Abstract
A case report is presented of a patient diagnosed with esophageal adenocarcinoma with an extremely aggressive clinical course. Discordant expression of HER2 in longitudinally-collected biopsies was noted, with the original biopsy testing negative and a follow up biopsy testing positive. Upon retest of the original biopsy however, heterogeneity of HER2 expression was found among tumor clones across three distinct fields. The patient did not survive long enough to receive chemotherapy with trastuzumab, which has shown efficacy in tumors with HER2 positive expression. Understanding of the biological heterogeneity of HER2 expression in the primary tumor is crucial to detect HER2 positive clones, which may yield improved patient outcomes.
Key Words: HER2, metastatic esophageal adenocarcinoma
Introduction
Gastroesophageal cancer is the second most common cause of cancer-related death in the world, although the relative incidence in the US is much lower when compared globally (1). Despite decades of basic and clinical research, the prognosis for metastatic esophageal cancer remains poor, with 5-year survival ranging between 5-20%, and median overall survival less than 1 year (2). The use of combination chemotherapy regimens as first-line therapy has been associated with survival advantage (3,4).
HER2, also known as ERBB-2, a member of human epithelial growth factor receptor family, has been most extensively studied in the context of breast cancer. Treatment with trastuzumab, a monoclonal antibody targeting HER2, has become the standard of care for breast cancer with HER2 amplification (5,6). The recent ToGA study which applied a novel approach to treatment of gastroesophageal cancer by incorporation of trastuzumab into the treatment regimen (7), demonstrated a survival advantage in the subset of patients with HER2 overexpression.
Here we report a case of esophageal cancer, which was initially diagnosed as HER2 negative by fluorescent in-situ hybridization (FISH). By contrast, a biopsy of one of the metastatic sites showed HER2 amplification. Surprisingly, upon retesting of the original biopsy, one of the 3 areas tested in the same biopsy was positive for amplification, while two other areas tested negative.
Case report
A 54-year-old male with a past medical history of heavy drinking and smoking presented in April, 2011 for progressively worsening dysphagia of 3 months’ duration. He underwent esophagogastroduodenoscopy (EGD), which revealed an ulcerated circumferential mass at 20 to 26 cm in the mid distal third of esophagus. Endoscopic ultrasound showed the mass was abutting the aorta, and biopsies confirmed poorly differentiated adenocarcinoma. Further workup with Positron Emission Tomography-Computed Tomography Scan (PET-CT) and magnetic resonance imaging (MRI) showed stage IV disease with metastases involving the right hilum, lower left lung lobe and pathological fracture of the right clavicle. HER2 testing did not detect amplification on the biopsy at initial diagnosis.
In May, 2011, the patient began radiation therapy to the esophagus concurrently with chemotherapy consisting of carboplatin/Paclitaxol/5-fluorouracil (5-FU). After completion of the course, he started to complain of weakness on his left side mainly involving his left lower extremity. An MRI of the brain in July, 2011, revealed at least 10 lesions. He received whole brain radiation, and weakness in the left leg improved significantly.
In August, 2011, repeat PET-CT showed progressive disease with involvement of right and left lobes of the liver, left lower lung, left gluteal muscle, left pectoral muscle and a polypoid area in the stomach.
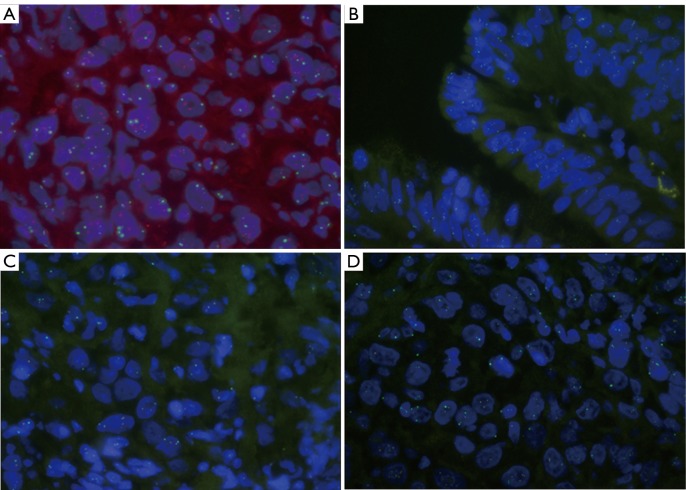
Between April when he was diagnosed and late August of 2011 when he was first seen at our Center for further workup and treatment, he had lost 70 pounds, albeit his performance status level remained at ECOG 1. He also suffered from severe pain and anorexia. In addition to symptomatic management, he was started on chemotherapy with a regimen consisting of 5-FU, Oxaliplatin, and Docetaxel. Although he tolerated treatment with this regimen very well initially, he continued to experience weight loss and dehydration, and a percutaneous endoscopic gastrostomy (PEG) tube was recommended. During the PEG tube insertion procedure, a firm, friable nodule approximately 1-2 cm was noted in the anterior proximal gastric antrum, and a biopsy was taken for further study. The new biopsy revealed an adenocarcinoma, consistent with primary tumor. However, in contrast to the initial negative result, HER2 was found to be amplified (Clarient, Figure 1A). In view of the discrepancy of the HER2 results, repeat testing of the initial tumor block was ordered and the HER2 study was carried out on 3 different areas by the same laboratory that had performed the initial testing. Surprisingly, it showed contradictory results with one of three areas exhibiting HER2 amplification (Figure 1B), while no amplification was noted in the other two areas, consistent with the original diagnosis (Figure 1C, D). Given the positive outcomes experienced by patients exhibiting HER2 positive expression in the the recent ToGA study, addition of trastuzumab treatment was planned for the subsequent chemotherapy cycle. Unfortunately, the patient died before he could be started on trastuzumab therapy.
Figure 1.
A. Gastric Nodule Biopsy (9/16/2011): HER2/neu by Fluorescence in Situ Hybridization HER2/CEP17 Ratio 3.0, Amplified; B. Esophageal Mass Biopsy (4/11/2011) HER2/neu by Fluorescence in Situ Hybridization HER2/CEP17 Ratio 2.5, Amplified; C. Esophageal Mass Biopsy (4/11/2011) HER2/neu by Fluorescence in Situ Hybridization HER2/CEP17 Ratio 1.4, UnAmplified; D. Esophageal Mass Biopsy (4/11/2011) HER2/neu by Fluorescence in Situ Hybridization HER2/CEP17 Ratio 1.2, Unamplified
Discussion
Treatment of metastatic gastroesophageal cancer remains very challenging despite the recent use of various combinations of chemotherapeutic agents. For example, in the pivotal phase 3 REAL-2 study, no significant advantage was found among four different three-drug combination regimens that were assessed, with a comparable median overall survival, reported between 9 to 11 months for all regimens (3).
Approximately 24% of gastroesophageal adenocarcinomas (GE-AC) overexpress HER2. HER2 overexpression in GE-AC has been associated with more aggressive biological behavior and poor outcome, but has not been associated with gender or age at time of diagnosis (8). Chemotherapy in combination with trastuzumab prolonged the median overall survival of gastroesophageal cancer significantly to 13.8 months, and higher expression of HER2 was predicted to confer a better prognosis, extending mean overall survival from under 12 months to approximately 16 months (7). Combination of trastuzumab and chemotherapy represents a new standard of care in HER2 positive gastroesophageal cancer. It is predictable that the anti-HER2 pathway will play a more important role in the foreseeable future with the introduction of new agents such as lapatinib or pertuzumab.
It is well known that HER2 positive breast cancer can become HER2 negative following treatment with trastuzumab (9). However, there is growing body of evidence that there is discordance of HER2 status between the primary breast cancer and metastatic sites or circulating tumor cells (10,11). A recent study on HER2 status in gastric cancer showed high concordance rates between primary and metastatic sites. Of 68 cases, only one case of HER2 negative gastric cancer showed HER2 positivity in a metastatic site. One possible explanation for the differences in rates of concordance between breast and GE carcinoma could be the biological differences between these two tumor types (12). Another potential explanation could be that the tumor biology can be heterogeneous and the systemic therapy has variable impact on selection and progression among tumor clones depending on their characteristics (13). Our case illustrates this unique biological phenomenon. The primary biopsy was studied retrospectively after the metastatic site tested positive for HER2 amplification. Interestingly, reexamination revealed heterogeneity of the HER2 status with both positive and negative clones detected. It is possible that a HER2 positive clone metastasized and progressed in spite of the systemic treatment. In the future, increasingly effective therapies will have greater potential for exerting selective pressure on the clones of gastroesophageal cancer. Improved understanding of the metastatic disease, in particular, the status of important biological markers, will provide invaluable prognostic and predictive information and subsequently direct therapeutic options.
In summary, we report a case of esophageal adenocarcinoma with an extremely aggressive clinical course. Repeat biopsy and HER2 testing led to a better understanding of its biological heterogeneity. In selected cases, it may be advisable to perform a rebiopsy and a retest for the HER2 status in order to direct further therapy.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Society AC: Cancer Facts & Figures. American Cancer Society, 2011. [Google Scholar]
- 2.Howlader N, Krapcho M, Neyman N, et al. (eds). SEER Cancer Statistics Review, 1975-2008. National Cancer Institute. Bethesda, MD, 2011. [Google Scholar]
- 3.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36-46 [DOI] [PubMed] [Google Scholar]
- 4.Cunningham D, Okines A, Ashley S.Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2010;362:858-9 [DOI] [PubMed] [Google Scholar]
- 5.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011;365:1273-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92 [DOI] [PubMed] [Google Scholar]
- 7.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97 [DOI] [PubMed] [Google Scholar]
- 8.Tanner M, Hollmén M, Junttila TT, et al. Amplification of HER2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol 2005;16:273-8 [DOI] [PubMed] [Google Scholar]
- 9.Mittendorf EA, Wu Y, Scaltriti M, et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin Cancer Res 2009;15:7381-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zidan J, Dashkovsky I, Stayerman C, et al. Comparison of HER2 overexpression in primary breast cancer and metastatic sites and its effect on biological targeting therapy of metastatic disease. Br J Cancer 2005;93:552-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fehm T, Müller V, Aktas B, et al. HER2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast Cancer Res Treat 2010;124:403-12 [DOI] [PubMed] [Google Scholar]
- 12.Bozzetti C, Negri FV, Lagrasta CA, et al. Comparison of HER2 status in primary and paired metastatic sites of gastric carcinoma. Br J Cancer 2011;104:1372-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vecchi M, Confalonieri S, Nuciforo P, et al. Breast cancer metastases are molecularly distinct from their primary tumors. Oncogene 2008;27:2148-58 [DOI] [PubMed] [Google Scholar]