Abstract
Background
Idiopathic pulmonary fibrosis (IPF) is a progressive disease. Effective treatment is not currently available and the prognosis is poor. The aim of our study was to identify clinical predictors of survival in patients with IPF.
Methods
By using medical record database of a university hospital, we reviewed the records of patients who had been diagnosed as having IPF from January 1996 through December 2007.
Results
Among 89 patients considered as having interstitial lung disease (ILD) on computed tomography (CT) of the chest, 22 were excluded because of the diagnosis of other ILDs or connective tissue disease, and finally, 67 met the criteria of IPF. The mean age at the diagnosis of IPF was 70 years (range, 41~87 years) and 43 (64%) were male. The mean survival time following the diagnosis of IPF was 40 months (range, 0~179 months). Among them, 28 cases were diagnosed as the progressive state of IPF on the follow-up CT examination, and the mean duration between diagnosis of IPF and progression was 31 months. Multivariate analysis using Cox regression model revealed that body mass index (BMI) less than 18.5 kg/m2 (p=0.030; hazard ratio [HR], 12.085; 95% confidence interval [CI], 1.277~114.331) and CT progression before 36 months from the diagnosis of IPF (p=0.042; HR, 13.564; 95% CI, 1.101~167.166) were independently associated with mortality.
Conclusion
Since low BMI at the diagnosis of IPF and progression on follow-up CT were associated with poor prognosis, IPF patients with low BMI and/or progression before 36 months following the diagnosis should be closely monitored.
Keywords: Idiopathic Pulmonary Fibrosis, Body Mass Index, Disease Progression
Introduction
Idiopathic pulmonary fibrosis (IPF) is the most common type of idiopathic interstitial lung diseases. It has chronic symptoms and is a progressive disease that develops gradual pulmonary interstitial fibrosis. It has poor prognosis and no effective treatment. In the past, lung biopsy was essential to confirm IPF. In particular, as IPF has temporal heterogeneity, open or thoracoscopic lung biopsy under general anesthesia was recommended to obtain relatively large tissues from various areas. In a clinical practice, however, as most patients with IPF are old and have substantial comorbidity such as cardiovascular disease, the risks of surgical lung biopsy may outweigh the benefits of establishing a secure diagnosis of IPF. According to the guideline presented by the American Thoracic Society (ATS) and European Respiratory Society (ERS) in 2000, a patient can be diagnosed with IPF if he/she satisfies all of three major diagnostic criteria (pulmonary function insufficiency of restrictive type, radiological characteristics of IPF on the high-resolution computed tomography (HRCT) of chest, no evidence of other diseases) and three or more of four minor diagnostic criteria when it is difficult to conduct a biopsy although biopsy is a principle1. In 2011, ATS, ERS, the Japanese Respiratory Society (JRS), and the Latin-America Thoracic Association (ALAT) presented a new guideline by referring to the recommendations of the Society of Thoracic Radiology and Pulmonary Pathology Society2. The diagnostic criteria (2011) are as follows: first, no other causative factor of pulmonary fibrosis should be present. Second, typical HRCT findings of chest should be available if lung biopsy is unavailable. Third, IPF should be confirmed by histopathology and radiology if lung biopsy is conducted. Thus, the new guideline emphasizes an interdisciplinary approach including clinical, and radiological, and pathological examinations. Accordingly, this study was retrospectively conducted to investigate factors affecting patients' prognosis in patients with IPF according to the 2011 diagnostic criteria.
Materials and Methods
The medical records of patients, who were diagnosed with IPF at Ewha Womans University Mokdong Hospital from January 1, 1996 to December 31, 2007, were reviewed. Among patients who had been suspected of having interstitial lung disease on chest CT, those who satisfied the diagnostic criteria2 for IPF presented by the ATS, ERS, JRS, and ALAT were selected as subjects. Pulmonary function test was conducted in a stable state using Vmax spectra 22 (SensorMedics, Yorba Linda, CA, USA) according to the ATS guideline3. The last follow-up date was May 7, 2010. The patients who survived until the last follow-up and patients who died before the last follow-up were classified into the survival group and death group, respectively. This study was reviewed and approved by the Institution Review Board of Ewha Womans University Mokdong Hospital.
Statistical analysis was conducted using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). Fisher's exact test or chi-square test was conducted to compare categorical variables between the two groups, whereas Student's t-test was conducted to compare continuous variables between the two groups. Survival curves were obtained using the Kaplan-Meier method, and the two survival curves were compared using Log rank method. The effect of variables on the survival was assessed using Cox proportional-hazards model.
Results
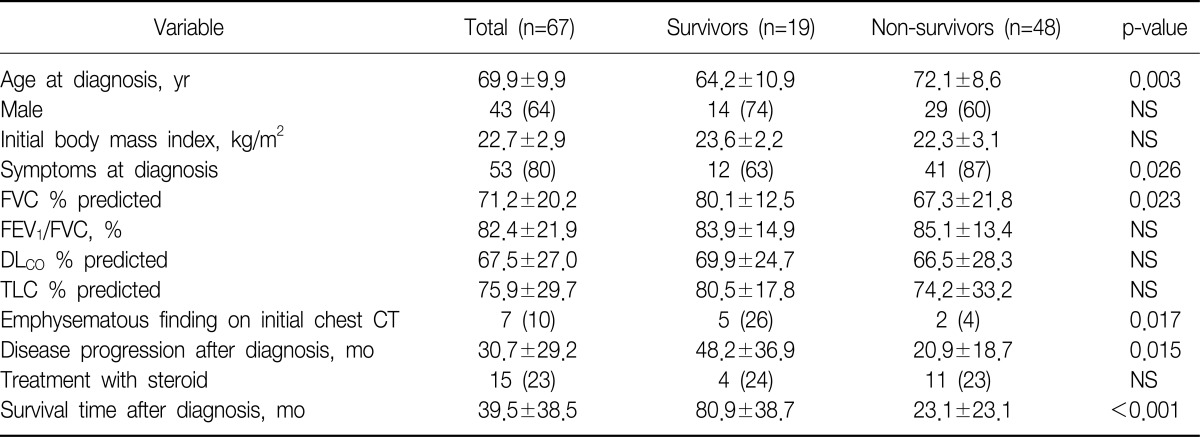
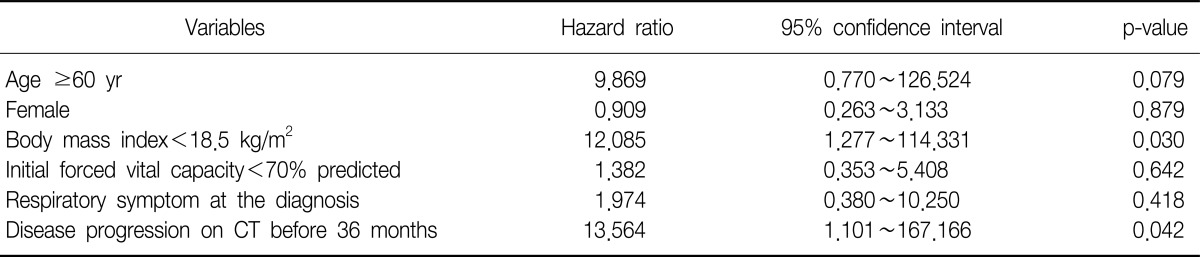
Among 89 patients who had been suspected of having interstitial lung disease on chest CT, 67 patients were diagnosed with IPF except for 22 patients who were diagnosed with other types of interstitial lung disease or connective tissue disease. The mean age of the patients at the time of diagnosis was 70 years (range, 41~87 years). The mean age of the survival and death groups were 64 years and 72 years, respectively, which showed a statistical significance (p=0.003). There were forty-three male patients (64.2%). They consisted of 14 patients (73.7%) from the survival group and 29 patients (60.4%) from the death group, which showed no gender difference (p=0.307). At the time of diagnosis, the mean body mass index (BMI) of the patients was 22.7 kg/m2, which showed no significant difference between the two groups (p=0.119). Fifty-three patients (80.3%) had symptom such as coughing, sputum, dyspnea, and hemoptysis at the time of diagnosis. They consisted of 12 patients (63.2%) from the survival group and 41 patients (87.2%) from the death group, which showed a significant difference (p=0.026). The mean forced vital capacity (FVC) was 71.2% predicted. The survival group had 80.1% predicted, whereas the death group had 67.3% predicted, which showed a significant difference (p=0.023). However, no significant differences in forced expired volume in one second/FVC, total lung capacity, and diffusing capacity for carbon monoxide (DLco) were found between the two groups. At the time of diagnosis, the 7 patients showed emphysematous change on chest CT. They consisted of 5 from the survival group and 2 from the death group, which showed a significant difference (p=0.017). The mean survival time was 40 months (range, 0~179 months), of whom 28 patients showed exacerbation on the follow-up chest CT, and the mean period between diagnosis and exacerbation was 31 months. The survival group had a mean period of 48 months, and the death group had a mean period of 21 months, which showed a significant difference (p=0.015) (Table 1). In multivariate analysis using Cox regression model, various factors that were reported to be related to prognosis in many previous studies were analyzed altogether. Among the factors such as age, gender, respiratory symptoms, BMI, FVC, and period between diagnosis and exacerbation, the prognosis was poorer in the patients with BMI<18.5 kg/m2 than in the patients with BMI≥18.5 kg/m2 (p=0.030; hazard ratio [HR], 12.085; 95% confidence interval [CI], 1.277~114.331). The prognosis was also poorer in the patients with period between diagnosis and exacerbation on follow-up HRCT <36 months than in the patients period between diagnosis and exacerbation ≥36 months (p=0.042; HR, 13.564; 95% CI, 1.101~167.166) (Table 2, Figure 1).
Table 1.
Characteristics of patients with IPF
The value of data was mean±standard deviation or number (%).
IPF: idiopathic pulmonary fibrosis; NS: not significant; FVC: forced vital capacity; FEV1: forced expiratory volume in 1 second; DLCO: diffusing capacity for carbon monoxide; TLC: total lung capacity; CT: computed tomography.
Table 2.
Prognostic factors of IPF by multiple Cox regression analyses
IPF: idiopathic pulmonary fibrosis; CT: computed tomography.
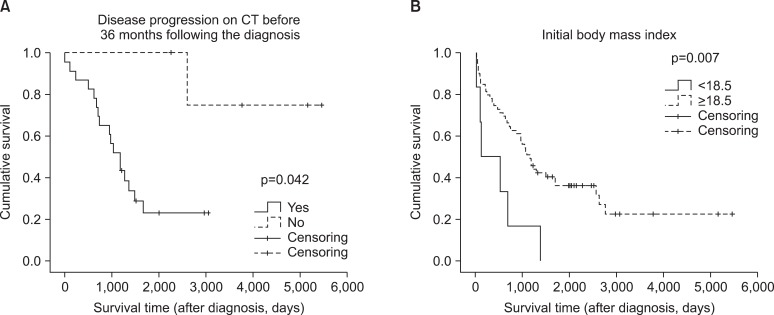
Figure 1.
Comparison of overall survival by Kaplan-Meier method. (A) Disease progression on computed tomography (CT) before 36 months following the diagnosis. (B) Initial body mass index. p-value by Log rank test.
Discussion
The survival time of patients with IPF varies depending on diagnosis time. That is, it depends on the time of radiological abnormality, symptom onset, and diagnosis. It was reported that reticular opacity of the bilateral base shown in chest X-ray occurred several years before the onset of symptoms, and the symptoms began 1,000 days after radiologic abnormalities in patients who were diagnosed with IPF via biopsy or chest X-ray4. In addition, many studies reported that the symptoms occurred 1~2 years before being diagnosed with IPF5-12. Thus, IPF was reported to be diagnosed five or more years after the onset of symptoms4. A comparison of the survival following its diagnosis showed that the median value of the survival time after symptom onset was approximately 48 months or less. Studies that were conducted following the classification of new idiopathic interstitial pneumonia reported that the median value of the survival time was 2~4 years, and that the 5-year survival rate was 20~40%6-12. In addition, patients with IPF had high mortality, but the change in pulmonary function was insignificant13,14. This is likely to be attributable to the fact that IPF showed stepwise progression rather than gradual progression, thereby resulting in high mortality15. Therefore, an intensive management of IPF is important during the aforementioned acute stage.
The acute exacerbation of IPF means the sudden deterioration of underlying pulmonary diseases, which can secondarily occurs following the occurrence of infection, pulmonary embolism, pneumothorax, and heart failure16. According to 2008 report on patients with idiopathic interstitial pneumonia, the death of patients with IPF were reported to be caused by respiratory failure 44%, pneumonia 31%, lung cancer 9%, cardiovascular disease 5%, and others 10% due to exacerbation. It was, however, difficult to distinguish acute exacerbation with pulmonary infection2. Meanwhile, acute exacerbation occurs without clear reason17-19, and this acute exacerbation stage occurs from time to time, and is also correlated with high mortality17-24. A follow-up study conducted on 168 patients with IPF reported that 21% of the patients died during a period of 76 weeks (median), of whom 47% died of acute exacerbation25. According to the results of studies on the prognosis of IPF, various factors, such as old age, male, dyspnea, smoking history, reduced pulmonary function, radiological abnormality, increased neutrophils or eosinophils in the bronchoalveolar lavage, honeycombing opacity on HRCT, and fibroblast foci on biopsy, were shown to be associated with poor prognosis26-28. In this study, however, as bronchoalveolar lavage and/or surgical biopsy were rarely conducted, it was impossible to investigate the correlation with these factors. Among the factors that have been known to be associated with prognosis, the age, gender, pulmonary function, and presence of symptoms showed no statistical significance. Meanwhile, the patients with underweight had poorer prognosis. However, few studies have been conducted to investigate the correlation of BMI with prognosis in patients with IPF. BMI, which is calculated by dividing body weight (kg) by height (m)2, is used as a marker that is measured by the World Health Organization for obesity management. As it can easily identify the nutritional state of patients, it is also used for checking malnutrition in addition to obesity. Patients are classified into underweight (<18.5 kg/m2), normal (18.5~24.99 kg/m2), overweight (25~29.99 kg/m2), and obese status (≥30 kg/m2)29. As the correlation of chronic obstructive pulmonary disease (COPD) with BMI has been well known, BODE index that is comprised of BMI, airway obstruction, dyspnea, and exercise capacity has played an important role as a predictor of all-cause mortality in patients with COPD. Patients with moderate or severe COPD commonly have underweight. This was reported to be associated with increased metabolism and energy consumption as a result of systemic inflammatory response associated with pulmonary diseases. In addition, decreased pulmonary function was reported to be associated with decreased survival time in cystic fibrosis patients with malnutrition status30-32. However, few studies have been conducted to investigate the correlation of BMI with survival time in patients with IPF. Cano et al.33 reported that BMI was associated with prognosis of patients with restrictive pulmonary impairment, and that the BMI was one of mortality predictors in 446 patients who had chronic respiratory failure due to various causes. Among the aforementioned patients, 162 patients had restrictive pulmonary impairment33, but most of whom were patients with chest wall disorders or kyphoscoliosis. Unfortunately, in that study, as the ratio of patients with interstitial lung disease or IPF was not shown, and the result was obtained from patients who had chronic respiratory failure due to various causes, it was difficult to directly correlate BMI with mortality risk caused by IPF33. It was reported that high BMI contributed to the extension of the survival time of patients with IPF34, and that malnutrition status caused thymic atrophy and reduced T-lymphocyte function, thereby increasing infection risk and decreasing the survival35,36. Although it is difficult to make a conclusion on the association of BMI with the prognosis of IPF based on the results of the aforementioned studies alone, a further study is required to investigate the association of BMI with the prognosis of IPF. The guidelines of the ATS and ERS (2011) classified risk factors of mortality into two groups; baseline factors include level of dyspnea, DLco<40% predicted, desaturation≤88% during 6-minute walk test, extent of honeycombing on HRCT, and pulmonary hypertension, and longitudinal factors include increase in level of dyspnea, decrease in FVC by >10% absolute value, decrease in DLco by >15% absolute value, and worsening of fibrosis on HRCT. However, no assessment criteria for follow-up interval of HRCT or extent of fibrosis on HRCT has been established yet2. It is necessary to diagnose disease progression by assessing the status of patients in an interval of 4~6 months. In the case of clinical exacerbation, it is necessary to check dyspnea, O2 saturation, FVC, and DLco in a shorter interval. If 6-minute walk test is available, it is also recommended2. As echocardiography, which is conducted in patients with interstitial lung disease to assess pulmonary arterial hypertension, is not accurate37, echocardiography alone is not recommended for assessing pulmonary arterial hypertension. Disease such as lung cancer, coronary artery disease, or pulmonary embolism may affect prognosis, but it is unclear if annual HRCT test to check concurrent disease is useful2. It was reported that IPF patients with concurrent emphysema had poorer prognosis than those without emphysema. A long-term oxygen therapy could be required to treat both diseases, and the both diseases could have pulmonary hypertension as a concurrent disease. As emphysema, however, did not significantly affect patient survival, it is unclear if emphysema is a factor representing poor prognosis2,38,39. In this study, five and two patients with emphysema were included in the survival and death groups, respectively, which showed that the number of patients with emphysema was higher in the survival group than in the death group. However, due to the small patient number, it was statistically insignificant. Although all patients in this study were evaluated for survival status, the number of subject was so small that selection bias could have occurred. In addition, the multivariate analysis showed that among factors that were considered correlated with prognosis in previous studies, the presence of respiratory symptom and decreased pulmonary function at the time of diagnosis were not associated with prognosis, but low BMI and radiological exacerbation were associated with prognosis. Although this result seems to be inconsistent with the previous results, it is meaningful as it shows a possibility that BMI could be associated with not only COPD but also IPF. Furthermore, it could be helpful for determining the interval to conduct HRCT.
In conclusion, patients who had low BMI at the time of diagnosis and patients who showed radiologic progression on follow-up CT before 36 months after diagnosis had poor prognosis. Thus, these patients should be more carefully managed to reduce the risk of acute exacerbation.
References
- 1.American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 2.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Thoracic Society. Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 4.Kim DS, Collard HR, King TE., Jr Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006;3:285–292. doi: 10.1513/pats.200601-005TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagai S, Nagao T, Kitaichi M, Izumi T. Clinical courses of asymptomatic cases with idiopathic pulmonary fibrosis and a histology of usual interstitial pneumonia. Eur Respir J. 1998;11:131s. [Google Scholar]
- 6.Bjoraker JA, Ryu JH, Edwin MK, Myers JL, Tazelaar HD, Schroeder DR, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;157:199–203. doi: 10.1164/ajrccm.157.1.9704130. [DOI] [PubMed] [Google Scholar]
- 7.Nagai S, Kitaichi M, Hamada K, Nagao T, Hoshino Y, Miki H, et al. Hospital-based historical cohort study of 234 histologically proven Japanese patients with IPF. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:209–214. [PubMed] [Google Scholar]
- 8.Collard HR, King TE, Jr, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168:538–542. doi: 10.1164/rccm.200211-1311OC. [DOI] [PubMed] [Google Scholar]
- 9.Flaherty KR, Mumford JA, Murray S, Kazerooni EA, Gross BH, Colby TV, et al. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168:543–548. doi: 10.1164/rccm.200209-1112OC. [DOI] [PubMed] [Google Scholar]
- 10.Jegal Y, Kim DS, Shim TS, Lim CM, Lee SD, Koh Y, et al. Physiology is a stronger predictor of survival than pathology in fibrotic interstitial pneumonia. Am J Respir Crit Care Med. 2005;171:639–644. doi: 10.1164/rccm.200403-331OC. [DOI] [PubMed] [Google Scholar]
- 11.Nicholson AG, Colby TV, Dubois RM, Hansell DM, Wells AU. The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med. 2000;162:2213–2217. doi: 10.1164/ajrccm.162.6.2003049. [DOI] [PubMed] [Google Scholar]
- 12.Travis WD, Matsui K, Moss J, Ferrans VJ. Idiopathic nonspecific interstitial pneumonia: prognostic significance of cellular and fibrosing patterns: survival comparison with usual interstitial pneumonia and desquamative interstitial pneumonia. Am J Surg Pathol. 2000;24:19–33. doi: 10.1097/00000478-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Raghu G, Brown KK, Bradford WZ, Starko K, Noble PW, Schwartz DA, et al. A placebo-controlled trial of interferon gamma-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2004;350:125–133. doi: 10.1056/NEJMoa030511. [DOI] [PubMed] [Google Scholar]
- 14.Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 15.Scientific Committee of the Korean Academy of Tuberculosis and Respiratory Diseases. 2008 National Survey of Idiopathic Interstitial Pneumonia in Korea. Tuberc Respir Dis. 2009;66:141–151. [Google Scholar]
- 16.Panos RJ, Mortenson RL, Niccoli SA, King TE., Jr Clinical deterioration in patients with idiopathic pulmonary fibrosis: causes and assessment. Am J Med. 1990;88:396–404. doi: 10.1016/0002-9343(90)90495-y. [DOI] [PubMed] [Google Scholar]
- 17.Kondoh Y, Taniguchi H, Kawabata Y, Yokoi T, Suzuki K, Takagi K. Acute exacerbation in idiopathic pulmonary fibrosis. Analysis of clinical and pathologic findings in three cases. Chest. 1993;103:1808–1812. doi: 10.1378/chest.103.6.1808. [DOI] [PubMed] [Google Scholar]
- 18.Ambrosini V, Cancellieri A, Chilosi M, Zompatori M, Trisolini R, Saragoni L, et al. Acute exacerbation of idiopathic pulmonary fibrosis: report of a series. Eur Respir J. 2003;22:821–826. doi: 10.1183/09031936.03.00022703. [DOI] [PubMed] [Google Scholar]
- 19.Akira M, Hamada H, Sakatani M, Kobayashi C, Nishioka M, Yamamoto S. CT findings during phase of accelerated deterioration in patients with idiopathic pulmonary fibrosis. AJR Am J Roentgenol. 1997;168:79–83. doi: 10.2214/ajr.168.1.8976924. [DOI] [PubMed] [Google Scholar]
- 20.Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:1040–1047. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- 21.Kim DS, Park JH, Park BK, Lee JS, Nicholson AG, Colby T. Acute exacerbation of idiopathic pulmonary fibrosis: frequency and clinical features. Eur Respir J. 2006;27:143–150. doi: 10.1183/09031936.06.00114004. [DOI] [PubMed] [Google Scholar]
- 22.Parambil JG, Myers JL, Ryu JH. Histopathologic features and outcome of patients with acute exacerbation of idiopathic pulmonary fibrosis undergoing surgical lung biopsy. Chest. 2005;128:3310–3315. doi: 10.1378/chest.128.5.3310. [DOI] [PubMed] [Google Scholar]
- 23.Kubo H, Nakayama K, Yanai M, Suzuki T, Yamaya M, Watanabe M, et al. Anticoagulant therapy for idiopathic pulmonary fibrosis. Chest. 2005;128:1475–1482. doi: 10.1378/chest.128.3.1475. [DOI] [PubMed] [Google Scholar]
- 24.Kondoh Y, Taniguchi H, Yokoi T, Nishiyama O, Ohishi T, Kato T, et al. Cyclophosphamide and low-dose prednisolone in idiopathic pulmonary fibrosis and fibrosing nonspecific interstitial pneumonia. Eur Respir J. 2005;25:528–533. doi: 10.1183/09031936.05.00071004. [DOI] [PubMed] [Google Scholar]
- 25.Martinez FJ, Safrin S, Weycker D, Starko KM, Bradford WZ, King TE, Jr, et al. The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med. 2005;142(12 Pt 1):963–967. doi: 10.7326/0003-4819-142-12_part_1-200506210-00005. [DOI] [PubMed] [Google Scholar]
- 26.King TE, Jr, Schwarz MI, Brown K, Tooze JA, Colby TV, Waldron JA, Jr, et al. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med. 2001;164:1025–1032. doi: 10.1164/ajrccm.164.6.2001056. [DOI] [PubMed] [Google Scholar]
- 27.Flaherty KR, Colby TV, Travis WD, Toews GB, Mumford J, Murray S, et al. Fibroblastic foci in usual interstitial pneumonia: idiopathic versus collagen vascular disease. Am J Respir Crit Care Med. 2003;167:1410–1415. doi: 10.1164/rccm.200204-373OC. [DOI] [PubMed] [Google Scholar]
- 28.Nicholson AG, Fulford LG, Colby TV, du Bois RM, Hansell DM, Wells AU. The relationship between individual histologic features and disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2002;166:173–177. doi: 10.1164/rccm.2109039. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. Obesity: preventing and managing the global epidemic. Report on a WHO Consultation. WHO Technical Report Series No. 894. Geneva: World Health Organization; 2000. [PubMed] [Google Scholar]
- 30.Nici L, Donner C, Wouters E, Zuwallack R, Ambrosino N, Bourbeau J, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173:1390–1413. doi: 10.1164/rccm.200508-1211ST. [DOI] [PubMed] [Google Scholar]
- 31.Stark LJ, Powers SW. Behavioral aspects of nutrition in children with cystic fibrosis. Curr Opin Pulm Med. 2005;11:539–542. doi: 10.1097/01.mcp.0000183051.18611.e4. [DOI] [PubMed] [Google Scholar]
- 32.Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med. 2006;173:475–482. doi: 10.1164/rccm.200505-840OE. [DOI] [PubMed] [Google Scholar]
- 33.Cano NJ, Pichard C, Roth H, Court-Fortuné I, Cynober L, Gérard-Boncompain M, et al. C-reactive protein and body mass index predict outcome in end-stage respiratory failure. Chest. 2004;126:540–546. doi: 10.1378/chest.126.2.540. [DOI] [PubMed] [Google Scholar]
- 34.Alakhras M, Decker PA, Nadrous HF, Collazo-Clavell M, Ryu JH. Body mass index and mortality in patients with idiopathic pulmonary fibrosis. Chest. 2007;131:1448–1453. doi: 10.1378/chest.06-2784. [DOI] [PubMed] [Google Scholar]
- 35.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 36.Savino W. The thymus gland is a target in malnutrition. Eur J Clin Nutr. 2002;56(Suppl 3):S46–S49. doi: 10.1038/sj.ejcn.1601485. [DOI] [PubMed] [Google Scholar]
- 37.Arcasoy SM, Christie JD, Ferrari VA, Sutton MS, Zisman DA, Blumenthal NP, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med. 2003;167:735–740. doi: 10.1164/rccm.200210-1130OC. [DOI] [PubMed] [Google Scholar]
- 38.Mejía M, Carrillo G, Rojas-Serrano J, Estrada A, Suárez T, Alonso D, et al. Idiopathic pulmonary fibrosis and emphysema: decreased survival associated with severe pulmonary arterial hypertension. Chest. 2009;136:10–15. doi: 10.1378/chest.08-2306. [DOI] [PubMed] [Google Scholar]
- 39.Cottin V, Nunes H, Brillet PY, Delaval P, Devouassoux G, Tillie-Leblond I, et al. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J. 2005;26:586–593. doi: 10.1183/09031936.05.00021005. [DOI] [PubMed] [Google Scholar]