ME
malic enzyme
- Q
ubiquinone
- Qr/Qt
fraction of the Q pool in the reduced state
- SHAM
salicylhydroxamic acid
Footnotes
This research was supported by a grant from the Australian Research Council to D.A.D.
LITERATURE CITED
- Amthor JS. Respiration and Crop Productivity. New York: Springer-Verlag; 1989. [Google Scholar]
- Atkin OK, Cummins WR. The effect of nitrogen source on growth, nitrogen economy and respiration of two high Arctic plant species differing in relative growth rate. Funct Ecol. 1994;8:389–399. [Google Scholar]
- Atkin OK, Villar R, Lambers H. Partitioning of electrons between the cytochrome and alternative pathways in intact roots. Plant Physiol. 1995;108:1179–1183. doi: 10.1104/pp.108.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcon-Bieto J, Lambers H, Day DA. Respiratory properties of developing bean and pea leaves. Aust J Plant Physiol. 1983;10:237–245. [Google Scholar]
- Chariot P, Ruet E, Authier FJ, Labes D, Poron F, Gherardi R. Cytochrome c oxidase deficiencies in the muscle of patients with inflammatory myopathies. Acta Neuropathol. 1996;91:530–536. doi: 10.1007/s004010050462. [DOI] [PubMed] [Google Scholar]
- Crane FL, Barr R. Determination of ubiquinones. Methods Enzymol. 1971;18:137–165. [Google Scholar]
- Day DA, Krab K, Lambers H, Moore AL, Siedow JN, Wagner AM, Wiskich JT. The cyanide-resistant oxidase: to inhibit or not to inhibit, that is the question. Plant Physiol. 1996;110:1–2. doi: 10.1104/pp.110.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day DA, Lambers H . Regulation of glycolysis and electron transport in roots. Physiol Plant. 1983;58:155–160. [Google Scholar]
- Day DA, Millar AH, Wiskich JT, Whelan J. Regulation of alternative oxidase activity by pyruvate in soybean mitochondria. Plant Physiol. 1994;106:1421–1427. doi: 10.1104/pp.106.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day DA, Moore AL, Day IB, Wiskich JT, Azcon-Bieto J. Regulation of non-phosphorylating electron transport pathways in soybean cotyledon mitochondria and its implications for fat metabolism. Plant Physiol. 1988;86:1199–1204. doi: 10.1104/pp.86.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day DA, Neuburger M, Douce R. Activation of NAD-linked malic enzyme in intact plant mitochondria by exogenous coenzyme A. Arch Biochem Biophys. 1984;231:233–242. doi: 10.1016/0003-9861(84)90383-7. [DOI] [PubMed] [Google Scholar]
- Day DA, Neuberger M, Douce R. Biochemical characterisation of chlorophyll-free mitochondria from pea leaves. Aust J Plant Physiol. 1985;12:219–228. [Google Scholar]
- Dry IB, Wiskich JT. Role of the external ATP/ADP ratio in the control of plant mitochondrial respiration. Arch Biochem. 1982;217:72–79. doi: 10.1016/0003-9861(82)90480-5. [DOI] [PubMed] [Google Scholar]
- Esau K. Anatomy of Seed Plants, Ed 2. New York: John Wiley & Sons; 1977. [Google Scholar]
- Guy RD, Berry JA, Fogel ML, Hoering TC. Differential fractionation of oxygen isotopes by cyanide-resistant and cyanide-sensitive respiration in plants. Planta. 1989;177:483–491. doi: 10.1007/BF00392616. [DOI] [PubMed] [Google Scholar]
- Guy RD, Berry JA, Fogel ML, Turpin DH, Weger HG (1992) Fractionation of the stable isotopes of oxygen during respiration by plants: the basis for a new technique. In H Lambers, LHW van der Plas, eds, Molecular, Biochemical and Physiological Aspects of Plant Respiration. Academic Publishing, The Hague, The Netherlands, pp 442–453
- Hoefnagel MHN, Millar AH, Wiskich JT, Day DA. Cytochrome and alternative respiratory pathways compete for electrons in the presence of pyruvate in soybean mitochondria. Arch Biochem Biophys. 1995;318:394–400. doi: 10.1006/abbi.1995.1245. [DOI] [PubMed] [Google Scholar]
- Hoefnagel MHN, Rich PR, Zhang Q, Wiskich JT. Substrate kinetics of the plant mitochondrial alternative oxidase and the effects of pyruvate. Plant Physiol. 1997;115:1145–1153. doi: 10.1104/pp.115.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt WF, Loomis RS. Respiration modelling and hypothesis testing with a dynamic model of sugar beet growth. Ann Bot. 1979;44:5–17. [Google Scholar]
- Kearns A, Whelan J, Young S, Elthon TE, Day DA. Tissue-specific expression of the alternative oxidase in soybean and siratro. Plant Physiol. 1992;99:712–717. doi: 10.1104/pp.99.2.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers H, Scheurwater I, Atkin OK (1996) Respiratory patterns in roots in relation to their functioning. In Y Waisel, A Eshel, V Kafakki, eds, Plant Roots: The Hidden Half, Ed 2. Marcel Dekker, New York, pp 323–362
- Lambers H, Steingröver E. Growth respiration of a flood-tolerant and a flood-intolerant Senecio species as affected by low oxygen tension. Physiol Plant. 1978;42:179–184. [Google Scholar]
- Lambers H, Szaniawski RK, de Visser R. Respiration for growth, maintenance and ion uptake: an evaluation of concepts, methods, values and their significance. Physiol Plant. 1983;58:556–563. [Google Scholar]
- Lennon AM, Pratt J, Leach G, Moore AL. Developmental regulation of respiratory activity in pea leaves. Plant Physiol. 1995;107:925–932. doi: 10.1104/pp.107.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry O, Rosebrough NJ, Farr AL, Randall RJ. Protein measurements with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- McDonnell E, Farrar JF. Respiratory characteristics of isolated barley mitochondria and intact barley roots. J Exp Bot. 1993;44:1485–1490. [Google Scholar]
- Meeuse BJD. Thermogenic respiration in aroids. Annu Rev Plant Physiol. 1975;26:117–126. [Google Scholar]
- Millar AH, Atkin OK, Lambers H, Wiskich JT, Day DA. A critique of the use of inhibitors to estimate partitioning of electrons between mitochondrial respiratory pathways in plants. Physiol Plant. 1995;95:523–532. [Google Scholar]
- Millar AH, Day DA. Nitric oxide inhibits the cytochrome oxidase but not the alternative oxidase of plant mitochondria. FEBS Lett. 1996;398:155–158. doi: 10.1016/s0014-5793(96)01230-6. [DOI] [PubMed] [Google Scholar]
- Millar AH, Day DA. Alternative solutions to radical problems. Trends Plant Sci. 1997;2:289–290. [Google Scholar]
- Millar AH, Finnegan PM, Whelan J, Drevon J-J, Day DA. Expression and kinetics of the mitochondrial alternative oxidase in nitrogen-fixing nodules of soybean roots. Plant Cell Environ. 1997;20:1273–1282. [Google Scholar]
- Millar AH, Hoefnagel MHN, Day DA, Wiskich JT. Specificity of the organic acid activation of alternative oxidase in plant mitochondria. Plant Physiol. 1996;111:613–618. doi: 10.1104/pp.111.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AH, Wiskich JT, Whelan J, Day DA. Organic acid activation of the alternative oxidase of plant mitochondria. FEBS Lett. 1993;329:259–262. doi: 10.1016/0014-5793(93)80233-k. [DOI] [PubMed] [Google Scholar]
- Moore AL, Dry IB, Wiskich JT. Measurement of the redox state of the ubiquinone pool in plant mitochondria. FEBS Lett. 1988;235:76–80. [Google Scholar]
- Nicoletti VG, Tendi EA, Lalicata C, Reale S, Costa A, Villa RF, Ragusa N, Stella AMG. Changes of mitochondrial cytochrome c oxidase and FoF1 ATP synthase subunits in rat cerebral cortex during aging. Neurochem Res. 1995;20:1465–1470. doi: 10.1007/BF00970595. [DOI] [PubMed] [Google Scholar]
- Obenland D, Dielthelm R, Shibles R, Stewart C. Relationship of alternative respiratory capacity and alternative oxidase amount during soybean seedling growth. Plant Cell Physiol. 1990;31:897–901. [Google Scholar]
- Palmer JM. The organisation and regulation of electron transport in plant mitochondria. Annu Rev Plant Physiol. 1976;27:133–157. [Google Scholar]
- Poorter H, van der Werf A, Atkin OK, Lambers H. Respiratory energy requirements of roots vary with the potential growth rate of the plant species. Physiol Plant. 1991;83:469–475. [Google Scholar]
- Purvis AC, Shewfelt RL. Does the alternative pathway ameliorate chilling injury in sensitive plant tissues? Physiol Plant. 1993;88:712–718. doi: 10.1111/j.1399-3054.1993.tb01393.x. [DOI] [PubMed] [Google Scholar]
- Ribas-Carbo M, Berry JA, Yakir D, Giles L, Robinson SA, Lennon AL, Siedow JN. Electron partitioning between the cytochrome and alternative pathways in plant mitochondria. Plant Physiol. 1995a;109:829–837. doi: 10.1104/pp.109.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas-Carbo M, Lennon AM, Robinson SA, Giles L, Berry JA, Siedow JN. The regulation of electron partitioning between the cytochrome and alternative pathways in soybean cotyledons and root mitochondria. Plant Physiol. 1997;113:903–911. doi: 10.1104/pp.113.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas-Carbo M, Wiskich JT, Berry JA, Siedow JN. Ubiquinone redox behaviour in plant mitochondria during electron transport. Arch Biochem Biophys. 1995b;317:156–160. doi: 10.1006/abbi.1995.1148. [DOI] [PubMed] [Google Scholar]
- Rich PR. Quinol oxidation in Arum maculatum mitochondria and its application to the assay, solubilisation and partial purification of the alternative oxidase. FEBS Lett. 1978;96:252–256. [Google Scholar]
- Robinson SA, Ribas-Carbo M, Yakir D, Giles L, Reuveni Y, Berry JA. Beyond SHAM and cyanide: opportunities for studying the alternative oxidase in plant respiration using oxygen isotope discrimination. Aust J Plant Physiol. 1995;22:487–496. [Google Scholar]
- Robinson SA, Yakir D, Ribas-Carbo M, Giles L, Osmond CB, Siedow JN, Berry JA. Measurements of the engagement of cyanide-resistant respiration in the Crassulacean acid metabolism plant Kalanchoe daigremontiana with the use of on-line oxygen isotope discrimination. Plant Physiol. 1992;100:1087–1091. doi: 10.1104/pp.100.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiezewska E, Dallner G, Andersson B, Ernster L. Biosynthesis of ubiquinone and plastoquinone in the endoplasmic reticulum-Golgi membranes of spinach leaves. J Biol Chem. 1993;268:1494–1499. [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach AL, Siedow JN. Covalent and non-covalent dimers of the cyanide-resistant alternative oxidase protein in higher plant mitochondria and their relationship to enzyme activity. Plant Physiol. 1993;103:845–854. doi: 10.1104/pp.103.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach AL, Siedow JN. Changes in the redox state of the alternative oxidase regulatory sulfhydryl/disulfide system during mitochondrial isolation: implications for inferences of activity in vivo. Plant Sci. 1997;123:19–28. [Google Scholar]
- Van den Bergen CWM, Wagner AM, Krab K, Moore AL. The relationship between electron flux and the redox poise of the quinone pool in plant mitochondria: interplay between quinol-oxidising and quinone-reducing pathways. Eur J Biochem. 1994;226:1071–1078. doi: 10.1111/j.1432-1033.1994.01071.x. [DOI] [PubMed] [Google Scholar]
- Vanlerberghe GC, Day DA, Wiskich JT, Vanlerberghe AE, McIntosh L. Alternative oxidase activity in tobacco leaf mitochondria: dependence upon tricarboxylic acid cycle-mediated redox regulation and pyruvate activation. Plant Physiol. 1995;109:353–361. doi: 10.1104/pp.109.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AM, Krab K. The alternative respiration pathway in plants: role and regulation. Physiol Plant. 1995;95:318–325. [Google Scholar]
- Wagner AM, Wagner MJ. Measurements of in vivo ubiquinone reduction levels in plant cells. Plant Physiol. 1995;108:277–283. doi: 10.1104/pp.108.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J-Q, Liang H-G. Studies on energy status and mitochondrial respiration during growth and senescence of mung bean cotyledons. Physiol Plant. 1993;89:805–810. [Google Scholar]
- Winkler MN, Mawson BT, Thorpe TA. Alternative and cytochrome pathway respiration during shoot bud formation in cultured Pinus radiata cotyledons. Physiol Plant. 1994;90:144–151. [Google Scholar]