Abstract
Food insecurity, micronutrient deficits, dyslipidemia, insulin resistance, obesity, cardiovascular disease, and bone disorders complicate the treatment of HIV infection. Nutrition and exercise interventions can be effective in ameliorating these symptoms that are associated with HIV and antiretroviral therapy (ART). In this literature review, we examine the most recent nutrition and exercise interventions for HIV-infected patients. Macronutrient supplementation can be useful in treating malnutrition and wasting. Multivitamin (vitamin B complex, vitamin C, and vitamin E) supplements and vitamin D may improve quality of life and decrease morbidity and mortality. Nutritional counseling and exercise interventions are effective for treating obesity, fat redistribution, and metabolic abnormalities. Physical activity interventions improve body composition, strength, and fitness in HIV-infected individuals. Taken collectively, the evidence suggests that a proactive approach to nutrition and physical activity guidance and interventions can improve outcomes and help abrogate the adverse metabolic, cardiovascular, and psychological consequences of HIV and its treatments.
Keywords: HIV, chronic disease and HIV, nutrition, physical activity, interventions, cardiometabolic disease, malnutrition, antiretroviral therapy (ART), multivitamin supplementation, vitamin D
INTRODUCTION
Since the advent of antiretroviral therapy (ART), patients with the human immunodeficiency virus (HIV) have longer life expectancies, but chronic illnesses such as cardiovascular and bone disease are becoming more prevalent in this population [1]. Individuals with HIV have higher rates of cardiovascular disease (CVD) than uninfected subjects, likely because of a combination of traditional risk factors, HIV-related inflammation, and adverse effects of antiretroviral therapy. In adults with HAART-related fat redistribution, several studies have suggested an increase in the risk of myocardial infarction relating to the level of viral control (increased inflammation) or to antiretroviral exposures [2]. Sudden cardiac death has been shown to be disproportionately represented in HIV-infected adults, accounting for 13% of all deaths and 86% of cardiac deaths [3]. Exogenous obesity, common among perinatally HIV-infected youth [4], can also contribute to CVD risk.
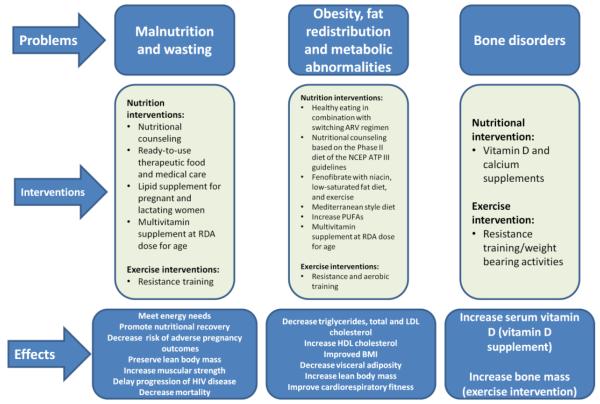
The Academy of Nutrition and Dietetics recognizes the importance of nutrition as a primary intervention in patients with HIV and suggests that HIV-infected patients receive nutritional counseling and assurance of food and nutrition security [5]. HIV infection leads to impaired immune function and nutrition independently affects the immune system [6]. Thus, interventions to improve nutritional status can positively impact the course of the disease [7]. In their review article on nutrition and disease progression, Chandrasekhar and Gupta found that there are challenges in studying the impact of nutrition in HIV-infected individuals due to heterogeneity in patient population, nutrient doses and combinations, baseline levels of deficiency, and study endpoints [8]. Their review of 31 clinical trials found few macronutrient and micronutrient supplementation trials improved CD4 count or decreased viral load. Nevertheless, nutrition and physical activity play an integral role in the overall care and treatment of HIV-infected patients [9, 10]. Exercise program can be effective in improving the health outcomes of patients with HIV undergoing ART to improve body composition and cardiovascular fitness, reduce the risk of diabetes and hyperlipidemia, thereby decreasing CVD risk. Figure 1 provides a summary of nutrition and physical activity interventions that can complement pharmacological interventions to treat malnutrition and wasting, obesity and metabolic abnormalities, and bone disorders.
Figure 1.
Interventions for Nutritional and Metabolic Disorders in HIV
SEARCH STRATEGY
Recent intervention studies with exercise and nutrition related outcomes in HIV-infected individuals were included in this review. We searched PUBMED (US National Library of Medicine, NIH, Bethesda, MD) for articles published between January 2010 and May 2012 using one of the key words ‘acquired immunodeficiency syndrome, acquired immune deficiency syndrome virus, human immunodeficiency virus, AIDS, HIV’ in combination with one of the key words ‘diet, nutrition, food, micronutrient, macronutrient, vitamin, physical therapy, exercise’. The search was limited to those written in English. We also checked the reference lists of relevant publications returned by the above key words. We excluded publications that did not relate to exercise or nutrition in HIV-infected individuals. Searches yielded 22 publications with nutrition and lifestyle interventions and 10 in exercise in HIV-infected children, adults, and pregnant women.
NUTRITIONAL INTERVENTIONS
Macronutrient interventions
Table 1 summarizes the current studies on nutritional interventions and their effects on HIV-infected individuals. Household food insecurity is a major contributor to nutritional problems in HIV-infected patients. Food security is defined as having physical, social, and economic access to sufficient food to meet dietary needs [11]. HIV-infected patients often live in poverty and can have increased energy needs, making food insecurity more common in HIV-infected individuals [12]. Food insecurity has a number of adverse consequences including macronutrient and micronutrient deficiencies, immunologic decline, mental health consequences, increased risk of vertical and horizontal transmission, obesity and increased morbidity and mortality [11, 13-15].
Table 1.
Nutrition Interventions to Address Chronic Disease and HIV
| Nutrition Intervention | Authors | Source, Population | Effect |
|---|---|---|---|
| Nutritional education and counseling | |||
| Nutritional counseling based on the Phase II diet of the National Cholesterol Education Program (NCEP) Adult Treatment Panel III |
Lazzaretti, et al. 2012 |
Brazil; > 17 years | Decreases fat intake78 Decreases triglycerides78 |
| Mediterranean style diet | Tsiodras, et al. 2009 |
Boston, MA; adults | Decreases insulin resistance82 Increases HDL cholesterol82 |
| Fenofibrate and niacin with low- saturated-fat diet and exercise |
Balasubramanyam, et al. 2011 |
Houston, TX; adults with fasting triglycerides > 150 mg/dl |
Increases HDL cholesterol80 Decreases non-HDL cholesterol80 Decreases triglycerides80 Improves hypoadiponectinemia80 |
| Food supplementation | |||
| Ready-to-use therapeutic foods | Ahoua, et al. 2011 | Kenya, Uganda; >15 years malnourished patients |
Promote nutritional recovery15 |
| Lipid nutrient supplementation | Kayira, et al. 2012 | Malawi; lactating women |
Reduction in weight loss17 |
| Fatty-acid supplementation | |||
| Polyunsaturated fatty acids | Oliveira, et al. 2011; Peters, et al. 2012 |
US, Canada, Europe; adults |
Reduces triglycerides83, 84 |
| Functional food | |||
| Probiotics | Hummelen, et al. 2010, 2011a 2011b; Irvine, et al. 2011 |
Africa, Brazil; adults | Alleviates gastrointestinal symptoms19-21 Improves tolerance to ART19-21 Decrease fatigue22 |
| Micronutrient supplementation | |||
| Multivitamin supplementation (vitamin B complex, vitamin C, and vitamin E) |
Fawzi, et al. 2004, 2007; Kawai, et al. 2010; Irlam, et al. 2012 |
Africa; pregnant and lactating women Resource-limited settings; adults and children |
Improves hemoglobin concentration26 Reduces risk of anemia26 Increases CD4 counts25 Delays progression of HIV disease25 Decreased risk of adverse pregnancy outcomes27 Reduces recurrence of pulmonary tuberculosis in those co-infected with tuberculosis and HIV30 |
| Vitamin A supplementation | Wiysonge, et al. 2011; Irlam, et al. 2012 |
Africa; pregnant women |
Improves birth weight of HIV-exposed uninfected infants33 Reduces all cause mortality in children30 |
| Vitamin D supplementation | Havens, et al. 2012; Childs, et al. 2012; Arpadi, et al. 2012; Longenecker, et al. 2012 |
US, Puerto Rico; young adults, Global; adults New York, NY; children Cleveland, OH; adults |
Decreases parathyroid hormone53, 54 Improves vitamin D status but no effect on bone density in children57 Increases serum 25(OH)D concentrations, improves cholesterol, increases HOMA-IR, no change in endothelial function55 |
Food-based interventions can treat malnourished HIV-infected patients and it is necessary to address both the individual needs of the patients and the food security of their households [16, 17]. Ready-to-use therapeutic foods effectively treat malnutrition of HIV-infected individuals in developing nations [18]. In a study of 1340 HIV-infected adults in sub Saharan Africa with body mass indices of less than 17 kg/m2, ready-to-use therapeutic food (2000 kilocalories per day) increased the chances of nutritional recovery in severely malnourished HIV-infected patients [18]. However, this retrospective analysis also showed that 48% of patients failed therapy because of lost-to-follow-up, poor adherence to ART, or severe malnutrition at baseline. This suggests that provision of adequate calories is only one component to effective interventions that improve nutritional status among HIV-infected patients.
Incentives provided through food supplements can be a helpful method to improve clinical adherence [19]. In the Breastfeeding, Antiretrovirals, and Nutrition (BAN) Study in Malawi, 3572 HIV-infected women were randomized to receive a lipid nutrient supplement (LNS) or not, while breastfeeding. This supplement provided 700 kilocalories of energy, 20 grams of protein, and micronutrients (except for vitamin A) to meet lactation needs. There was a significant reduction in weight loss associated with the LNS, regardless of receipt of ART [20]. This well designed, randomized controlled clinical trial suggests that providing macronutrients during nutritionally vulnerable periods curtails weight loss in HIV-infected women. In contrast, Kindra found that a daily serving of a nutritional supplement that provided 280 kilocalories of energy and 8 grams of protein in a randomized controlled clinical trial had no significant impact on maternal or infant outcomes. The latter study may not have provided sufficient calories to support the additional energy needs of pregnant HIV-infected women [21]. In a small group of mothers with low BMI at baseline in the aforementioned study, the supplement was associated with preservation of lean body mass.
Probiotics can potentially play a role in the care and treatment of HIV-infected patients by alleviating gastrointestinal symptoms and improving tolerance to ART. However, their effects on the immune system are not well documented [22-24]. Probiotic yogurt can decrease fatigue and facilitate achieving daily nutrient requirements in HIV-infected individuals in Tanzania, although this study was not randomized and was retrospective in nature [25].
For HIV-infected individuals who suffer from malnutrition and food insecurity, nutritional supplements can be effective if administered in adequate amounts. HIV populations at higher risk include those with increased metabolic demands such as pregnant and lactating women, children, and those with active infections. Factors other than access to sufficient food sources should be taken into account when considering interventions aimed at nutritional deficits. Future intervention efforts should define optimal nutritional requirements so that supplements can be dosed in adequate amounts or administered by meal replacements. In developing nations, where malnutrition is of great concern, research should focus on locally based and sustainable foods. Food supplements that are economically feasible and efficient have the potential to improve nutritional intake, curtail weight loss, and alleviate HIV-associated symptoms.
Micronutrient Interventions
Micronutrient malnutrition - deficiencies in vitamins and minerals - can lead to fatigue, anemia, reduced immunity, CVD, and increased mortality [26]. The World Health Organization [27] supports the use of micronutrient supplements at Recommended Dietary Allowance (RDA) levels in HIV-infected individuals [27]. Since these recommendations were published in 2003, there have been a number of trials that studied the consequence of micronutrient supplementation in HIV-infected patients [28]. Many of these publications originate from developing countries. For pregnant and lactating HIV-infected women, multivitamin (vitamin B complex, vitamin C, and vitamin E) supplements are recommended. Prior studies from Africa show that multivitamins improve hemoglobin concentrations, reduce the risk of anemia, increase CD4 counts, delay progression of HIV disease, and decrease mortality [29, 30]. More recent studies show that multivitamin supplements at a single dose of the RDA are as effective as multiple doses of the RDA in decreasing the risk of adverse pregnancy outcomes in developing nations [31]. Based on a comprehensive review of multivitamin interventions for HIV-infected pregnant and lactating women, Siegfried [26] recommended that multivitamin supplements at standard doses be taken during the antenatal period and at least for 6 weeks post-partum. In a randomized, controlled, double-blind, hospital based trial of 847 children age one to five years, Ndeezi found that twice the RDA of 14 micronutrients for six months is well tolerated, but does not affect mortality, growth, or CD4 counts [32]. In adults with HIV, a multivitamin supplementation trial reduced the recurrence of pulmonary TB and increased weight gain in those co-infected with TB and HIV [33]. Taken collectively, these recent studies generally support micronutrient supplementation at the level of the RDA in all HIV populations. Higher dosing is unlikely to contribute significantly to better outcomes.
There is mixed evidence regarding the effects of vitamin A supplementation alone. Early studies suggested its benefits in reducing the risk of mother to child transmission (MTCT) [34, 35]. Yet in a meta-analysis of 5 randomized intervention trials that included 7,528 pregnant or post-partum HIV-infected women, vitamin A did not have an effect on MTCT, preterm birth, stillbirth, infant death at 24 month, maternal CD4 counts or mortality, but it was associated with improved birth weight [36]. A review of vitamin A supplementation in adults in developing nations showed none of the 6 trials proved vitamin A to be beneficial in slowing HIV disease progression in adults, but vitamin A supplementation halved all cause mortality in a meta-analysis of 3 trials in children. No adverse effects of vitamin A were found in any of the trials [33]. The basis for the differential effects of vitamin A in adults versus children might relate to underlying differences in nutritional needs and susceptibility to deficiencies in infants and children.
There is considerable research and clinical interest in vitamin D deficiency and its effects on HIV-related outcomes. This interest has peaked because of the observation that HIV-infected patients have higher fracture rates [37, 38] and because of data that shows vitamin D deficiency can contribute to immune dysfunction, CVD and metabolic risk [39-41]. Vitamin D deficiency is prevalent in about two thirds of HIV-infected adults and there is a 36% prevalence of deficiency in children [42, 43]. It is associated with mortality and AIDS-defining events [44]. Vitamin D deficiency is associated with a number of demographic, nutritional, and HIV-specific conditions [42-53]. African Americans have the highest prevalence of vitamin D deficiency, regardless of HIV status [49, 54, 55]. Studies have associated vitamin D deficiency with over 9 times the risk of developing atherosclerotic CVD compared with those of normal vitamin D status in both developed and developing nations [45, 52, 53]. Other CVD risk factors, such as increased carotid intima media thickness (cIMT) [46] and arterial dysfunction [47] are more common in HIV-infected patients with vitamin D deficiency. Vitamin D deficiency is associated with higher viral load [51] and immune suppression [43] and clinically with higher rates of thrush and respiratory infections [42-53]. In a systematic review, Childs and colleagues found that efavirenz is associated with a reduction in 25-hydroxy vitamin D levels, tenofovir with secondary hyperparathyroidism, and combination ART with increased bone turnover and low bone mineral density. Although the positive associations of optimal vitamin D levels and clinical outcomes are encouraging, the aforementioned studies are cross-sectional and observational.
A limited number of vitamin D intervention trials shows that there is a small decrease in parathyroid hormone (PTH), but no appreciable effect on bone mineral density (BMD) with vitamin D supplements [56]. Havens, in a randomized, double-blind, placebo-controlled trial found that 50,000 IU vitamin D supplement every four weeks decreased PTH in patients 18-25 years old who were being treated with tenofovir [57], however, they did not evaluate BMD or other endpoints. Their findings suggest that vitamin D supplementation may offset the effect of tenofovir on calcium balance and bone metabolism. Longenecker, in a similar study design, found that a daily supplement of 4,000 IU vitamin D increased serum 25-hydroxy vitamin D levels but increased insulin resistance and had no impact on endothelial function [58]. Kakalia found that a daily supplement with 1600 IU vitamin D did not impact CD4 counts in children with relatively preserved immune function. This study was small and not placebo-controlled and did not evaluate the effect of vitamin D in children with greater immune suppression who may benefit most [59]. In a randomized, placebo-controlled multicenter clinical trial of HIV-infected children and adolescents, one gram of calcium per day and 100,000 IU of vitamin D every 2 months increased serum 25-hydroxy vitamin D levels but did not affect bone mass accrual [60]. The National Endocrine Society recognizes that ART increases the catabolism of 25-hydroxy vitamin D and 1, 25-hydroxy vitamin D levels and recommends that children and adults on ART be given two to three times more vitamin D for their age group [61].
Zinc, selenium, and glutathione [62] have been studied in intervention trials to determine their effects on immune function. Long-term (18-month) zinc supplementation may reduce the likelihood of opportunistic infection in adults and children and improve CD4 counts in adults. Supplementation seems to be well tolerated. However, it does not decrease viral load, MTCT, or improve mortality [63, 64]. Additional studies on children and pregnant women are needed and there is little information on dose and duration of supplementation, and very few reports in developing nations where nutritional baseline deficits are greater. Selenium and other antioxidants deficiencies are fairly common in HIV-infected patient, especially in developing nations. Selenium supplements are currently being tested to determine its effectiveness in preventing immunological failure in HIV-infected individuals [65, 66]. Other antioxidants such as cysteine and glutathione are being testing in a small randomized, crossover designed study of 12 HIV-infected adults and 20 healthy controls. Baseline glutathione levels were lower among HIV and cysteine or glutamine increased the levels of glutathione in the HIV group to levels in the controls [67]. These findings may be particularly important in HIV-infected patients as they are at risk for low levels of antioxidants.
Cross-sectional and retrospective studies show a number of positive associations between specific micronutrients and clinical outcomes in HIV. The evidence from recent intervention literature taken collectively shows that there is a role for multivitamin supplements at the level of the RDA in most HIV populations. The impact of specific micronutrient supplements as determined through randomized, controlled trials are few and less clear and are probably most beneficial in those populations with increased metabolic demands such as pregnancy, lactation, childhood and underlying nutrient deficits. One of the challenges of investigating micronutrient supplementation is that the dosage and composition of supplements varies across studies. There is a need to determine the optimal composition and dosage of micronutrients. The safety and efficacy of micronutrient supplementation in the short-term and long-term must be further evaluated and studies should aim to recruit diverse groups of participants.
Interventions for obesity, fat redistribution, and metabolic abnormalities
Dyslipidemia, insulin resistance, obesity and lipodystrophy are common in HIV-infected individuals, especially those on highly active antiretroviral therapy (HAART), and are associated with an increased risk of atherosclerotic CVD [68-73]. Biomarkers of endothelial dysfunction and inflammation are significantly higher in both HIV-infected adults and children compared to healthy controls [71, 74-76]. Risk factors associated with these biomarkers (and presumptive CVD risk) include higher waist to hip ratios, body fat, hyperlipidemia and HIV viral load [71, 74]. Thus, interventions that result in decreasing metabolic risk and vascular inflammation may improve the CVD risk profile.
Following exposure to ART, there are increases in total, LDL, and HDL cholesterol in both adults and children. Evaluating metabolic changes in children as they start or change ART can be helpful to determine specific effects of ART because children have fewer confounding psychosocial factors (such as smoking, alcohol, obesity) that can independently impact metabolic outcomes. Children newly exposed to ART experienced a rapid rise in LDL cholesterol over the first 6 months that continued through 12 months [77]. Ten percent of a cohort of 449 children in the United Kingdom had LDL-cholesterols over the 95% for age and protease inhibitors (PIs) caused greater rises in total cholesterol than non-nucleoside reverse transcriptase inhibitors (NNRTIs). The authors concluded dietary and exercise interventions and a change in ART may help address these metabolic abnormalities [78]. In children with incident hypercholesterolemia, Jacobson found that a switch in antiretroviral regimen was associated with cholesterol levels that returned back to normal [79]. There was limited power to detect effects of switching to specific ARTs, however, higher viral load at baseline was associated with normalization of cholesterol. According to the Department of Health and Human Services (DHHS) Panel on Antiretroviral Guidelines for Adults and Adolescents, switching from one PI to another PI or to the same PI at a lower dosing frequency may reduce dyslipidemia [80].
Nutritional counseling programs can also prove useful in preventing dyslipidemia for adults initiating HAART. Lazzaretti recently conducted a 12-month randomized controlled clinical trial where adults who initiated treatment of HAART received quarterly nutritional guidance from a registered dietician. This intervention focused on nutrition therapy for dyslipidemia based on the Phase II diet of the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP) guidelines [81]. Individuals in the intervention group reduced their fat intake (from 31% to 21% of total daily calories). As a result, their plasma triglycerides were reduced from 135 mg/dL to 101 mg/dL. Plasma cholesterol and low-density lipoprotein cholesterol (85 mg/dL to 106 mg/dL) increased in the control group and were unchanged in the diet group. After one year, only 21% of patients who received nutritional counseling had lipid profiles compatible with dyslipidemia compared with 68% of controls. In another 12-month randomized clinical trial with 53 adults on HAART, an individualized nutritional counseling program based on a client-centered approach led to a reduction in fat consumption and increase in fiber intake [82]. These dietary changes did not translate into improved biochemical metabolic status. In a factorial designed, double blind randomized control trial, a combination of fenofibrate and niacin with low-saturated fat diet and exercise was the most effective in increasing HDL-C, decreasing non-HDL-C and hypertriglyceridemia, and ameliorating hypoadiponectinemia in patients with HIV/ART-associated dyslipidemia, than each of the other singular interventions [83].
Recent evidence shows that the Mediterranean style diet, as measured by the Mediterranean Diet Score [84], is associated with less insulin resistance and higher HDL-cholesterol and may be beneficial to HIV patients with HAART-induced metabolic syndrome and lipodystrophy [85]. The Mediterranean style diet emphasizes high consumption of foods of vegetable origin, high fat intake from monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs), and low saturated fat intake. PUFAs, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) reduce triglyceride levels significantly compared with placebo in HIV-infected patients with HAART-associated hypertriglyceridemia [86, 87]. There is also evidence that chromium supplementation can improve insulin resistance and metabolic abnormalities resulting from ART [88]. There was a strong correlation between baseline insulin levels and decrease in insulin among the chromium supplemented group only, however the baseline HOMA-IR was lower in the placebo group. Further studies with properly matched intervention and placebo groups are needed to study the relationship between chromium supplementation and metabolic abnormalities. In HIV-infected patients treated with thymidine analogue nucleoside reverse transcriptase inhibitors (tNRTI), lipodystrophy can be treated by switching to tenofovir as both limb and mitochondrial DNA fat increased. However, in this Phase II trial, uridine supplementation, a nutrient that may prevent or improve the mitochondrial effects of tNRTIs, improved mitochondrial RNA but increased biomarkers associated with inflammation, diminished mitochondrial DNA in fat and did not improve limb fat [89].
HIV-infected patients at all ages are at risk for cardiometabolic disease. There is evidence that close nutritional monitoring by professionals experienced in administering nutritional guidance can effectively improve metabolic parameters. Nutritional counseling can also augment the effects of physical activity interventions and medications used to treat metabolic disorders. There is some evidence that specific diets can also have a role. Future research efforts should analyze the long-term effects of nutritional interventions on LDL and HDL cholesterol and triglycerides in children and adults.
PHYSICAL ACTIVITY INTERVENTIONS
HIV and its therapies and complications can lead to muscle wasting, loss of bone mineral density, and metabolic complications. HIV-infected populations suffer from impaired aerobic capacity, decreased maximum oxygen consumption, decreased flexibility, decreased muscular strength, insulin resistance, and lipodystrophy, which may be a result of the disease, therapies, or a combination of both [68, 71, 90-93]. Exercise can be coupled with pharmacological treatment and nutritional counseling to alleviate some of the symptoms associated with HIV infection. There have been a number of earlier, non-controlled studies that show resistance and aerobic training improve body composition, strength, and fitness and are well tolerated in the HIV population [94-98]. In a randomized, controlled study of 27 HIV-infected adults, Farinatti confirmed that an aerobic, resistance, and flexibility exercise program in HIV-infected adults on HAART can increase muscular strength and reduce heart rate without any negative effects on immune function [99]. However, they did not evaluate metabolic outcomes in this study. Evaluating the effects of physical activity on metabolic outcomes is critical because of ongoing risks based on chronic ART exposures and viral infection. Randomized, controlled trials are even more limited.
There are now a few reports on the benefits of physical activity on metabolic outcomes in HIV. In a systematic review of randomized controlled trials, Fillipas reported that aerobic exercise decreases total body fat and improves lipid profiles in HIV-infected individuals [100]. In a recent randomized clinical trial in HIV-infected adults, a one-hour, three times weekly supervised gym class with monthly nutritional counseling was compared to a monthly workshop to discuss the importance of physical activity and nutrition [101]. All quality of life domains, except pain, were improved in both the intervention and control group but general health, vitality, and mental health were significantly higher for the exercise group. The exercise group also had significant improvements in lean mass, resting heart rate, hip circumference, CD4 cell count, metabolic equivalents, glucose levels, and maximum oxygen consumption. In addition, total cholesterol levels that were above the limits of the National Cholesterol Education Program III before the intervention, decreased to within normal limits after 6 months of the exercise intervention. This is one of the largest randomized controlled trials on exercise in HIV-infected patients and one of the first in this population to show improvements in metabolic outcomes with exercise. In another randomized controlled study of participants with insulin resistance and central adiposity, the implementation of a four-month exercise training program consisting of 1.5 to 2 hours of aerobic and resistance exercise three times a week augmented the insulin-sensitizing benefits of pioglitazone and the intervention with exercise was associated with a decrease in both trunk and limb fat [102]. Although the effect of pioglitazone and exercise in combination is promising, the investigators did not study the effects of exercise alone. These findings indicate that an exercise program can be effective in improving the health outcomes of patients with HIV undergoing ART to reduce the risk of diabetes and hyperlipidemia.
Although the number of children with perinatally-acquired HIV is decreasing in developed nations, they continue to represent a significant number of HIV-infected patients world-wide [103]. Studies on physical activity in this group are limited. A single-arm feasibility and effectiveness study that evaluated a 3-month structured exercise program combined with a 3-month home based program among HIV-infected children found, similarly, that a combination resistance and aerobic activity administered twice weekly increases muscle endurance, cardiorespiratory fitness, and lean body mass [104]. No changes in metabolic outcomes were appreciated, although the sample size was small. Baseline aerobic capacity among HIV-infected children is lower than controls and this decrease in aerobic capacity is related to increased body fat and longer receipt of HAART [93]. No randomized exercise trials in children have been published.
On the other end of the life spectrum, physical activity can help with the age-associated declines in bone mineral density and CD4 counts among HIV-infected individuals [105, 106]. The implementation of a moderate to vigorous aerobic and resistance exercise program 3 times per week can improve cardiovascular, metabolic, and muscle function in older individuals living with HIV [107]. A one-year biweekly resistance exercise program in HIV-infected individuals over the age of 60 years found that exercise improves muscular strength, glucose levels, and lipids [108]. Although the study was not randomized, it was prospective and controlled. In the control group of participants not living with HIV, the rate of increase of muscular strength was greatest in the first six months while the HIV-infected individuals showed a linear rate of increase indicating that the training program should be continued for more than one year to achieve maximal benefits.
Alternative physical activity interventions are also being studied. A recent study by Cade found that in a randomized controlled trial, yoga is effective at reducing systolic and diastolic blood pressure [109]. HIV-infected adults on ART participated in one-hour yoga sessions 2-3 times per week aimed to encourage adherence, self-control, mental focus, self-awareness and physical resilience. This study has important implications on its effects on blood pressure, but there were no changes in body composition or metabolic parameters. Since, yoga is a low cost, non-invasive, and widely available intervention, its effects on blood pressure reduction may contribute to decreasing the risk of CVD.
In addition to the potential benefits of improving metabolic and body composition outcomes, physical activity may have added value in helping fatigue, depression, and anxiety – pervasive symptoms among HIV-infected patients [110, 111]. In a recent review article, Jong and colleagues found that exercise is an effective way to reduce HIV-related fatigue, depression, and anxiety and improve quality of life [111].
One of the major limitations of exercise programs in controlling HIV-related symptoms is the poor compliance among participants. Petroczi found that about 55% of patients adhere to an exercise program and perceived well-being is highly associated with adherence [112]. Thus behavioral interventions can potentially serve to increase compliance with physical activity and diet recommendations. El-Bassel suggests that a multiple-behavior intervention is more effective than a single-behavior intervention to achieve the desired changes in physical activity and diet [113]. Rotheram-Borus suggests using a family based intervention for the treatment of HIV-related complications [114]. A cognitive–behavioral stress management training combined with expressive–supportive therapy helped to improve health behaviors (high levels of medication adherence, appropriate nutritional intake and physical activity, safer sexual practices, and reduced alcohol use/abuse) among minority and underserved HIV-infected women [115]. Future studies are needed to investigate other methods of improving adherence.
The evidence for the benefits of regular physical activity for people living with HIV is limited but persuasive. Exercise augments fitness, metabolic endpoints, quality of life and overall function for those who are compliant with an exercise program. However, adherence to a regular fitness program is a major challenge for many, and strategies to engage patients in physical activity are needed. Specialized equipment to assist the individual track progress, or programs delivered in the community, work or school-setting are approaches that may help [95, 116-120]. Table 2 summarizes novel strategies aimed at delivering or measuring the compliance to physical activity. All programs should target the current recommendations of 30 minutes a day, 5 days per week of moderate to vigorous physical activity [121].
Table 2.
Potential Strategies to Increase Physical Activity Among Individuals with HIV Infection that are Effective in Other Populations
| Strategy | Authors | Goal | Conclusion |
|---|---|---|---|
| Educational materials and lessons |
Davies CA., et al. 2012 Allen JC, Lewis JB, Tagliaferro AR. 2012 |
Increase physical activity by providing information on decision- making and knowledge of goals |
The ability of internet-delivered interventions to produce meaningful change in long-term physical activity remains unclear115 Worksite health promotion reduced coronary heart disease risk factors119 |
| School based physical education classes |
Ardoy DN, et al. 2011 | Improve cardiovascular health in young people by increasing physical activity in the school setting |
Four high-intensity PE sessions/week, improved physical fitness, particularly aerobic fitness113 |
| Hospital-based physical activity sessions in children |
Miller, et al. 2010 Mutimura E, et. al. 2008 |
Improve physical fitness measures through a supervised program |
Twice weekly aerobic and resistance training sessions resulted in improved physical fitness and increased lean mass101 Exercise training is a safe, practical, and an effective treatment for evolving metabolic and cardiovascular syndromes associated with HIV120 |
| Multi-component physical activity and nutrition education with structured exercise sessions |
Hardy OT, et al. 2012 Lloyd JJ, Wyatt KM, Creanor S., 2012 |
Improve CVD and anthropometric outcomes Assess weight and behavioral outcomes |
Significant improvement in HDL116 Decreased consumption of energy-dense snacks and increased healthy snacks; less TV/screen time and increased moderate-vigorous physical activity, improved anthropometric measures117 |
| Specialized equipment | Snyder A, Colvin B, Gammack JK, 2011 |
Pedometer to increase motivation and increase amount of time spent on physical activity in older adults |
Motivation to increase overall mobility; Improved of functional measures114 |
CONCLUSION
The world is coping with the ever-burgeoning problem of overweight and obesity, yet also recognizes that malnutrition continues to be a challenge. HIV infection has become a paradigm for these seemingly disparate entities. Since the advent of HAART, HIV-infected patients have enjoyed greater life expectancy and some populations have even averted HIV infection because of successful preventive strategies. However, the underlying risks for malnutrition have not disappeared due to imperfect viral suppression and inadequate access to ART. Cardiovascular and other metabolic diseases will continue to affect this population as life expectancy grows. Many, although not all, of the benefits of nutrition and physical activity interventions are well substantiated. Since HIV-infected individuals are burdened with a lifetime of medications, more studies should focus on nutritional support being provided with food instead of pills. Physical activity, another non-pharmacologic intervention, should be promoted to improve health in a multidimensional way. The frequency, intensity and time spent on physical activity needs to be better defined to determine the right load that yields optimal results. Similar to nutritional interventions, physical activity should be incorporated into daily life activities. Evidence of the long-term effects of these interventions on the incidence and prevalence of malnutrition, nutritional deficiencies, CVD and its associated symptoms and mortality are essential. Future nutrition and physical activity studies should focus on discovery through well-controlled trials and dissemination of results from the individual to global populations.
Acknowledgments
This work was supported by grants from National Institutes of Health (NHLBI 1 R01 HL095127, NICHD 1 R01 HD060325, 1R01 NR012885), the Micah Batchelor Award for Research Excellence, and the Coulter Jones Foundation.
Footnotes
Disclosure: No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1.Fitch K, Grinspoon S. Nutritional and metabolic correlates of cardiovascular and bone disease in HIV-infected patients. Am J Clin Nutr. 2011;94(6):1721S–8S. doi: 10.3945/ajcn.111.012120. doi:10.3945/ajcn.111.012120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr., Phair JP. Cardiovascular disease in HIV infection. Curr Opin HIV AIDS. 2011;6(4):266–71. doi: 10.1097/COH.0b013e328347876c. doi:10.1097/COH.0b013e328347876c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tseng ZH, Secemsky EA, Dowdy D, Vittinghoff E, Moyers B, Wong JK, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012;59(21):1891–6. doi: 10.1016/j.jacc.2012.02.024. doi:10.1016/j.jacc.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arbeitman LOBR, Somarriba G, Ludwig D, Messiah S, Neri D, Scott G, Miller T, editors. Prevalence of Obesity in HIV-Infected Children in a Miami Cohort. Pediatric Academic Societies; Boston: 2012. [Google Scholar]
- 5.Fields-Gardner C, Campa A, American Dietetics A Position of the American Dietetic Association: Nutrition Intervention and Human Immunodeficiency Virus Infection. J Am Diet Assoc. 2010;110(7):1105–19. doi: 10.1016/j.jada.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 6.Chandra RK. Nutrition, immunity and infection: from basic knowledge of dietary manipulation of immune responses to practical application of ameliorating suffering and improving survival. Proc Natl Acad Sci U S A. 1996;93(25):14304–7. doi: 10.1073/pnas.93.25.14304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes S, Kelly P. Interactions of malnutrition and immune impairment, with specific reference to immunity against parasites. Parasite Immunol. 2006;28(11):577–88. doi: 10.1111/j.1365-3024.2006.00897.x. doi:10.1111/j.1365-3024.2006.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandrasekhar A, Gupta A. Nutrition and disease progression pre-highly active antiretroviral therapy (HAART) and post-HAART: can good nutrition delay time to HAART and affect response to HAART? Am J Clin Nutr. 2011;94(6):1703S–15S. doi: 10.3945/ajcn.111.019018. doi:10.3945/ajcn.111.019018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raiten DJ. Nutrition and pharmacology: general principles and implications for HIV. Am J Clin Nutr. 2011;94(6):1697S–702S. doi: 10.3945/ajcn.111.019109. doi:10.3945/ajcn.111.019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raiten DJ, Mulligan K, Papathakis P, Wanke C. Am J Clin Nutr. vol 6. United States; 2011. Executive summary--nutritional care of HIV-infected adolescents and adults, including pregnant and lactating women: what do we know, what can we do, and where do we go from here? pp. 1667S–76S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ivers LC, Cullen KA. Food insecurity: special considerations for women. Am J Clin Nutr. 2011;94(6):1740S–4S. doi: 10.3945/ajcn.111.012617. doi:10.3945/ajcn.111.012617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosmiski L. Energy expenditure in HIV infection. Am J Clin Nutr. 2011;94(6):1677S–82S. doi: 10.3945/ajcn.111.012625. doi:10.3945/ajcn.111.012625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Musoke PM, Fergusson P. Severe malnutrition and metabolic complications of HIV-infected children in the antiretroviral era: clinical care and management in resource-limited settings. Am J Clin Nutr. 2011;94(6):1716S–20S. doi: 10.3945/ajcn.111.018374. doi:10.3945/ajcn.111.018374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiser SD, Young SL, Cohen CR, Kushel MB, Tsai AC, Tien PC, et al. Conceptual framework for understanding the bidirectional links between food insecurity and HIV/AIDS. Am J Clin Nutr. 2011;94(6):1729S–39S. doi: 10.3945/ajcn.111.012070. doi:10.3945/ajcn.111.012070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larson NI, Story MT. Food insecurity and weight status among U. S. children and families: a review of the literature. Am J Prev Med. 2011;40(2):166–73. doi: 10.1016/j.amepre.2010.10.028. doi:10.1016/j.amepre.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 16.Bahwere P, Deconinck H, Banda T, Mtimuni A, Collins S. Impact of household food insecurity on the nutritional status and the response to therapeutic feeding of people living with human immunodeficiency virus. Patient Prefer Adherence. 2011;5:619–27. doi: 10.2147/PPA.S25672. doi:10.2147/ppa.s25672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yager JE, Kadiyala S, Weiser SD. HIV/AIDS, food supplementation and livelihood programs in Uganda: a way forward? PLoS One. 2011;6(10):e26117. doi: 10.1371/journal.pone.0026117. doi:10.1371/journal.pone.0026117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahoua L, Umutoni C, Huerga H, Minetti A, Szumilin E, Balkan S, et al. Nutrition outcomes of HIV-infected malnourished adults treated with ready-to-use therapeutic food in sub-Saharan Africa: a longitudinal study. J Int AIDS Soc. 2011;14:2. doi: 10.1186/1758-2652-14-2. doi:10.1186/1758-2652-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kundu CK, Samanta M, Sarkar M, Bhattacharyya S, Chatterjee S. Food supplementation as an incentive to improve pre-antiretroviral therapy clinic adherence in HIV-positive children--experience from eastern India. J Trop Pediatr. 2012;58(1):31–7. doi: 10.1093/tropej/fmr026. doi:10.1093/tropej/fmr026. [DOI] [PubMed] [Google Scholar]
- *20.Kayira D, Bentley ME, Wiener J, Mkhomawanthu C, King CC, Chitsulo P, et al. A lipid-based nutrient supplement mitigates weight loss among HIV-infected women in a factorial randomized trial to prevent mother-to-child transmission during exclusive breastfeeding. Am J Clin Nutr. 2012;95(3):759–65. doi: 10.3945/ajcn.111.018812. doi:10.3945/ajcn.111.018812. This study reports that a lipid-based supplement diminishes weight loss in HIV-infected pregnant women living in a resource-limited setting. Optimization of maternal nutrition may ultimately impact pregnancy and infant outcomes.
- 21.Kindra G, Coutsoudis A, Esposito F. Effect of nutritional supplementation of breastfeeding HIV positive mothers on maternal and child health: findings from a randomized controlled clinical trial. BMC Public Health. 2011;11:946. doi: 10.1186/1471-2458-11-946. doi:10.1186/1471-2458-11-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hummelen R, Vos AP, van’t Land B, van Norren K, Reid G. Altered host-microbe interaction in HIV: a target for intervention with pro- and prebiotics. Int Rev Immunol. 2010;29(5):485–513. doi: 10.3109/08830185.2010.505310. doi:10.3109/08830185.2010.505310. [DOI] [PubMed] [Google Scholar]
- 23.Hummelen R, Hemsworth J, Changalucha J, Butamanya NL, Hekmat S, Habbema JD, et al. Effect of micronutrient and probiotic fortified yogurt on immune-function of anti-retroviral therapy naive HIV patients. Nutrients. 2011;3(10):897–909. doi: 10.3390/nu3100897. doi:10.3390/nu3100897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hummelen R, Changalucha J, Butamanya NL, Koyama TE, Cook A, Habbema JD, et al. Effect of 25 weeks probiotic supplementation on immune function of HIV patients. Gut Microbes. 2011;2(2):80–5. doi: 10.4161/gmic.2.2.15787. [DOI] [PubMed] [Google Scholar]
- 25.Irvine SL, Hummelen R, Hekmat S. Probiotic yogurt consumption may improve gastrointestinal symptoms, productivity, and nutritional intake of people living with human immunodeficiency virus in Mwanza, Tanzania. Nutr Res. 2011;31(12):875–81. doi: 10.1016/j.nutres.2011.10.005. doi:10.1016/j.nutres.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Siegfried N, Irlam JH, Visser ME, Rollins NN. Micronutrient supplementation in pregnant women with HIV infection. Cochrane Database Syst Rev. 2012;3 doi: 10.1002/14651858.CD009755. CD009755. doi:10.1002/14651858.cd009755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO Nutrient Requirements for People Living with HIV/AIDS: Report of a technical consultation. 2003.
- 28.Forrester JE, Sztam KA. Micronutrients in HIV/AIDS: is there evidence to change the WHO 2003 recommendations? Am J Clin Nutr. 2011;94(6):1683S–9S. doi: 10.3945/ajcn.111.011999. doi:10.3945/ajcn.111.011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fawzi WW, Msamanga GI, Spiegelman D, Wei R, Kapiga S, Villamor E, et al. A randomized trial of multivitamin supplements and HIV disease progression and mortality. N Engl J Med. 2004;351(1):23–32. doi: 10.1056/NEJMoa040541. doi:10.1056/NEJMoa040541. [DOI] [PubMed] [Google Scholar]
- 30.Fawzi WW, Msamanga GI, Kupka R, Spiegelman D, Villamor E, Mugusi F, et al. Multivitamin supplementation improves hematologic status in HIV-infected women and their children in Tanzania. Am J Clin Nutr. 2007;85(5):1335–43. doi: 10.1093/ajcn/85.5.1335. [DOI] [PubMed] [Google Scholar]
- *31.Kawai K, Kupka R, Mugusi F, Aboud S, Okuma J, Villamor E, et al. A randomized trial to determine the optimal dosage of multivitamin supplements to reduce adverse pregnancy outcomes among HIV-infected women in Tanzania. Am J Clin Nutr. 2010;91(2):391–7. doi: 10.3945/ajcn.2009.28483. doi:10.3945/ajcn.2009.28483. This randomized trial found no difference in the effects of single compared with multiple RDA multivitamin supplements on the risks of adverse pregnancy outcomes among HIV-infected pregnant women. Micronutrient deficiencies and nutrient-nutrient interactions are of great concern to this population of HIV-infected women and their offspring.
- 32.Ndeezi G, Tylleskar T, Ndugwa CM, Tumwine JK. Effect of multiple micronutrient supplementation on survival of HIV-infected children in Uganda: a randomized, controlled trial. J Int AIDS Soc. 2010;13:18. doi: 10.1186/1758-2652-13-18. doi:10.1186/1758-2652-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irlam JH, Visser MM, Rollins NN, Siegfried N. Micronutrient supplementation in children and adults with HIV infection. Cochrane Database Syst Rev. 2010;(12) doi: 10.1002/14651858.CD003650.pub3. CD003650. doi:10.1002/14651858.CD003650.pub3. [DOI] [PubMed] [Google Scholar]
- 34.Semba RD, Miotti PG, Chiphangwi JD, Saah AJ, Canner JK, Dallabetta GA, et al. Maternal vitamin A deficiency and mother-to-child transmission of HIV-1. Lancet. 1994;343(8913):1593–7. doi: 10.1016/s0140-6736(94)93056-2. [DOI] [PubMed] [Google Scholar]
- 35.Azais-Braesco V, Pascal G. Vitamin A in pregnancy: requirements and safety limits. Am J Clin Nutr. 2000;71(5 Suppl):1325S–33S. doi: 10.1093/ajcn/71.5.1325s. [DOI] [PubMed] [Google Scholar]
- 36.Wiysonge CS, Shey M, Kongnyuy EJ, Sterne JA, Brocklehurst P. Vitamin A supplementation for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database Syst Rev. 2011;1 doi: 10.1002/14651858.CD003648.pub3. CD003648. doi:10.1002/14651858.CD003648.pub3. [DOI] [PubMed] [Google Scholar]
- 37.Bonjoch A, Figueras M, Estany C, Perez-Alvarez N, Rosales J, del Rio L, et al. High prevalence of and progression to low bone mineral density in HIV-infected patients: a longitudinal cohort study. AIDS. 2010;24(18):2827–33. doi: 10.1097/QAD.0b013e328340a28d. doi:10.1097/QAD.0b013e328340a28d. [DOI] [PubMed] [Google Scholar]
- 38.Ofotokun I, McIntosh E, Weitzmann MN. HIV: inflammation and bone. Curr HIV/AIDS Rep. 2012;9(1):16–25. doi: 10.1007/s11904-011-0099-z. doi:10.1007/s11904-011-0099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lagishetty V, Liu NQ, Hewison M. Vitamin D metabolism and innate immunity. Mol Cell Endocrinol. 2011;347(1-2):97–105. doi: 10.1016/j.mce.2011.04.015. doi:10.1016/j.mce.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gagnon C, Lu ZX, Magliano DJ, Dunstan DW, Shaw JE, Zimmet PZ, et al. Low Serum 25-Hydroxyvitamin D Is Associated with Increased Risk of the Development of the Metabolic Syndrome at Five Years: Results from a National, Population-Based Prospective Study (The Australian Diabetes, Obesity and Lifestyle Study: AusDiab) J Clin Endocrinol Metab. 2012;97(6):1953–61. doi: 10.1210/jc.2011-3187. doi:10.1210/jc.2011-3187. [DOI] [PubMed] [Google Scholar]
- 41.Muscogiuri G, Sorice GP, Ajjan R, Mezza T, Pilz S, Prioletta A, et al. Can vitamin D deficiency cause diabetes and cardiovascular diseases? Present evidence and future perspectives. Nutr Metab Cardiovasc Dis. 2012;22(2):81–7. doi: 10.1016/j.numecd.2011.11.001. doi:10.1016/j.numecd.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Adeyemi OM, Agniel D, French AL, Tien PC, Weber K, Glesby MJ, et al. Vitamin D deficiency in HIV-infected and HIV-uninfected women in the United States. J Acquir Immune Defic Syndr. 2011;57(3):197–204. doi: 10.1097/QAI.0b013e31821ae418. doi:10.1097/QAI.0b013e31821ae418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rutstein R, Downes A, Zemel B, Schall J, Stallings V. Vitamin D status in children and young adults with perinatally acquired HIV infection. Clin Nutr. 2011;30(5):624–8. doi: 10.1016/j.clnu.2011.02.005. doi:10.1016/j.clnu.2011.02.005. [DOI] [PubMed] [Google Scholar]
- **44.Viard JP, Souberbielle JC, Kirk O, Reekie J, Knysz B, Losso M, et al. Vitamin D and clinical disease progression in HIV infection: results from the EuroSIDA study. AIDS. 2011;25(10):1305–15. doi: 10.1097/QAD.0b013e328347f6f7. doi:10.1097/QAD.0b013e328347f6f7. This study shows evidence that vitamin D deficiency is an important cofactor in HIV disease progression and this is of importance as vitamin D deficiency is frequent and also associated with morbidity and mortality in this population.
- 45.Mehta S, Mugusi FM, Spiegelman D, Villamor E, Finkelstein JL, Hertzmark E, et al. Vitamin D status and its association with morbidity including wasting and opportunistic illnesses in HIV-infected women in Tanzania. AIDS Patient Care STDS. 2011;25(10):579–85. doi: 10.1089/apc.2011.0182. doi:10.1089/apc.2011.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ross AC, Judd S, Kumari M, Hileman C, Storer N, Labbato D, et al. Vitamin D is linked to carotid intima-media thickness and immune reconstitution in HIV-positive individuals. Antivir Ther. 2011;16(4):555–63. doi: 10.3851/IMP1784. doi:10.3851/imp1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shikuma CM, Seto T, Liang CY, Bennett K, Degruttola V, Gerschenson M, et al. Vitamin D Levels and Markers of Arterial Dysfunction in HIV. AIDS Res Hum Retroviruses. 2011 doi: 10.1089/aid.2011.0086. doi:10.1089/aid.2011.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sroussi HY, Burke-Miller J, French AL, Adeyemi OM, Weber KM, Lu Y, et al. Association among Vitamin D, Oral Candidiasis, and Calprotectinemia in HIV. J Dent Res. 2012;91(7):666–70. doi: 10.1177/0022034512446342. doi:10.1177/0022034512446342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crutchley RD, Gathe J, Jr., Mayberry C, Trieu A, Abughosh S, Garey KW. Risk factors for vitamin D deficiency in HIV-infected patients in the south central United States. AIDS Res Hum Retroviruses. 2012;28(5):454–9. doi: 10.1089/aid.2011.0025. doi:10.1089/aid.2011.0025. [DOI] [PubMed] [Google Scholar]
- 50.O’Brien KO, Donangelo CM, Ritchie LD, Gildengorin G, Abrams S, King JC. Serum 1,25-dihydroxyvitamin D and calcium intake affect rates of bone calcium deposition during pregnancy and the early postpartum period. Am J Clin Nutr. 2012;96(1):64–72. doi: 10.3945/ajcn.111.029231. doi:10.3945/ajcn.111.029231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim JH, Gandhi V, Psevdos G, Jr., Espinoza F, Park J, Sharp V. Evaluation of vitamin D levels among HIV-infected patients in New York City. AIDS Res Hum Retroviruses. 2012;28(3):235–41. doi: 10.1089/AID.2011.0040. doi:10.1089/aid.2011.0040. [DOI] [PubMed] [Google Scholar]
- 52.Lai H, Detrick B, Fishman EK, Gerstenblith G, Brinker JA, Hollis BW, et al. Vitamin D deficiency is associated with the development of subclinical coronary artery disease in African Americans with HIV infection: a preliminary study. J Investig Med. 2012;60(5):801–7. doi: 10.231/JIM.0b013e318250bf99. doi:10.231/JIM.0b013e318250bf99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lai H, Gerstenblith G, Fishman EK, Brinker J, Kickler T, Tong W, et al. Vitamin D Deficiency Is Associated With Silent Coronary Artery Disease in Cardiovascularly Asymptomatic African Americans With HIV Infection. Clin Infect Dis. 2012;54(12):1747–55. doi: 10.1093/cid/cis306. doi:10.1093/cid/cis306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31(1):48–54. doi: 10.1016/j.nutres.2010.12.001. doi:10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 55.Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88(2):558S–64S. doi: 10.1093/ajcn/88.2.558S. [DOI] [PubMed] [Google Scholar]
- 56.Childs K, Welz T, Samarawickrama A, Post FA. Effects of vitamin D deficiency and combination antiretroviral therapy on bone in HIV-positive patients. AIDS. 2012;26(3):253–62. doi: 10.1097/QAD.0b013e32834f324b. doi:10.1097/QAD.0b013e32834f324b. [DOI] [PubMed] [Google Scholar]
- *57.Havens PL, Stephensen CB, Hazra R, Flynn PM, Wilson CM, Rutledge B, et al. Vitamin D3 decreases parathyroid hormone in HIV-infected youth being treated with tenofovir: a randomized, placebo-controlled trial. Clin Infect Dis. 2012;54(7):1013–25. doi: 10.1093/cid/cir968. doi:10.1093/cid/cir968. This study of over 200 young adults randomized to receive vitamin D or not showed decreases in PTH levels of those one tenofovir, regardless of vitamin D levels at baseline. This suggests that vitamin D supplementation may offset the effect of tenofovir on calcium balance and bone metabolism.
- 58.Longenecker CT, Hileman CO, Carman TL, Ross AC, Seydafkan S, Brown TT, et al. Vitamin D supplementation and endothelial function in vitamin D deficient HIV-infected patients: a randomized placebo-controlled trial. Antivir Ther. 2012;17(4):613–21. doi: 10.3851/IMP1983. doi:10.3851/imp1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *59.Kakalia S, Sochett EB, Stephens D, Assor E, Read SE, Bitnun A. Vitamin D supplementation and CD4 count in children infected with human immunodeficiency virus. J Pediatr. 2011;159(6):951–7. doi: 10.1016/j.jpeds.2011.06.010. doi:10.1016/j.jpeds.2011.06.010. This randomized clinical trial concluded that supplementation with 1600 IU of vitamin D per day does not impact CD4 count and suggested that doses above current recommendations may be more appropriate.
- *60.Arpadi SM, McMahon DJ, Abrams EJ, Bamji M, Purswani M, Engelson ES, et al. Effect of supplementation with cholecalciferol and calcium on 2-y bone mass accrual in HIV-infected children and adolescents: a randomized clinical trial. Am J Clin Nutr. 2012;95(3):678–85. doi: 10.3945/ajcn.111.024786. doi:10.3945/ajcn.111.024786.. This randomized clinical trial concluded that 1g of calcium per day and 100,000 IU oral cholecalciferol every 2 months improves vitamin D serum levels but does not affect bone mass accrual in HIV-infected children and adolescents.
- 61.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30. doi: 10.1210/jc.2011-0385. doi:10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 62.Nunnari G, Coco C, Pinzone MR, Pavone P, Berretta M, Di Rosa M, et al. The role of micronutrients in the diet of HIV-1-infected individuals. Front Biosci (Elite Ed) 2012;4:2442–56. doi: 10.2741/e556. [DOI] [PubMed] [Google Scholar]
- 63.Baum MK, Lai S, Sales S, Page JB, Campa A. Randomized, controlled clinical trial of zinc supplementation to prevent immunological failure in HIV-infected adults. Clin Infect Dis. 2010;50(12):1653–60. doi: 10.1086/652864. doi:10.1086/652864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zeng L, Zhang L. Efficacy and safety of zinc supplementation for adults, children and pregnant women with HIV infection: systematic review. Trop Med Int Health. 2011 doi: 10.1111/j.1365-3156.2011.02871.x. doi:10.1111/j.1365-3156.2011.02871.x. [DOI] [PubMed] [Google Scholar]
- 65.Kamwesiga J, Mutabazi V, Kayumba J, Tayari JC, Smyth R, Fay H, et al. Effect of selenium supplementation on CD4 T-cell recovery, viral suppression, morbidity and quality of life of HIV-infected patients in Rwanda: study protocol for a randomized controlled trial. Trials. 2011;12:192. doi: 10.1186/1745-6215-12-192. doi:10.1186/1745-6215-12-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stone CA, Kawai K, Kupka R, Fawzi WW. Role of selenium in HIV infection. Nutr Rev. 2010;68(11):671–81. doi: 10.1111/j.1753-4887.2010.00337.x. doi:10.1111/j.1753-4887.2010.00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Borges-Santos MD, Moreto F, Pereira PC, Ming-Yu Y, Burini RC. Plasma glutathione of HIV(+) patients responded positively and differently to dietary supplementation with cysteine or glutamine. Nutrition. 2012;28(7-8):753–6. doi: 10.1016/j.nut.2011.10.014. doi:10.1016/j.nut.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 68.Geffner ME, Patel K, Miller TL, Hazra R, Silio M, Van Dyke RB, et al. Factors associated with insulin resistance among children and adolescents perinatally infected with HIV-1 in the pediatric HIV/AIDS cohort study. Horm Res Paediatr. 2011;76(6):386–91. doi: 10.1159/000332957. doi:10.1159/000332957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Silva EF, Lewi DS, Vedovato GM, Garcia VR, Tenore SB, Bassichetto KC. Nutritional and clinical status, and dietary patterns of people living with HIV/AIDS in ambulatory care in Sao Paulo, Brazil. Rev Bras Epidemiol. 2010;13(4):677–88. doi: 10.1590/s1415-790x2010000400012. [DOI] [PubMed] [Google Scholar]
- 70.Souza DT, Rondo PH, Reis LC. The nutritional status of children and adolescents with HIV/AIDS on antiretroviral therapy. J Trop Pediatr. 2011;57(1):65–8. doi: 10.1093/tropej/fmq035. doi:10.1093/tropej/fmq035. [DOI] [PubMed] [Google Scholar]
- **71.Miller TI, Borkowsky W, DiMeglio LA, Dooley L, Geffner ME, Hazra R, et al. Metabolic abnormalities and viral replication are associated with biomarkers of vascular dysfunction in HIV-infected children. HIV Med. 2012;13(5):264–75. doi: 10.1111/j.1468-1293.2011.00970.x. doi:10.1111/j.1468-1293.2011.00970.x. This multi-centered study presents the anthropometric, metabolic, inflammatory and endothelial dysfunction abnormalities in children and adolescents with HIV.
- 72.Bonfanti P, De Socio GV, Ricci E, Antinori A, Martinelli C, Vichi F, et al. The feature of Metabolic Syndrome in HIV naive patients is not the same of those treated: Results from a prospective study. Biomed Pharmacother. 2012 doi: 10.1016/j.biopha.2012.01.005. doi:10.1016/j.biopha.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 73.Stanley TL, Grinspoon SK. Body composition and metabolic changes in HIV-infected patients. J Infect Dis. 2012;205(Suppl 3):S383–90. doi: 10.1093/infdis/jis205. doi:10.1093/infdis/jis205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miller TL, Somarriba G, Orav EJ, Mendez AJ, Neri D, Schaefer N, et al. Biomarkers of vascular dysfunction in children infected with human immunodeficiency virus-1. J Acquir Immune Defic Syndr. 2010;55(2):182–8. doi: 10.1097/QAI.0b013e3181e222c9. doi:10.1097/QAI.0b013e3181e222c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baker JV, Duprez D. Biomarkers and HIV-associated cardiovascular disease. Curr Opin HIV AIDS. 2010;5(6):511–6. doi: 10.1097/COH.0b013e32833ed7ec. doi:10.1097/COH.0b013e32833ed7ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Worm SW, Hsue P. Role of biomarkers in predicting CVD risk in the setting of HIV infection? Curr Opin HIV AIDS. 2010;5(6):467–72. doi: 10.1097/COH.0b013e32833f2ea6. doi:10.1097/COH.0b013e32833f2ea6. [DOI] [PubMed] [Google Scholar]
- 77.Sztam KA, Jiang H, Jurgrau A, Deckelbaum RJ, Foca MD. Early increases in concentrations of total, LDL, and HDL cholesterol in HIV-infected children following new exposure to antiretroviral therapy. J Pediatr Gastroenterol Nutr. 2011;52(4):495–8. doi: 10.1097/MPG.0b013e3181f5e9d4. doi:10.1097/MPG.0b013e3181f5e9d4. [DOI] [PubMed] [Google Scholar]
- 78.Rhoads MP, Lanigan J, Smith CJ, Lyall EG. Effect of specific ART drugs on lipid changes and the need for lipid management in children with HIV. J Acquir Immune Defic Syndr. 2011;57(5):404–12. doi: 10.1097/QAI.0b013e31821d33be. doi:10.1097/QAI.0b013e31821d33be. [DOI] [PubMed] [Google Scholar]
- 79.Jacobson DL, Williams P, Tassiopoulos K, Melvin A, Hazra R, Farley J. Clinical management and follow-up of hypercholesterolemia among perinatally HIV-infected children enrolled in the PACTG 219C study. J Acquir Immune Defic Syndr. 2011;57(5):413–20. doi: 10.1097/QAI.0b013e31822203f5. doi:10.1097/QAI.0b013e31822203f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Adolescents PoAGfAa. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2012.
- *81.Lazzaretti RK, Kuhmmer R, Sprinz E, Polanczyk CA, Ribeiro JP. Dietary intervention prevents dyslipidemia associated with highly active antiretroviral therapy in human immunodeficiency virus type 1-infected individuals: a randomized trial. J Am Coll Cardiol. 2012;59(11):979–88. doi: 10.1016/j.jacc.2011.11.038. doi:10.1016/j.jacc.2011.11.038. This randomized trial of nutritional education through NCEP guidelines showed caloric intake from fat was decreased and resulted in lower levels of triglycerides and a lower percent of patients with dyslipidemia at 1 year post intervention. This is an encouraging study that shows close nutritional monitoring and guidance can be effective in decreased CVD risk among HIV-infected adults.
- 82.Almeida LB, Segurado AC, Duran AC, Jaime PC. Impact of a nutritional counseling program on prevention of HAART-related metabolic and morphologic abnormalities. AIDS Care. 2011;23(6):755–63. doi: 10.1080/09540121.2010.525789. doi:10.1080/09540121.2010.525789. [DOI] [PubMed] [Google Scholar]
- * 83.Balasubramanyam A, Coraza I, Smith EO, Scott LW, Patel P, Iyer D, et al. Combination of niacin and fenofibrate with lifestyle changes improves dyslipidemia and hypoadiponectinemia in HIV patients on antiretroviral therapy: results of “heart positive,” a randomized, controlled trial. J Clin Endocrinol Metab. 2011;96(7):2236–47. doi: 10.1210/jc.2010-3067. doi:10.1210/jc.2010-3067.. This study of over 200 HIV-infected adults shows that the addition of dietary counseling can improve metabolic outcomes in adults who are on other pharmacologic interventions. This suggests a role of dietary counseling even in those HIV-infected patients who require medications to treat their dyslipidemia.
- 84.Panagiotakos DB, Pitsavos C, Arvaniti F, Stefanadis C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev Med. 2007;44(4):335–40. doi: 10.1016/j.ypmed.2006.12.009. doi:10.1016/j.ypmed.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 85.Tsiodras S, Poulia KA, Yannakoulia M, Chimienti SN, Wadhwa S, Karchmer AW, et al. Adherence to Mediterranean diet is favorably associated with metabolic parameters in HIV-positive patients with the highly active antiretroviral therapy-induced metabolic syndrome and lipodystrophy. Metabolism. 2009;58(6):854–9. doi: 10.1016/j.metabol.2009.02.012. doi:10.1016/j.metabol.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oliveira JM, Rondo PH. Omega-3 fatty acids and hypertriglyceridemia in HIV-infected subjects on antiretroviral therapy: systematic review and meta-analysis. HIV Clin Trials. 2011;12(5):268–74. doi: 10.1310/hct1205-268. doi:10.1310/hct1205-268. [DOI] [PubMed] [Google Scholar]
- 87.Peters BS, Wierzbicki AS, Moyle G, Nair D, Brockmeyer N. The effect of a 12-week course of omega-3 polyunsaturated fatty acids on lipid parameters in hypertriglyceridemic adult HIV-infected patients undergoing HAART: a randomized, placebo-controlled pilot trial. Clin Ther. 2012;34(1):67–76. doi: 10.1016/j.clinthera.2011.12.001. doi:10.1016/j.clinthera.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 88.Aghdassi E, Arendt BM, Salit IE, Mohammed SS, Jalali P, Bondar H, et al. In patients with HIV-infection, chromium supplementation improves insulin resistance and other metabolic abnormalities: a randomized, double-blind, placebo controlled trial. Curr HIV Res. 2010;8(2):113–20. doi: 10.2174/157016210790442687. [DOI] [PubMed] [Google Scholar]
- 89.McComsey GA, O’Riordan M, Choi J, Libutti D, Rowe D, Storer N, et al. Mitochondrial function, inflammation, fat and bone in HIV lipoatrophy: randomized study of uridine supplementation or switch to tenofovir. Antivir Ther. 2012;17(2):347–53. doi: 10.3851/IMP1928. doi:10.3851/imp1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cade WT, Peralta L, Keyser RE. Aerobic capacity in late adolescents infected with HIV and controls. Pediatr Rehabil. 2002;5(3):161–9. doi: 10.1080/1363849021000039362. doi:10.1080/1363849021000039362. [DOI] [PubMed] [Google Scholar]
- 91.Cade WT, Fantry LE, Nabar SR, Keyser RE. Decreased peak arteriovenous oxygen difference during treadmill exercise testing in individuals infected with the human immunodeficiency virus. Arch Phys Med Rehabil. 2003;84(11):1595–603. doi: 10.1053/s0003-9993(03)00275-2. [DOI] [PubMed] [Google Scholar]
- 92.Oursler KK, Sorkin JD, Smith BA, Katzel LI. Reduced aerobic capacity and physical functioning in older HIV-infected men. AIDS Res Hum Retroviruses. 2006;22(11):1113–21. doi: 10.1089/aid.2006.22.1113. doi:10.1089/aid.2006.22.1113. [DOI] [PubMed] [Google Scholar]
- 93.Somarriba G, Lopez-Mitnik G, Ludwig D, Neri D, Schaefer N, Lipshultz S, et al. Physical Fitness in Children Infected with the Human Immunodeficiency Virus: Associations with Highly Active Antiretroviral Therapy. AIDS Research and Human Retroviruses. 2012 doi: 10.1089/aid.2012.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dudgeon WD, Phillips KD, Carson JA, Brewer RB, Durstine JL, Hand GA. Counteracting muscle wasting in HIV-infected individuals. HIV Med. 2006;7(5):299–310. doi: 10.1111/j.1468-1293.2006.00380.x. doi:10.1111/j.1468-1293.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- 95.Miller TL. A hospital-based exercise program to improve body composition, strength, and abdominal adiposity in 2 HIV-infected children. AIDS Read. 2007;17(9):450–2. 5, 8. [PubMed] [Google Scholar]
- 96.O’Brien K, Nixon S, Tynan AM, Glazier R. Aerobic exercise interventions for adults living with HIV/AIDS. Cochrane Database Syst Rev. 2010;(8) doi: 10.1002/14651858.CD001796.pub3. CD001796. doi:10.1002/14651858.CD001796.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Glover EI, Phillips SM. Resistance exercise and appropriate nutrition to counteract muscle wasting and promote muscle hypertrophy. Curr Opin Clin Nutr Metab Care. 2010;13(6):630–4. doi: 10.1097/MCO.0b013e32833f1ae5. doi:10.1097/MCO.0b013e32833f1ae5. [DOI] [PubMed] [Google Scholar]
- 98.Mesquita Soares TC, Galvao De Souza HA, De Medeiros Guerra LM, Pinto E, Pipolo Milan E, Moreira Dantas P, et al. Morphology and biochemical markers of people living with HIV/AIDS undergoing a resistance exercise program: clinical series. J Sports Med Phys Fitness. 2011;51(3):462–6. [PubMed] [Google Scholar]
- 99.Farinatti PT, Borges JP, Gomes RD, Lima D, Fleck SJ. Effects of a supervised exercise program on the physical fitness and immunological function of HIV-infected patients. J Sports Med Phys Fitness. 2010;50(4):511–8. This aerobic and resistance training intervention study in adults with HIV concluded that a supervised exercise program was safe, improved muscular and aerobic fitness and has no negative effects on immunological function.
- 100.Fillipas S, Cherry CL, Cicuttini F, Smirneos L, Holland AE. The effects of exercise training on metabolic and morphological outcomes for people living with HIV: a systematic review of randomised controlled trials. HIV Clin Trials. 2010;11(5):270–82. doi: 10.1310/hct1105-270. doi:10.1310/hct1105-270. [DOI] [PubMed] [Google Scholar]
- 101.Ogalha C, Luz E, Sampaio E, Souza R, Zarife A, Neto MG, et al. A randomized, clinical trial to evaluate the impact of regular physical activity on the quality of life, body morphology and metabolic parameters of patients with AIDS in Salvador, Brazil. J Acquir Immune Defic Syndr. 2011;57(Suppl 3):S179–85. doi: 10.1097/QAI.0b013e31821e9bca. doi:10.1097/QAI.0b013e31821e9bca. [DOI] [PubMed] [Google Scholar]
- **102.Yarasheski KE, Cade WT, Overton ET, Mondy KE, Hubert S, Laciny E, et al. Exercise training augments the peripheral insulin-sensitizing effects of pioglitazone in HIV-infected adults with insulin resistance and central adiposity. Am J Physiol Endocrinol Metab. 2011;300(1):E243–51. doi: 10.1152/ajpendo.00468.2010. doi:10.1152/ajpendo.00468.2010.. This study showed that exercise training augments the insulin response to pioglitazone (as assessed by hyperinsulinemic euglycemic clamp) and exercise training was associated with reduction in total body and limb fat. Exercise may be an important complementary treatment for patients requiring pharmacologic treatments for insulin resistance.
- 103.UNAIDS Global Report: UNAIDS Report on the Global AIDS Epidemic. 2010.
- 104.Miller TL, Somarriba G, Kinnamon DD, Weinberg GA, Friedman LB, Scott GB. The effect of a structured exercise program on nutrition and fitness outcomes in human immunodeficiency virus-infected children. AIDS Res Hum Retroviruses. 2010;26(3):313–9. doi: 10.1089/aid.2009.0198. doi:10.1089/aid.2009.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mansky KC. Aging, human immunodeficiency virus, and bone health. Clin Interv Aging. 2010;5:285–92. doi: 10.2147/cia.s13852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Somarriba G, Neri D, Schaefer N, Miller TL. The effect of aging, nutrition, and exercise during HIV infection. HIV AIDS (Auckl) 2010;2:191–201. doi: 10.2147/HIV.S9069. doi:10.2147/hiv.s9069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yahiaoui A, McGough EL, Voss JG. Development of evidence-based exercise recommendations for older HIV-infected patients. J Assoc Nurses AIDS Care. 2012;23(3):204–19. doi: 10.1016/j.jana.2011.06.001. doi:10.1016/j.jana.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 108.Souza PM, Jacob-Filho W, Santarem JM, Zomignan AA, Burattini MN. Effect of progressive resistance exercise on strength evolution of elderly patients living with HIV compared to healthy controls. Clinics (Sao Paulo) 2011;66(2):261–6. doi: 10.1590/S1807-59322011000200014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cade WT, Reeds DN, Overton ET, Herrero P, Waggoner AD, Davila-Roman VG, et al. Effects of human immunodeficiency virus and metabolic complications on myocardial nutrient metabolism, blood flow, and oxygen consumption: a cross-sectional analysis. Cardiovasc Diabetol. 2011;10:111. doi: 10.1186/1475-2840-10-111. doi:10.1186/1475-2840-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ciccolo JT, Jowers EM, Bartholomew JB. The benefits of exercise training for quality of life in HIV/AIDS in the post-HAART era. Sports Med. 2004;34(8):487–99. doi: 10.2165/00007256-200434080-00001. [DOI] [PubMed] [Google Scholar]
- 111.Jong E, Oudhoff LA, Epskamp C, Wagener MN, van Duijn M, Fischer S, et al. Predictors and treatment strategies of HIV-related fatigue in the combined antiretroviral therapy era. AIDS. 2010;24(10):1387–405. doi: 10.1097/QAD.0b013e328339d004. doi:10.1097/QAD.0b013e328339d004. [DOI] [PubMed] [Google Scholar]
- 112.Petroczi A, Hawkins K, Jones G, Naughton DP. HIV Patient Characteristics that Affect Adherence to Exercise Programmes: An Observational Study. Open AIDS J. 2010;4:148–55. doi: 10.2174/1874613601004010148. doi:10.2174/1874613601004010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.El-Bassel N, Jemmott JB, 3rd, Landis JR, Pequegnat W, Wingood GM, Wyatt GE, et al. Intervention to influence behaviors linked to risk of chronic diseases: a multisite randomized controlled trial with African-American HIV-serodiscordant heterosexual couples. Arch Intern Med. 2011;171(8):728–36. doi: 10.1001/archinternmed.2011.136. doi:10.1001/archinternmed.2011.136. This study is a multi-centered, multi-disciplinary team approach to improve multiple health behaviors in adults living with HIV. The authors concluded that this approach improved adherence to physical activity, improved healthy diet consumption and increased medical compliance.
- 114.Rotheram-Borus MJ, Swendeman D, Lee SJ, Li L, Amani B, Nartey M. Interventions for families affected by HIV. Transl Behav Med. 2011;1(2):313–26. doi: 10.1007/s13142-011-0043-1. doi:10.1007/s13142-011-0043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Weiss SM, Tobin JN, Antoni M, Ironson G, Ishii M, Vaughn A, et al. Enhancing the health of women living with HIV: the SMART/EST Women’s Project. Int J Womens Health. 2011;3:63–77. doi: 10.2147/IJWH.S5947. doi:10.2147/ijwh.s5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ardoy DN, Fernandez-Rodriguez JM, Ruiz JR, Chillon P, Espana-Romero V, Castillo MJ, et al. [Improving physical fitness in adolescents through a school-based intervention: the EDUFIT study] Rev Esp Cardiol. 2011;64(6):484–91. doi: 10.1016/j.recesp.2011.01.009. doi:10.1016/j.recesp.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 117.Snyder A, Colvin B, Gammack JK. Pedometer use increases daily steps and functional status in older adults. J Am Med Dir Assoc. 2011;12(8):590–4. doi: 10.1016/j.jamda.2010.06.007. doi:10.1016/j.jamda.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 118.Davies CA, Spence JC, Vandelanotte C, Caperchione CM, Mummery WK. Meta-analysis of internet-delivered interventions to increase physical activity levels. Int J Behav Nutr Phys Act. 2012;9(1):52. doi: 10.1186/1479-5868-9-52. doi:10.1186/1479-5868-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hardy OT, Wiecha J, Kim A, Salas C, Briceno R, Moody K, et al. Effects of a multicomponent wellness intervention on dyslipidemia among overweight adolescents. J Pediatr Endocrinol Metab. 2012;25(1-2):79–82. doi: 10.1515/jpem.2011.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lloyd JJ, Wyatt KM, Creanor S. Behavioural and weight status outcomes from an exploratory trial of the Healthy Lifestyles Programme (HeLP): a novel school-based obesity prevention programme. BMJ Open. 2012;2(3) doi: 10.1136/bmjopen-2011-000390. doi:10.1136/bmjopen-2011-000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–34. doi: 10.1249/mss.0b013e3180616b27. doi:10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]