Abstract
In patients with colorectal carcinoma, studies have reported improved survival with increasing numbers of retrieved lymph nodes. These findings are puzzling, as increased node sampling was not correlated with significant change in disease staging. Although the physiologic processes underlying this correlation between number of lymph nodes sampled and survival remain unknown, the reported correlation has caused modifications to clinical and non-clinical practices. Herein, we review the literature and discuss potential etiologies responsible for the observed increased survival statistics. Literature regarding colorectal lymph node anatomy, molecular aspects of colorectal cancer, changes in tumor characteristics and utilization of lymph node sample numbers are evaluated. In addition, we present the mathematical concepts available for probabilistic prediction of diagnostic confidence based upon sample size. From evaluation of the aggregate literature, certain facts emerge which are not easily identified within the individual studies. Colorectal carcinoma appears to encompass a number of individual disease entities with different physiologic characteristics and likelihoods of metastasis. In addition, it appears the improved survival is likely multifactorial including effects from intrinsic tumor biology and tumor-host interactions along with ever changing clinical practices. Finally, because lymph node count is dependent on a number of variables and is correlated, but unlikely to be causally associated with survival, use of this number as a quality indicator is unwarranted. Based on statistical considerations, the current recommended goal of 12-15 recovered lymph nodes without evidence of metastatic disease provides approximately 80% negative predictive value for colorectal carcinoma metastasis.
Key Words: Lymph node count, colorectal carcinoma, cancer survival, colorectal carcinoma biology
Introduction
Colorectal cancer is the third most common cancer in the United States. The American Cancer Society estimated 150,000 new cases of colorectal cancer and nearly 50,000 colorectal cancer deaths for 2011 (1). With a disease affecting so many lives, there has been a substantial interest in its pathology. Of all the characteristics of the disease examined, lymph node status is the most significant predictor for determining patient survival in patients with colorectal cancer (2-4). Recently, multiple studies have correlated improved survival with increasing number of retrieved lymph nodes (5-10). These improved outcomes were originally attributed to better staging, which subsequently lead to better treatment with chemotherapy. However, a number of more recent studies have challenged this hypothesis (11,12).
A review of the literature reveals conflicting information. Some authors suggest increased numbers of harvested nodes could be a marker for better medical or surgical treatment (11,12), while others have shown a link between number of nodes and specific patient parameters including patient age, tumor location, molecular abnormalities, and tumor stage. It also has been observed that node positive disease above a certain staging threshold is not correlated with increased numbers of retrieved lymph nodes (13). Such observations suggest the association between increased number of nodes retrieved and increased survival rests upon multiple complex mechanisms including tumor-host interactions. To date, no definitive study has explained this apparently paradoxical finding. Within the context of this confusion, attempts have been made to optimize pathology practice. For instance, the College of American Pathologists recommends at least 12 lymph nodes be sampled in a colorectal cancer resection specimen (14). Based on such statements, there has been a push to use the number of retrieved lymph nodes as an indicator of quality of care.
In the context of such complexity and implication for the practices of surgery and pathology, an overarching review of the pertinent literature should prove valuable. Herein we review literature regarding colonic anatomy, molecular aspects of colorectal carcinoma, as well as current trends in tumor characteristics. We also propose a novel algorithm to predict the level of diagnostic confidence obtainable for a given number of sampled lymph nodes and mathematically describe some of the “rules of thumb” currently in use.
Colonic anatomy and lymph node drainage
A brief review of the anatomy of the vascular supply and lymphatic drainage of the colon provides a framework for discussion of colonic oncologic pathology. The vascular supply of the large colon is derived from the superior and inferior mesenteric arteries. The superior mesenteric artery supplies the portion of the colon derived from the midgut (cecum, appendix, ascending colon, right two-thirds of the transverse colon) while the inferior mesenteric artery supplies the segments derived from the hindgut (left third of the transverse colon, descending colon, sigmoid, rectum, and upper anal canal) (15). The unnamed branches of these arteries ramify between the muscle layers of the portion of colon which they supply, and continue to subdivide before ultimately terminating in the circular smooth muscle layers of the bowel wall as branches of the appendices epiploicae (15). The majority of the venous drainage of the colon occurs through the hepatic portal vein via the superior and inferior mesenteric veins, though a small portion of the rectum is drained into the internal iliac vein and the pudendal vein, via the middle rectal veins and the inferior rectal veins, respectively (15).
The route of lymphatic drainage of the colon largely mirrors that of the arterial circulation (Figure 1) in contrast to much of anatomy where lymphatic drainage mirrors the venous circulation (15). The lymphatic vessels of the cecum, ascending and proximal transverse colon drain into lymph nodes associated with the superior mesenteric artery while vessels of the distal transverse - and sigmoid colon, along with those from the rectum drain into nodes associated with the inferior mesenteric artery (15). Lymph nodes of the colon form four groups: the epicolic, paracolic, intermediate, and preterminal colic nodes. Epicolic nodes are minute nodules on the serosal surface of the colon. Paracolic nodes lie along the medial borders of the ascending, and descending colon as well as along the mesenteric borders of the transverse, and sigmoid colon. Intermediate nodes lie along the ileocolic, right colic, middle colic, left colic, sigmoid, and superior rectal arteries (15). Finally, preterminal nodes lie along the main trunks of the superior and inferior mesenteric arteries and drain into para-aortic nodes at the origin of these vessels. The drainage pattern of the lymphatic fluid from node to node begins with the nodes closest to the colon and progresses from multiple nodes through fewer and fewer nodes as the anastomoses between higher order nodes decrease. This process leads to a nomenclature of hierarchical designation for lymph nodes. Consequently, the para-aortic nodes are usually regarded as the highest nodes of the territory which they drain. Within the nodes at lower levels there is substantial redundancy in colonic coverage by lymphatic drainage possibly explaining the difficulty in determining sentinel lymph nodes as used in other organ resections (15). As such, a radical lymphadenectomy during resection for colorectal cancer requires the removal of the highest possible lymph nodes draining the area of the colon in which the tumor is located (15). Though Miscusi et al. showed in a small sample size that approximately 34 lymph nodes normally exist within the mesorectum (16), no studies have been performed that attempted to find the average number of lymph nodes present in the mesocolon.
Figure 1.
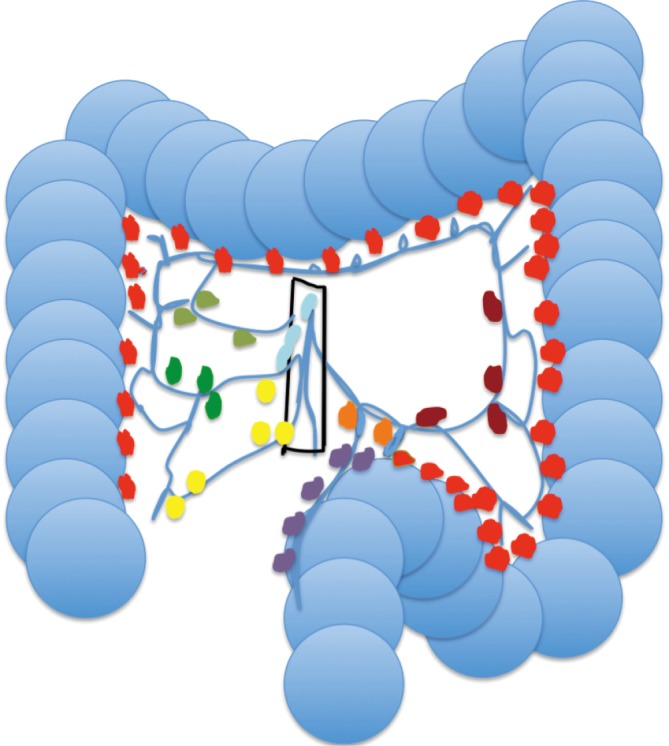
Diagram of the colon and lymphatic drainage. Lymph node key: right colic (dark green); superior mesenteric (aqua); middle colic (light green); left colic (dark red); sigmoid (purple); inferior mesenteric (orange). Paracolic lymph nodes (red and purple), intermediate lymph nodes (yellow, light green, dark green, orange, dark red), and preterminal lymph nodes (light blue). Epicolic nodes are not shown due to minute dimensions
Colorectal carcinoma and anatomic sites
There is a growing amount of evidence suggesting colon carcinomas of the right and left colon should be considered distinct entities. A number of differences between the characteristics of right-sided colorectal carcinomas (RCC) and left-sided colorectal carcinomas (LCC) have been repeatedly demonstrated within the literature. Right-sided tumors are commonly exophytic and present with complications of anemia or abdominal pain. In comparison, left-sided tumors more often cause obstructive symptoms. RCC tends to be: grossly more exophytic in appearance, of the mucinous histologic type and cytogenetically diploid, as well as demonstrate higher rates of microsatellite instability. LCCs, on the other hand, usually possess an infiltrative growth pattern, show chromosomal instability and are more often aneuploid (17).
Such differences would suggest RCC and LCC might behave differently. Intriguingly, the literature exploring such potential differences shows conflicting data with regard to patient survival based on laterality of tumor location, but is unambiguous that differences do exist. For example, Benedix et al. (18) demonstrated that right-sided tumors tended to occur in older women with more co-morbidities. These tumors tended to show a more poorly differentiated histology and there was an overall worse prognosis in patients with RCC as compared to LCC. Mequid et al. using retrospective survival analysis of data from the Surveillance, Epidemiology, and End Results Program (SEER) database between 1988 and 2003, showed that RCC had a 5% increased mortality risk relative to LCC (19). The Mequid study treated many of the parameters observed by the Benedix group as confounders, and as such, more stringently controlled for age, gender, race, tumor stage, tumor size, histologic grade, number of lymph nodes examined, and year of diagnosis. From these two studies, it appears there is a difference in behavior of RCC and LCC, but it is not precisely clear what the extent of the difference is.
Other studies appear to contradict these reports, although they also have shown differences in morbidity and mortality between patients with right-sided versus left-sided cancers. Weiss et al. also used SEER data to show that there was no statistically significant difference in mortality between RCC and LCC for all stages combined and for stage I disease. However, there was a significant decrease in mortality seen with stage II RCC as compared to LCC. This is in contrast to an increased mortality seen in stage III right-sided cancers when compared to the left colon (17). In addition, this group performed an extensive adjustment of confounding factors as well as limiting the sample to a more homogenous group of patients with a narrow age distribution (66 years and older as a result of Medicare linkage) and those considered for surgery with curative therapy (by excluding AJCC stage IV and those undergoing palliative procedures). While this body of literature implies differences in tumor biology based on anatomic location, it does not unambiguously define those differences. More studies are necessary to fully elucidate the phenomenon in question.
Molecular basis of colorectal cancer
Differences between right- and left-sided colorectal cancers are observed at the molecular level as well as the gross anatomic level. The primary mechanism through which molecular alterations lead to colorectal cancer appears to be genomic instability. Genomic instability may take a number of forms which are generally classified as chromosomal instability, DNA-repair defects, and aberrant DNA methylation (20).
Chromosomal instability is the most common type of genomic instability associated with the development of colorectal cancers. These somatic defects are characteristic of roughly 80-85% of sporadic colorectal cancers (20). The primary mechanism through which these genetic alterations occur is loss of heterozygosity at a number of gene loci. The most common genetic mutation in colorectal cancer inactivates the gene that encodes the adenomatous polyposis coli (APC) protein. APC acts as part of the β-catenin degradation complex that controls levels of β-catenin through proteolysis. When the APC gene on chromosome 5q is mutated, there is a loss of functional APC protein which allows for the inappropriate and constitutive activation of the β-catenin -Wnt signaling pathway, which is regarded as the initiating event in colorectal cancer (20).
Aberrant DNA methylation is an epigenetic mechanism of gene inactivation leading to genomic instability and associated carcinogenesis. 5-methylcytosine is a fifth DNA base that is introduced by DNA methylases within CpG islands of dinucleotides (20). In the normal genome, this occurs in non-coding regions of DNA and serves to “silence” un-needed portions of the genome. In the colorectal-cancer genome there is moderate depletion of overall cytosine methylation, but an increased amount of aberrant methylation within certain promoter-associated CpG islands. This can lead to aberrant promoter-associated methylation, which in turn induces epigenetic silencing of gene expression. A subgroup of loci that becomes aberrantly methylated is known as the CpG island methylator phenotype (CIMP) that is seen in about 15% of colorectal cancers and all tumors with aberrant methlyation of mutL homolog 1 (MLH1) (20).
A third form of genomic instability occurs through defects in DNA-repair mechanisms. These defects lead to inactivation of genes required for repair of base-base mismatches in DNA, a group known as mismatch-repair genes. This inactivation can be inherited, as in hereditary non-polyposis colon cancer (HNPCC) or acquired, as seen in tumors with previously mentioned methylation-associated silencing of a gene encoding a DNA mismatch repair protein (20). The loss of mismatch-repair function is most easily recognized by the presence of microsatellite instability. This phenomenon leads to the inability to repair strand slippage within repetitive DNA sequences and leads to changes in the size of mononucleotide or dinucleotide repeats (microsatellites) scattered throughout the genome. The most commonly seen genes mutated are MLH1, mutS homolog 2 (MSH2), postmeiotic segregation increased 2 (PMS2) and mutS homolog 6 (MSH6) (20,21).
Microsatellite instability, colon cancer, and lymph nodes
A number of studies have shown differences in the pathologic features, survival, and even number of lymph nodes retrieved based on the degree of microsatellite instability observed (20-24). Of note, colorectal carcinomas with high-frequency microsatellite instability (MSI-H), as defined by more than 30% of microsatellite loci showing instability, tend to have a less aggressive course than microsatellite stable (MSS) tumors and tumors with low-frequency microsatellite instability (21-23).
MSI-H tumors tend to be associated with certain characteristics. They tend to occur more frequently in the proximal right colon. MSI-H tumors tend to appear poorly differentiated, often accompanied by a mucinous or medullary architecture and a prominent peritumoral lymphocytic infiltrate (21-23). Interestingly, tumors with MSI-H tend to show an increased number of lymph nodes (25) as compared to tumors that are MSS. They also demonstrate a more favorable clinical course.
Changing trends
Trends are changing with regard to lymph nodes in colorectal cancer staging. Multiple papers reported significantly increased overall survival and disease-free survival as the number of lymph nodes retrieved increased regardless of whether the lymph nodes were positive or negative for metastatic disease (5-11). This increase in survival was initially attributed to more accurate staging; that is, increased numbers of retrieved nodes more accurately reflected the true node status of the patient. Thus, less under-staging results in appropriately utilized chemotherapy.
Increased numbers of harvested nodes increased the rate of node positivity, but with diminishing returns. Some studies showed a link between higher lymph node counts and node positivity. However, sampling beyond a certain number failed to significantly increase the sensitivity of diagnosing metastatic disease (12). More recent data also support this, showing that there appears to be an upper limit where more lymph nodes retrieved do not improve staging, and thus logically, should not affect survival. For instance, Baxter et al. recently demonstrated that in patients with pT3 colon carcinoma those with 7 nodes examined were equally as likely as those with 30 nodes examined to be node positive (13). In addition, they discovered that patients with very high lymph node counts (greater than 18) were actually less likely to have positive nodes than those with intermediate counts (12-17 lymph nodes). Ervine et al. in an exhaustive study of all lymph nodes in 391 consecutive cases, found only 1% in which upstaging would have been appropriate. The team further suggested that even these would likely have been upstaged without the additional node sampling due to other tumor findings (26). This suggestion has been confirmed in additional reports which (11) imply that up-staging is not the mechanism responsible for increased survival. There are likely other confounding factors associated with survival and the number of nodes retrieved. These may include tumor biological factors, tumor-host interaction and lymph nodes as a marker for improved surgical and medical care. Selection bias also may play a role in confounding, with pathologists searching less diligently for all nodes in specimens that show large numbers of lymph nodes grossly involved by tumor.
Changes also are occurring within disease trends specifically related to tumor laterality. While overall mortality rates of colon cancer have been decreasing over the last decade, right-sided tumors have been shown to be an increasing proportion of tumors (17-19). Recently, the idea has been proposed to separate right and left-sided tumors into distinct entities based upon some of the observed differences described above (17,19).
Total numbers of retrieved lymph nodes also has shifted over the last two decades. Many studies have noted that compared to a number of years ago, the average number of lymph nodes harvested per specimen has increased. This is likely due to increased awareness of the importance of the lymphadenectomy and proper staging. However, although this number is trending upwards (11,13,27,28), the majority of populations and institutions studied are not meeting current recommendations of 12 or more lymph nodes. In a population study performed in Canada, Baxter et al. found that only 37% of colorectal carcinoma patients were achieving this number (29). Likewise, Lagoudianakis et al. showed 41.6% compliance (30), while Bilimoria et al. showed greater than 60% of institutions did not meet the recommended 12 lymph node benchmark (31). A number of factors have been associated with increased number of lymph nodes retrieved in resection specimens for colorectal carcinoma, including length of resected bowel segment, patient age, and tumor location (28,30,32).
In addition, the prognostic capability of the more simplistic staging systems, such as American Joint Committee on Cancer (AJCC) staging system, recently has been questioned. Although attempting to further delineate prognostic groups, the creation of additional sub-stages in the AJCC seventh edition has led to what Weiser et al. considers “loss of the clear rank ordering which is the hallmark of categorical staging systems” (33). In their study, they created nomograms which incorporate number of nodes retrieved, number of positive nodes, age of patient, and tumor grade in addition to the T stage. These nomograms are felt to be better predictors of patient prognosis than the traditional TNM stage system, but are more complex to use (34).
While the focus traditionally has been on the effects the number of lymph nodes retrieved have on the prognosis of patients with colorectal carcinoma, current recommendations take a more pre-emptive approach. Prevention of invasive carcinoma and, if present, the detection of early stage cancers, through the use of a number of tests, either singly or in combination are expected to yield profound survival improvement (30). Gordon (35) summarizes that screening for CRC is justified because: it is a common and serious disease, various screening tests achieve accurate detection of early-stage disease, evidence shows that removing adenomatous polyps and detecting early stage disease will reduce mortality from disease, and benefits of screening outweigh its harms. Current guidelines recommend that those 50 years and older should undergo screening using either annual fecal occult blood testing (FOBT) with flexible sigmoidoscopy every five years or colonoscopy every 10 years, or some combination of both. Currently studies have shown that only about 40% of those eligible choose to undergo this screening. Recently, two less invasive tests, the CT colonography and Fecal DNA testing have been introduced, with the fecal DNA test showing a higher sensitivity and specificity for colorectal cancer detection than FOBT (35,36). It is hoped this will lead to improved overall screening for colorectal carcinoma with a subsequent improvement in survival.
Statistical considerations
There have been statistical studies attempting to provide rational guidelines for the number of lymph nodes that should be sampled in various situations (37,38). These studies made no experimental attempts to determine total nodes present or the number of positive nodes in a given cancer, but instead utilized mathematical principles to back-calculate probabilities. Each study made an implicit assumption that no selection bias exists in node sampling. Based on studies targeted toward discovering every single node present in a given specimen (39,40), this assumption is most certainly an inaccurate approximation, as the nodes not found through manual dissection and inspection are often much smaller. These smaller nodes yield a very different rate of metastatic disease than those easily palpated at the pathologist’s dissection table.
Another approach to designing guidelines for node sampling has been the correlation of various nodal findings with other case characteristics such as tumor size, invasiveness, and location as well as patient characteristics (28,30,32). These studies were correlative since they did not attempt to determine, by dissection, the true total underlying lymph node counts, and consequently their findings potentially could be nullified by alterations in practice or diagnostic definition.
From a probabilistic perspective, the sampling of lymph nodes for the determination of staging is a theoretically straightforward problem, following the same mathematics as any other series of random selections. For each sampled node, the probability of a negative result will depend upon the total number of nodes available to sample and the number of positive lymph nodes present as follows: Probability of sampling a negative node = [(n–x)/n], Where: n = total number of nodes available to sample, x = number of positive lymph nodes present within the specimen.
Each successively sampled lymph node will reduce the number of lymph nodes available for future sampling by 1. For multiple nodes sampled, the overall Negative Predictive Value (NPV) for metastatic disease will depend upon the product of the individual probabilities for each sampled lymph node. A generic equation expressing NPV for metastatic disease can be stated as follows: NPV = 1–[(n–x)! (n–s)!]/[n! (n–x–s)!], Where: n = total number of nodes available to sample, x = number of positive lymph nodes present in the sample, s = number of lymph nodes sampled.
Estimation of an appropriate minimum lymph node sample number for a desired NPV can be made if the number of expected positive lymph nodes in a case of node positive disease as well as the total number of lymph nodes present is known. Brown et al. performed a study yielding these data (39). From their work, it is possible to estimate the average number of lymph nodes present per centimeter of resected colon (three) as well as the average number of positive nodes present in surgical cases that have become metastatic (nine). While these numbers seem high, it should be kept in mind that these were collected in an exhaustive search utilizing fat-clearing methods. Utilizing these numbers, we have plotted the Negative Predictive Value (NPV) versus number of lymph nodes sampled and conclude that sampling 12-15 lymph nodes produces an NPV of 78-83% (Figure 2).
Figure 2.
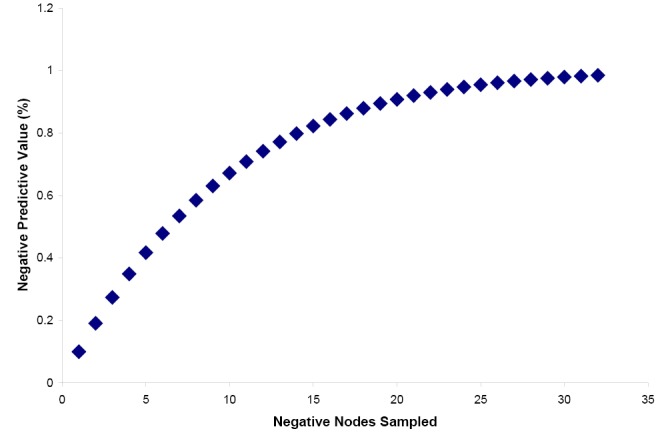
Negative Predictive Value vs. Number of Negative Lymph Nodes Sampled. Plot demonstrates the theoretical negative predictive value (NPV) for lymph node metastatic disease based upon the number of lymph nodes sampled, assuming each node sampled is negative for metastatic disease. The plot is based upon the equation presented in the text with the expectations that the average colonic resection contains a total of 90 lymph nodes and the average case of metastatic disease contains nine positive nodes. These expectations are based upon reference 39
Difficulties in staging
Current surgical guidelines (41) recommend excising the primary feeder arterial vessel supplying the involved section of colon, along with the corresponding mesocolon, lymph nodes and lymphatics. These guidelines recommend 5 cm of normal bowel surrounding the primary lesion. The resected material is then sent for pathology evaluation.
There are many factors involved in staging colon cancer, of which the most prognostically significant is the lymph node status (2-4). The College of American Pathologists currently recommends examination of 12 lymph nodes as a benchmark for proper staging (14); this is supported by numerous studies including the analysis above, although some even advocate a higher number (5,27). All specimens (like all patients) are not equal, however. Many times the standard approach to both surgery and staging must be modified.
From the pathologist’s perspective, no guidelines exist to standardize the process involved in lymph node search. Some institutions occasionally use fat-clearing methods, such as soaking the mesenteric tissue in a chemical soup that dissolves away the fat leaving behind the lymph nodes and other non-fatty tissue. However, most institutions do not routinely use fat clearing methods, and some use fat-clearing methods only when 12 nodes cannot be found. Brown et al. (39), as well as Scott (40), reported significantly increased lymph node harvests utilizing fat clearing techniques. They showed an average of 20.9 lymph nodes harvested using the traditional manual method. They were able to additionally examine 68.6 lymph nodes after clearance of mesenteric fat and submission of all remaining tissue. Of these additional nodes, 82% were smaller than 2 mm. Unarguably, while these techniques are useful, they are also time consuming, labor intensive, and utilize chemicals that are both toxic and expensive.
In addition, a recent study demonstrated that not only is the lymph node harvest non-uniform, microscopic lymph node counting is also not a uniformly reproducible process. This study demonstrated that lymph node counting varies not only between pathologists but between the same pathologist over a given time period (42).
Metastasis
Metastasis occurs when genetically unstable cancer cells are able to travel to new anatomic locations and adapt to a tissue microenvironment that is distant from the primary tumor. This process involves both the selection of traits that are beneficial to cancer cells and the concurrent development of traits within the tissue stroma that provides an appropriate milieu for invasion by metastatic cells (43-47). This process eventually allows for the incipient cancer cells to form macroscopic metastasis.
Lymph node status is the most important prognostic factor when staging colorectal cancer, because the detection of nodal metastasis will determine whether or not a patient receives adjuvant chemotherapy. Consequently, accurate staging for patients is of utmost import. Even with careful node dissection and examination, around 30% of all pN0 colon cancer patients still develop local, regional and/or distant disease recurrence (2). This finding may be due to lack of distinction within the pN0 stage between complete node negativity and micrometastatic disease. Recently, both micrometastases and isolated tumor cells are staged as pN0micro+. Although pN0 stage has traditionally been associated with better prognosis than higher N stage, studies have demonstrated that, as expected, there is increased risk associated with micrometastases. Studies have attempted to evaluate the impact micrometastasis and isolated tumor cells have on survival in otherwise node-negative colorectal cancer (2-4). For example, Bilchik et al. reported a significantly increased recurrence rate of 22% with micrometastases vs. 6% without micrometastases (48). Likewise, Faerden et al. reported 23% vs. 7% recurrence rate at 5 years in patients with and without micrometastases, respectively, as well as a 75% 5-year disease free survival with micrometastases vs. 93% 5-year disease free survival in patients without micrometastatic disease (P=0.012) (3). These studies demonstrate stage pN0 should be treated very differently from pN0micro+ and suggest a need for certain patients with pN0micro+ disease to receive some additional therapy. Currently, the Enroute+ study is accruing patients to determine the best therapy modality in patients with micrometastases. This randomized, multicenter trial will use ex vivo sentinel node mapping and immunohistochemistry to determine if patients harbor micrometastases, and if so, randomizing them for either adjuvant chemotherapy or no direct therapy (2).
Issues of quality
It is well known that, although only 36-41% of hospitals are routinely meeting the minimal sampling of 12 node recommendation, hospitals have improved their lymph node counts over the last two decades (11,13,27,28). Increasing the sampled number of lymph nodes leads to increased accuracy in node status and determination of the appropriate therapy for patients. As discussed above, increased lymph node counts are associated with significantly increased survival. Thus, the lymph node count has been touted by some to be a measure of quality by payers and policymakers.
However, there are concerns regarding implementation of 12 lymph nodes as a quality indicator. First, the studies which support a minimum harvest of 12 lymph nodes are primarily observational and cannot fully explain the association between increased lymph node count and improved survival (49). Data showing improved survival in both node-negative and node-positive patients with high numbers of lymph nodes suggests there is a biologic association or tumor-host association that may be an independent prognostic factor. If these associations are due to confounding, using lymph node counts as a quality indicator will have little impact.
Secondly, using the recommended minimum of 12 lymph nodes as a benchmark for quality assumes that lymph node numbers are relatively similar between patients. This is clearly not the case. Lymph node numbers have been shown to significantly vary by a variety of parameters. The number of lymph nodes retrieved has been directly proportional to length and width of the specimen as well as T stage/depth of invasion (32). Right-sided tumors and those with microsatellite instability are also associated with increased yield. Older patients have lower lymph node numbers, which may stem from decreasing immune function or changes in surgical technique. It also has been suggested that because of the low survival benefit or greater co-morbidity, surgeons are less likely to perform extensive resections on elderly patients (32). Low lymph node counts have been correlated with use of neoadjuvant therapy (50). Therefore, variation in lymph node count is less likely to be an indication of quality, but rather an indication of the heterogeneity of both patients and tumors.
The implementation of quality indicators may have additional unintended consequences as Simunovic and Baxter explain (51). Setting 12 lymph nodes as a quality indicator could lead pathologists to stop their search once this number is attained, thus leaving lymph nodes behind and potentially altering prognostic indicators. Surgeons may tend to resect slightly larger specimens in order to increase the likelihood of reaching this number, possibly causing increased morbidity. There is concern over what aspect of care the quality indicator would address. Both surgeons and pathologists are responsible for factors related to the number of lymph nodes examined. Meeting a quality indicator benchmark does not guarantee that lymph nodes were not overlooked. Likewise, if an institution does not meet this benchmark, it is very difficult to determine the cause and rectify the inadequacy.
This benchmark also could lead to increased utilization of fat-clearing techniques. These techniques are not ‘bad’ intrinsically, but they modify the population of lymph nodes examined. Most studies either specified that they did not use fat-clearing techniques, or they were based on data from large databases, which do not specify if these techniques were or were not used. Most hospitals do not routinely use these techniques. Therefore, the question of whether the prognosis is altered by the use of fat-clearing techniques to recover 12 lymph nodes remains unanswered. In addition, these techniques require exposure to hazardous chemicals and increased expense. Simunovic and Baxter proposed standardizing both the surgeon’s lymphadenectomy and pathologist’s lymph node examination before considering implementing lymph node counts as quality indicators (49-51).
Additional considerations and discussion
Review of the link between increased numbers of lymph nodes retrieved and increased survival amongst patients with colorectal carcinoma (5-8,52) illustrates the distinction between causality and association. It has been proposed that this association was secondary to more accurate clinical and pathologic staging, a phenomenon known as stage migration (9,10). However, more recent work appears to contradict this hypothesis by showing that the increased number of lymph nodes retrieved has not led to an increase in node-positive disease (12,13,51). Another possible association, the type of practice setting (academic institution versus community hospital) in which surgeries are performed, also has been disproved by some groups (51).
We speculate that the association of increased survival with increased number of lymph nodes retrieved likely results from the tumor biology of colorectal carcinoma in the context of changing disease incidence. The literature has shown an increasing incidence of right-sided colon cancers over the last decade, which may be due to changing practice patterns among surgeons and clinicians. In right-sided tumors, there are greater numbers of lymph nodes retrieved, and right-sided tumors tend to be associated microscopically with a dense peritumoral infiltrate of lymphocytes. It has also been shown that right-sided tumors are associated with a high-rate of microsatellite instability (MSI) and an improved overall prognosis as compared to tumors not associated with MSI. Improved survival may be due to increasing detection and therapy for cancers with better prognoses that happen to yield resection specimens with more easily identified lymph nodes.
In spite of a large body of literature, a number of questions remain. For one, studies that further investigate host-tumor interactions are needed. Though the literature has demonstrated an increase in the number of lymph nodes retrieved in right-sided colon cancers, and their association with MSI is well known, no research has been performed that easily explains this association. The increased number of lymph nodes retrieved in tumors located in the right colon may be secondary to cytokines released either from tumor cells, or in response to tumor cells (24,28). Whether the increased lymph node harvest is due to greater numbers of nodes or more easily located lymph nodes is unclear.
In addition, no experimental studies have definitively shown what the average expected lymph node retrieval should yield for a given specimen without use of fat-clearing solutions. This study has been performed for the mesorectum (16), but, to the best of our knowledge, no such study has been attempted for the mesocolon. Furthermore, a number of groups have used mathematical principles (37,38) or extensive mesenteric dissection techniques (39,40) to estimate the total number of lymph nodes. However, these remain estimates and do not account for selection bias inherent in a mesenteric lymph node dissection. Performing experiments that would more accurately ascertain the expected number of lymph nodes to retrieve for a given specimen may prove useful. This may aid the development of a more uniform approach to the mesenteric lymph node dissection, including standardization of the use of fat-clearing solutions for all colorectal cancer resection specimens, or using supplemental techniques only in cases that the desired lymph node number is not obtained. In addition, coming to a more rigorously calculated expected number of lymph nodes retrieved for a given specimen may result in the discovery of a more optimal disease specific number of lymph nodes with a better negative predictive value than the current blanket recommendation of 12-15 lymph nodes.
Another important issue surrounds what is actually being measured when lymph nodes are counted. When restricting their search to SEER-Medicare patient data, as opposed to all SEER date, Weiss et al. (17) were able to account for such confounders as patient co-morbidities, patient acuity, and clinician attributes. They showed that these factors did not contribute to the improved survival seen in patients with increased number of lymph nodes retrieved. However, this does not adequately explain why only 36-41% of hospitals are routinely attaining the minimum 12 lymph node recommendations. This may be due to a continued lack of awareness or training among both pathologists and surgeons, and may even be due to institutional cultures that are difficult to adjust. Studies are needed to better understand the barriers at play in the 59-64% of hospitals in which the 12 lymph node recommendation is not being achieved. This information could be used to evaluate more fully what variables, (i.e., the patient, surgeon, pathologist, or institution) best explains why the majority of hospitals are not retrieving the minimum suggested number of lymph nodes.
Finally, it has been proposed that the lymph node count may be used as a measure of quality, and as such, may be used in the future by third-party payers as a benchmark for reimbursement. As mentioned earlier, there are a number of variables that contribute to the overall lymph node count in colorectal cancer specimens. We acknowledge the ease and perceived objectivity associated with using lymph node counts as a measure of specimen adequacy. However, we agree with multiple authors who have cautioned against using lymph node number as a measure of quality.
In conclusion, we believe that the current CAP recommendation of 12-15 lymph nodes examined in a colorectal specimen is appropriate. Our calculations suggest sampling 12-15 lymph nodes will yield a roughly 80% negative predictive value (NPV) for metastasis of colorectal carcinoma. There has been an increase in the use of multiple tools for better screening and earlier detection of colorectal carcinoma, which may improve the ability to detect cancers at an earlier stage. This will likely be augmented through education regarding the importance of screening and increase in access to appropriate medical care and diagnoses. There are a number of variables that dictate the number of lymph nodes retrieved in any given specimen. Therefore, even though lymph node counts provide a single, objective data point, the value these numbers yield remains unclear. The truest indicator of quality care remains the patient’s outcome.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.American Cancer Society. Cancer Facts & Figures 2011. Atlanta: American Cancer Society [Internet] 2011 [cited 2012]; [about 56 p.]. Available online: http://www.cancer.org/Research/CancerFactsFigures/CancerFactsFigures/cancer-facts-figures-2011
- 2.Lips D, Koebrugge B, Liefers G, et al. The influence of micrometastases on prognosis and survival in stage I-II colon cancer patients: the Enroute+ study. BMC Surgery [Internet] 2011 [cited 2012];11:[about 9 p.]. Available online: http://www.biomedcentral.com/1471-2482/11/11 [DOI] [PMC free article] [PubMed]
- 3.Faerden AE, Sjo OH, Bukholm IR, et al. Lymph node micrometastases and isolated tumor cells influence survival in stage I and II colon cancer. Dis Colon Rectum 2011;54:200-6 [DOI] [PubMed] [Google Scholar]
- 4.Liefers GJ, Cleton-Jansen AM, van de Velde CJ, et al. Micrometastases and survival in stage II colorectal cancer. N Engl J Med 1998;339:223-8 [DOI] [PubMed] [Google Scholar]
- 5.Choi H, Law W, Poon J. The optimal number of lymph nodes examined in stage II colorectal cancer and impact of on outcomes. BMC Cancer [Internet] 2010 [cited 2012];10:[about 9 p.]. Available online: http://www.biomedcentral.com/1471-2407/10/267 [DOI] [PMC free article] [PubMed]
- 6.Kotake K, Honjo S, Sugihara K, et al. Number of lymph nodes retrieved is an important determinant of survival of patients with stage II and stage III colorectal cancer. Jpn J Clin Oncol. 2012;42:29-35 [DOI] [PubMed] [Google Scholar]
- 7.Chang GJ, Rodriguez-Bigas M, Skibber J, et al. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst. 2007;99:433-41 [DOI] [PubMed] [Google Scholar]
- 8.Le Voyer TE, Sigurdson ER, Hanlon AL, et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol 2003;21:2912-9 [DOI] [PubMed] [Google Scholar]
- 9.Tepper JE, O’Connell MJ, Niedzwiecki D, et al. Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol 2001;19:157-63 [DOI] [PubMed] [Google Scholar]
- 10.Ratto C, Sofo L, Ippoliti M, et al. Accurate lymph-node detection in colorectal specimens resected for cancer is of prognostic significance. Dis Colon Rectum 1999;42:143-54; discussion 154-8 [DOI] [PubMed] [Google Scholar]
- 11.Parsons HM, Tuttle TM, Kuntz KM, et al. Association between lymph node evaluation for colon cancer and node positivity over the past 20 years. JAMA 2011;306:1089-97 [DOI] [PubMed] [Google Scholar]
- 12.Bui L, Rempel E, Reeson D, et al. Lymph node counts, rates of positive lymph nodes, and patient survival for colon cancer surgery in Ontario, Canada: a population-based study. J Surg Oncol 2006;93:439-45 [DOI] [PubMed] [Google Scholar]
- 13.Baxter NN, Ricciardi R, Simunovic M, et al. An evaluation of the relationship between lymph node number and staging in pT3 colon cancer using population-based data. Dis Colon Rectum 2010;53:65-70 [DOI] [PubMed] [Google Scholar]
- 14.Compton CC, Fielding L, Burgart L, et al. Prognostic factors in colorectal cancer: College of American Pathologists consensus statement 1999. Arch Pathol Lab Med 2000;124:979-94 [DOI] [PubMed] [Google Scholar]
- 15.Healy J, Borley N. Large Intestine. In: Standrin S, editor. Gray’s anatomy (39th edition). Edinburgh: Elsevier Churchill Livingstone 2005:1177-84. [Google Scholar]
- 16.Miscusi G, di Gioia C, Patrizi G, et al. Anatomical lymph node mapping in normal mesorectal adipose tissue. Dis Colon Rectum 2010;53:1640-4 [DOI] [PubMed] [Google Scholar]
- 17.Weiss JM, Pfau PR, O’Connor ES, et al. Mortality by stage for right- versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results- medicare data. J Clin Oncol 2011;29:4401-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benedix F, Kube R, Meyer F, et al. Comparison of 17,641 patients with right-sided and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum 2010;53:57-64 [DOI] [PubMed] [Google Scholar]
- 19.Meguid RA, Slidell MB, Wolfgang CL, et al. Is there a difference in survival between right- versus left-sided colon cancers? Ann Surg Oncol 2008;15:2388-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markowitz SD, Bertagnolli MM. Molecular basis of colorectal cancer. N Engl J Med 2009;361:2449-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology 2010;138:2073-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gryfe R, Kim H, Hsieh E, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med 2000;342:69-77 [DOI] [PubMed] [Google Scholar]
- 23.Gafà R, Maestri I, Matteuzzi M, et al. Sporadic colorectal adenocarcinomas with high-frequency microstatellite instability: pathobiologic features, hMLH1 and hMSH2 expression, and clinical outcome. Cancer 2000;89:2025-37 [PubMed] [Google Scholar]
- 24.Søreide K, Nedrebø BS, Søreide JA, et al. Lymph node harvest in colon cancer: influence of microsatellite instability and proximal tumor location. World J Surg 2009;33:2695-703 [DOI] [PubMed] [Google Scholar]
- 25.Eveno C, Nemeth J, Soliman H, et al. Association between a high number of isolated lymph nodes in T1 to T4 N0M0 colorectal cancer and the microsatellite instability phenotype. Arch Surg 2010;145:12-7 [DOI] [PubMed] [Google Scholar]
- 26.Ervine A, Houghton J, Park R.Should lymph nodes from colorectal cancer resection specimens be processed in their entirety? J Clin Pathol 2012;65:114-6 [DOI] [PubMed] [Google Scholar]
- 27.Goldstein NS. Lymph node recoveries from 2427 pT3 colorectal resection specimens spanning 45 years: recommendations for a minimum number of recovered lymph nodes based on predictive probabilities. Am J Surg Pathol 2002;26:179-89 [DOI] [PubMed] [Google Scholar]
- 28.Gonsalves WI, Kanuri S, Tashi T, et al. Clinicopathologic factors associated with lymph node retrieval in resectable colon cancer: a veterans’ affairs central cancer registry (VACCR) database analysis. J Surg Oncol 2011;104:667-71 [DOI] [PubMed] [Google Scholar]
- 29.Baxter NN, Virnig DJ, Rothenberger DA, et al. Lymph node evaluation in colorectal cancer patients: a population-based study. J Natl Cancer Inst 2005;97:219-25 [DOI] [PubMed] [Google Scholar]
- 30.Lagoudianakis E, Pappas A, Koronakis N, et al. Lymph node harvesting in colorectal carcinoma specimens. Tumori 2011;97:74-8 [DOI] [PubMed] [Google Scholar]
- 31.Bilimoria KY, Bentrem DJ, Stewart AK, et al. Lymph node evaluation as a colon cancer quality measure: a national hospital report card. J Natl Cancer Inst 2008;100:1310-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen SS, Haupt BX, Ro JY, et al. Number of lymph nodes examined and associated clinicopathologic factors in colorectal carcinoma. Arch Pathol Lab Med 2009;133:781-6 [DOI] [PubMed] [Google Scholar]
- 33.Weiser MR, Gönen M, Chou JF, et al. Predicting survival after curative colectomy for cancer; individualizing colon cancer staging. J Clin Oncol 2011;29:4796-802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiser MR, Landmann RG, Kattan MW, et al. Individualized prediction of colon cancer recurrence using a nomogram. J Clin Oncol 2008;26:380-5 [DOI] [PubMed] [Google Scholar]
- 35.Gordon PH. Screening for colorectal carcinoma. Curr Oncol 2010;17:34-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson CD, Chen MH, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med 2008;359:1207-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gönen M, Schrag D, Weiser MR. Nodal staging score: a tool to assess adequate staging of node-negative colon cancer. J Clin Oncol 2009;27:6166-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joseph NE, Sigurdson ER, Hanlon AL, et al. Accuracy of determining nodal negativity in colorectal cancer on the basis of the number of nodes retrieved on resection. Ann Surg Oncol 2003;10:213-8 [DOI] [PubMed] [Google Scholar]
- 39.Brown HG, Luckasevic TM, Medich DS, et al. Efficacy of manual dissection of lymph nodes in colon cancer resections. Mod Pathol 2004;17:402-6 [DOI] [PubMed] [Google Scholar]
- 40.Scott KW, Grace RH. Detection of lymph node metastases in colorectal carcinoma before and after fat clearance. Br J Surg 1989;76:1165-7 [DOI] [PubMed] [Google Scholar]
- 41.Nelson H, Petrelli N, Carlin A, et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst 2001;93:583-96 [DOI] [PubMed] [Google Scholar]
- 42.Parkash V, Bifulco C, Feinn R, et al. To count and how to count, that is the question; interobserver and intraobserver variability among pathologists in lymph node counting. Am J Clin Pathol 2010;134:42-9 [DOI] [PubMed] [Google Scholar]
- 43.Martinez-Outschoorn UE, Pavlides S, Howell A, et al. Stromal-epithelial metabolic coupling in cancer; integrating autophagy and metabolism in the tumor microenvironment. Int J Biochem Cell Biol 2011;43:1045-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lentini A, Abbruzzese A, Provenzano B, et al. Transglutaminases: key regulators of cancer metastasis. Amino Acids [Internet] 2012 [cited 2012]: [about 8 p.] Available online: http://www.springerlink.com/content/q823p37h54948516 [DOI] [PubMed]
- 45.Bonuccelli G, Tsirigos A, Whitaker-Menezes D, et al. Ketones and lactate “fuel” tumor growth and metastasis: evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle 2010;9:3506-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong R. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res [Internet] 2011 [cited 2012];30:[about 14 p.]. Available online: http://www.jeccr.com/content/30/1/87 [DOI] [PMC free article] [PubMed]
- 47.Sreekumar R, Sayan B, Mirnezami A, et al. MicroRNA control of invasion and metastasis pathways. Front Genet [Internet] 2011 [cited 2012];2:[about 5 p.]. Available online: http://www.frontiersin.org/Non-Coding_RNA/10.3389/fgene.2011.00058/full [DOI] [PMC free article] [PubMed]
- 48.Bilchik A, Nissan A, Wainberg Z, et al. Surgical quality and nodal ultrastaging is associated with long-term disease-free survival in early colorectal cancer: an analysis of two international multicenter prospective trials. Ann Surg 2010;252:467-74; discussion 474-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baxter NN. Is lymph node count an ideal quality indicator for cancer care? J Surg Oncol 2009;99:265-8 [DOI] [PubMed] [Google Scholar]
- 50.Baxter NN, Morris AM, Rothenberger DA, et al. Impact of preoperative radiation for rectal cancer on subsequent lymph node evaluation; a population-based analysis. Int J Radiat Oncol Biol Phys 2005;61:426-31 [DOI] [PubMed] [Google Scholar]
- 51.Simunovic M, Baxter N.Lymph node counts in colon cancer surgery: lessons for users of quality indicators. JAMA 2007;298:2194-5 [DOI] [PubMed] [Google Scholar]
- 52.Chen SL, Bilchik AJ. More extensive nodal dissection improves survival for stages I to III of colon cancer: a population-based study. Ann Surg 2006;244:602-10 [DOI] [PMC free article] [PubMed] [Google Scholar]


