Abstract
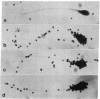
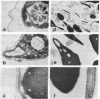
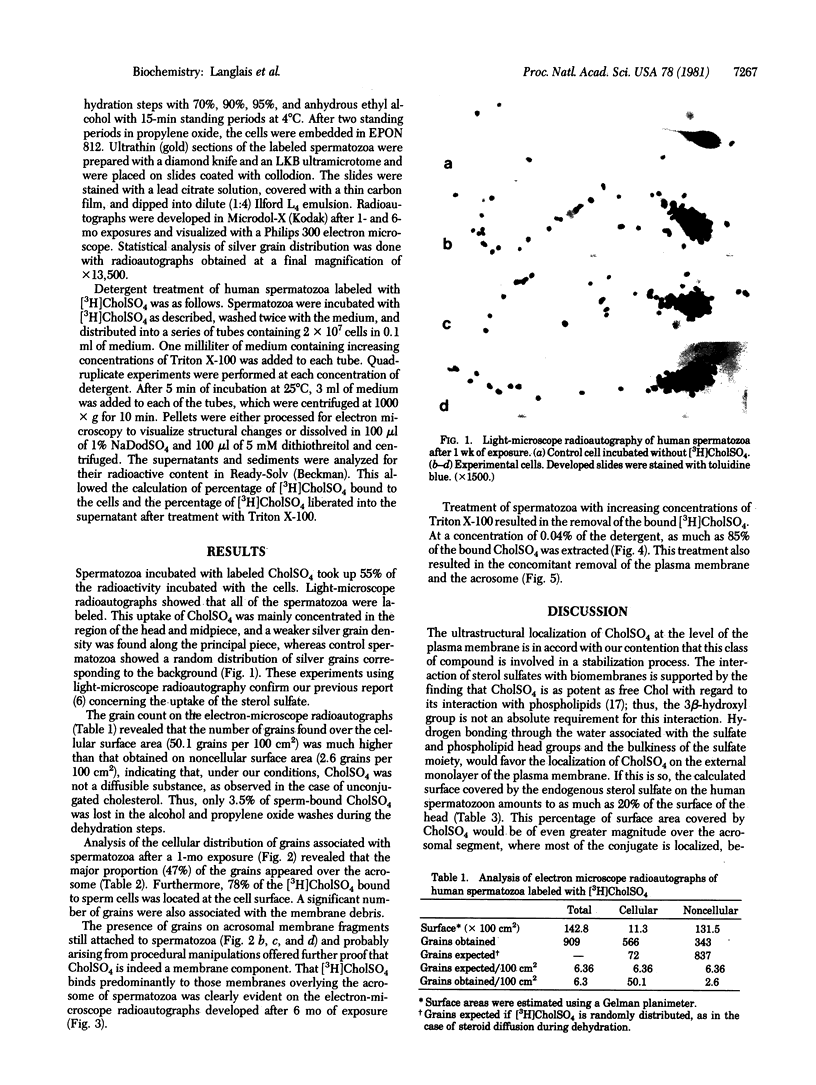
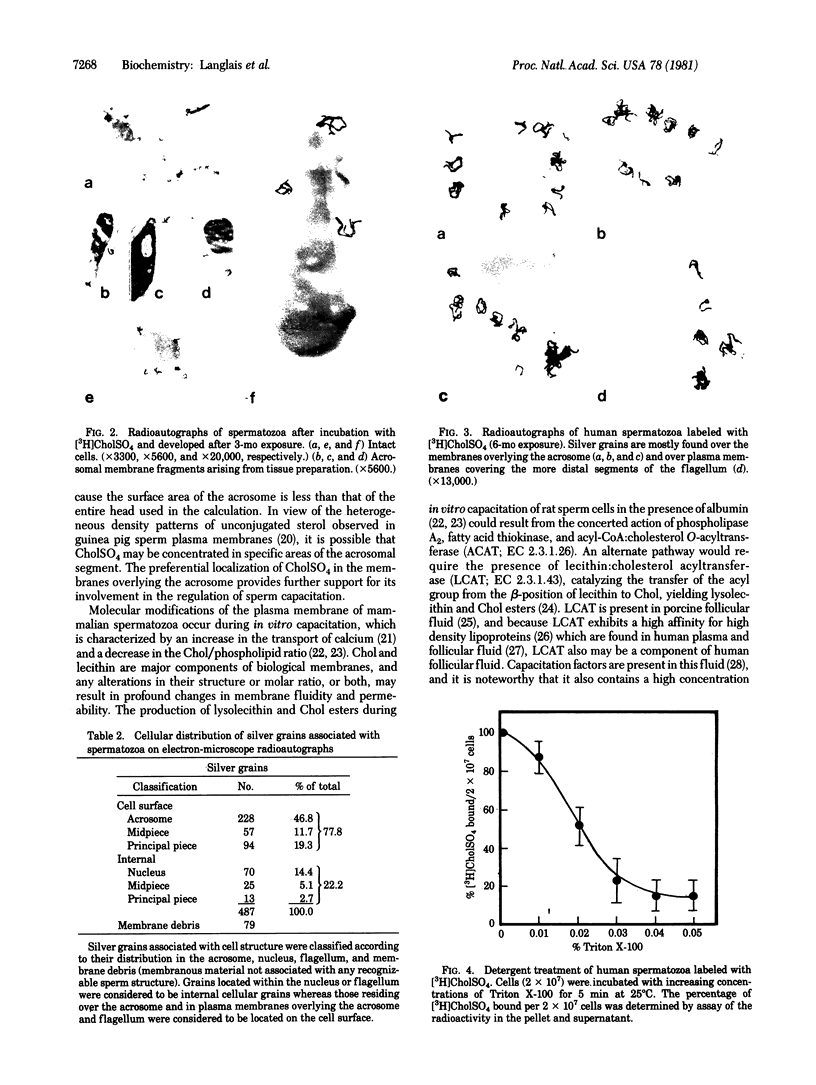
Cholesteryl sulfate is a normal constituent of human spermatozoa. The in vitro uptake of tritiated cholesteryl sulfate resulted in the labeling of all spermatozoa as demonstrated by light-microscope radioautography. The binding of the sterol sulfate was localized mainly in the head and midpiece. Radioautography at the level of the electron microscope revealed that the sterol sulfate is localized on the plasma membrane, mostly in the region of the acrosome. Further proof of this localization was obtained by selective dissolution of the plasma membrane and acrosome of the spermatozoa with low concentrations of Triton X-100. This treatment resulted in the simultaneous removal of tritiated cholesteryl sulfate bound to the spermatozoa. A hypothesis is presented concerning the role of cholesteryl sulfate as a membrane stabilizer and enzyme inhibitor during the maturation of spermatozoa in the epididymis. According to this hypothesis, the cleavage of the sulfate moiety within the female reproductive tract triggers a cascade of events leading to sperm capacitation and fertilization.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers J. J., Adolphson J. L., Chen C. H. Radioimmunoassay of human plasma lecithin-cholesterol acyltransferase. J Clin Invest. 1981 Jan;67(1):141–148. doi: 10.1172/JCI110006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers J. J., Lin J., Roberts G. P. Effect of human plasma apolipoproteins on the activity of purified lecithin: cholesterol acyltransferase. Artery. 1979 Jan;5(1):61–75. [PubMed] [Google Scholar]
- Barros C., Austin C. R. In vitro fertilization and the sperm acrosome reaction in the hamster. J Exp Zool. 1967 Dec;166(3):317–323. doi: 10.1002/jez.1401660304. [DOI] [PubMed] [Google Scholar]
- Bedford J. M. Sperm capacitation and fertilization in mammals. Biol Reprod Suppl. 1970;2:128–158. [PubMed] [Google Scholar]
- Beier H. M. Oviducal and uterine fluids. J Reprod Fertil. 1974 Mar;37(1):221–237. doi: 10.1530/jrf.0.0370221. [DOI] [PubMed] [Google Scholar]
- Bleau G., Chapdelaine A., Roberts K. D. The assay of cholesterol sulfate in biological material by enzymatic radioisotopic displacement. Can J Biochem. 1972 Mar;50(3):277–286. doi: 10.1139/o72-039. [DOI] [PubMed] [Google Scholar]
- Bleau G., Lalumiùre G., Chapdelaine A., Roberts K. Red cell surface structure. Stabilization by cholesterol sulfate as evidenced by scanning electron microscopy. Biochim Biophys Acta. 1975 Jan 28;375(2):220–223. doi: 10.1016/0005-2736(75)90190-x. [DOI] [PubMed] [Google Scholar]
- Bleau G., Vandenheuvel W. J., Andersen O. F., Gwatkin R. B. Desmosteryl sulphate of hamster spermatozoa, a potent inhibitor of capacitation in vitro. J Reprod Fertil. 1975 Apr;43(1):175–178. doi: 10.1530/jrf.0.0430175. [DOI] [PubMed] [Google Scholar]
- Briggs M. H. Steroid hormones and the fertilizing capacity of spermatozoa. Steroids. 1973 Oct;22(4):547–553. doi: 10.1016/0039-128x(73)90010-x. [DOI] [PubMed] [Google Scholar]
- Burck P. J., Zimmerman R. E. The inhibition of acrosin by sterol sulphates. J Reprod Fertil. 1980 Jan;58(1):121–125. doi: 10.1530/jrf.0.0580121. [DOI] [PubMed] [Google Scholar]
- Davis B. K., Byrne R., Bedigian K. Studies on the mechanism of capacitation: albumin-mediated changes in plasma membrane lipids during in vitro incubation of rat sperm cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1546–1550. doi: 10.1073/pnas.77.3.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. K., Byrne R., Hungund B. Studies on the mechanism of capacitation. II. Evidence for lipid transfer between plasma membrane of rat sperm and serum albumin during capacitation in vitro. Biochim Biophys Acta. 1979 Dec 12;558(3):257–266. doi: 10.1016/0005-2736(79)90260-8. [DOI] [PubMed] [Google Scholar]
- Demel R. A., Bruckdorfer K. R., van Deenen L. L. Structural requirements of sterols for the interaction with lecithin at the air water interface. Biochim Biophys Acta. 1972 Jan 17;255(1):311–320. doi: 10.1016/0005-2736(72)90030-2. [DOI] [PubMed] [Google Scholar]
- Elias P. M., Friend D. S., Goerke J. Membrane sterol heterogeneity. Freeze-fracture detection with saponins and filipin. J Histochem Cytochem. 1979 Sep;27(9):1247–1260. doi: 10.1177/27.9.479568. [DOI] [PubMed] [Google Scholar]
- Furukawa Y., Nishida T. Stability and properties of lecithin-cholesterol acyltransferase. J Biol Chem. 1979 Aug 10;254(15):7213–7219. [PubMed] [Google Scholar]
- Gabara B., Gledhill B. L., Croce C. M., Cesarini J. P., Koprowski H. Ultrastructure of rabbit spermatozoa after treatment with lysolecithin and in the presence of hamster somatic cells. Proc Soc Exp Biol Med. 1973 Sep;143(4):1120–1124. doi: 10.3181/00379727-143-37482. [DOI] [PubMed] [Google Scholar]
- Glomset J. A. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968 Mar;9(2):155–167. [PubMed] [Google Scholar]
- HABERMANN E. Manometrische Bestimmung von Phospholipase A. Biochem Z. 1957;328(6):474–484. [PubMed] [Google Scholar]
- Hax W. M., van Venrooij G. E., Denier van der Gon J. J., Elbers P. F. Cell communication induced by lysolecithin. J Membr Biol. 1973 Aug 30;13(1):61–78. doi: 10.1007/BF01868220. [DOI] [PubMed] [Google Scholar]
- Howell J. I., Lucy J. A. Cell fusion induced by lysolecithin. FEBS Lett. 1969 Aug;4(3):147–150. doi: 10.1016/0014-5793(69)80218-8. [DOI] [PubMed] [Google Scholar]
- Iwamatsu T., Chang M. C. In vitro fertilization of mouse eggs in the presence of bovine follicular fluid. Nature. 1969 Nov 29;224(5222):919–920. doi: 10.1038/224919a0. [DOI] [PubMed] [Google Scholar]
- Lalumière G., Bleau G., Chapdelaine A., Roberts K. D. Cholesteryl sulfate and sterol sulfatase in the human reproductive tract. Steroids. 1976 Feb;27(2):247–260. doi: 10.1016/0039-128x(76)90101-x. [DOI] [PubMed] [Google Scholar]
- Legault Y., Bleau G., Chapdelaine A., Roberts K. D. Steroid sulfatase activity of the hamster reproductive tract during the estrous cycle. Biol Reprod. 1980 Nov;23(4):720–725. doi: 10.1095/biolreprod23.4.720. [DOI] [PubMed] [Google Scholar]
- Legault Y., Bleau G., Chapdelaine A., Roberts K. D. The binding of sterol sulfates to hamster spermatozoa. Steroids. 1979 Jul;34(1):89–99. doi: 10.1016/0039-128x(79)90128-4. [DOI] [PubMed] [Google Scholar]
- Legault Y., Bouthillier M., Bleau G., Chapdelaine A., Roberts K. D. The sterol and sterol sulfate content of the male hamster reproductive tract. Biol Reprod. 1979 Jun;20(5):1213–1219. doi: 10.1095/biolreprod20.5.1213. [DOI] [PubMed] [Google Scholar]
- Lichtenstein A. H., Brecher P. Properties of acyl-CoA:cholesterol acyltransferase in rat liver microsomes. Topological localization and effects of detergents, albumin, and polar steroids. J Biol Chem. 1980 Oct 10;255(19):9098–9104. [PubMed] [Google Scholar]
- Lopata A., Patullo M. J., Chang A., James B. A method for collecting motile spermatozoa from human semen. Fertil Steril. 1976 Jun;27(6):677–684. doi: 10.1016/s0015-0282(16)41899-6. [DOI] [PubMed] [Google Scholar]
- Lucy J. A. The fusion of biological membranes. Nature. 1970 Aug 22;227(5260):815–817. doi: 10.1038/227815a0. [DOI] [PubMed] [Google Scholar]
- Lui C. W., Meizel S. Further evidence in support of a role for hamster sperm hydrolytic enzymes in the acrosome reaction. J Exp Zool. 1979 Feb;207(2):173–185. doi: 10.1002/jez.1402070202. [DOI] [PubMed] [Google Scholar]
- McRorie R. A., Williams W. L. Biochemistry of mammalian fertilization. Annu Rev Biochem. 1974;43(0):777–803. doi: 10.1146/annurev.bi.43.070174.004021. [DOI] [PubMed] [Google Scholar]
- Mukherjee A. B., Lippes J. Effect of human follicular and tubal fluids on human, mouse and rat spermatozoa in vitro. Can J Genet Cytol. 1972 Mar;14(1):167–174. doi: 10.1139/g72-019. [DOI] [PubMed] [Google Scholar]
- Nakagawa M., Kojima S. Effect of cholesterol sulfate and sodium dodecyl sulfate on lecithin-cholesterol acyltransferase in human plasma. J Biochem. 1976 Oct;80(4):729–733. doi: 10.1093/oxfordjournals.jbchem.a131333. [DOI] [PubMed] [Google Scholar]
- Notation A. D., Ungar F. Regulation of rat testis steroid sulfatase. A kinetic study. Biochemistry. 1969 Feb;8(2):501–506. doi: 10.1021/bi00830a007. [DOI] [PubMed] [Google Scholar]
- ROBERTS K. D., BANDI L., CALVIN H. I., DRUCKER W. D., LIEBERMAN S. EVIDENCE THAT STEROID SULFATES SERVE AS BIOSYNTHETIC INTERMEDIATES. IV. CONVERSION OF CHOLESTEROL SULFATE IN VIVO TO URINARY C-19 AND C-21 STEROIDAL SULFATES. Biochemistry. 1964 Dec;3:1983–1988. doi: 10.1021/bi00900a034. [DOI] [PubMed] [Google Scholar]
- Rosado A., Velázquez A., Lara-Ricalde R. Cell polarography. II. Effect of neuraminidase and follicular fluid upon the surface characteristics of human spermatozoa. Fertil Steril. 1973 May;24(5):349–354. doi: 10.1016/s0015-0282(16)39672-8. [DOI] [PubMed] [Google Scholar]
- Shalgi R., Kraicer P., Rimon A., Pinto M., Soferman N. Proteins of human follicular fluid: the blood-follicle barrier. Fertil Steril. 1973 Jun;24(6):429–434. [PubMed] [Google Scholar]
- Simpson E. R., Rochelle D. B., Carr B. R., MacDonald P. C. Plasma lipoproteins in follicular fluid of human ovaries. J Clin Endocrinol Metab. 1980 Dec;51(6):1469–1471. doi: 10.1210/jcem-51-6-1469. [DOI] [PubMed] [Google Scholar]
- Singh J. P., Babcock D. F., Lardy H. A. Increased calcium-ion influx is a component of capacitation of spermatozoa. Biochem J. 1978 Jun 15;172(3):549–556. doi: 10.1042/bj1720549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soupart P., Anderson M. L., Albert D. H., Coniglio J. G., Repp J. E. Accumulation, nature, and possible functions of the malachite green affinity material in ejaculated human spermatozoa. Fertil Steril. 1979 Oct;32(4):450–454. doi: 10.1016/s0015-0282(16)44303-7. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R. In vitro capacitation of golden hamster spermatozoa by homologous and heterologous blood sera. Biol Reprod. 1970 Oct;3(2):147–153. doi: 10.1093/biolreprod/3.2.147. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R., Noda Y. D., Fujimoto M., Nicolson G. L. The distribution of negative surface charges on mammalian spermatozoa. Am J Anat. 1972 Dec;135(4):497–519. doi: 10.1002/aja.1001350405. [DOI] [PubMed] [Google Scholar]
- Yao J. K., Chang S. C., Ryan R. J., Dyck P. J. A model for studying LCAT reaction: in vitro cholesterol esterification in pig ovarian follicular fluid. Lipids. 1978 Apr;13(4):225–231. doi: 10.1007/BF02533660. [DOI] [PubMed] [Google Scholar]
- Yao J. K., Chang S. C., Ryan R. J., Dyck P. J. Serum lipoproteins and albumin in the lecithin:cholesterol acyltransferase reaction. Biochem Biophys Res Commun. 1980 Jul 31;95(2):738–744. doi: 10.1016/0006-291x(80)90848-7. [DOI] [PubMed] [Google Scholar]