Abstract
Bone loss has been observed within the first six months after hematopoietic cell transplantation (HCT) in both children and adults. While there is some evidence that bone formation may be reduced in children after HCT, it is currently unknown if bone resorption is increased. The objective of this prospective study was to evaluate changes in markers of bone resorption over the first six months after pediatric HCT. Twenty-six participants (8 females) age 10.9±3.4 years entered the study prior to HCT. Bone resorption was measured by urine deoxypyridinoline (DPD) and pyridinoline (PYD), and by plasma N-telopeptide (NTX) and C-telopeptide (CTX). Seventeen participants who completed day +30 visit and either day +100 or +180 visits were included in the analysis. DPD increased between days +30 and +100 (mean change 11.3 nmol/nmol creatinine; p=0.012) and between days +30 and +180 (13.7 nmol/nmol creatinine; p=0.036). PYD increased between days +30 and +100 (32 nmBCE/L; p=0.019). CTX increased between baseline and day +100 (5.9 μg/L; p=0.012). Changes in NTX levels were not statistically significant. This study shows that markers of bone resorption increase in children after HCT, suggesting that increased resorption may be a contributing factor to the pathophysiology of bone loss after pediatric HCT.
Keywords: bone resorption, pediatric, bone marrow transplantation, bone, osteoporosis
Introduction
With improved survival, the population of patients treated for secondary effects of pediatric HCT has increased significantly. One of the secondary complications of HCT is osteoporosis (1-7). Up to 20% of children treated with HCT will have low bone mineral density (BMD) (Z-score < −2) prior to reaching adulthood (5, 6), and approximately 20% have been shown to have low BMD as early as 6 months after HCT (4). This low BMD is clinically significant because a decrease in BMD Z-score predisposes children to an increased risk of fracture (8, 9). In addition, if the critical period of peak bone mass accrual during adolescence is interrupted, patients are less likely to reach an optimal peak BMD and may be more likely to suffer from osteoporotic fractures later in life (10).
Knowledge of the mechanisms of bone loss following HCT in children is very limited. In adult patients, osteoporosis is due to both a decrease in bone formation and an increase in bone resorption (11, 12). In children post-HCT, these mechanisms have only been evaluated in one study that found a decrease in the biomarkers of bone formation, but no significant increase in bone resorption (4). However, the aforementioned study included only one biomarker of bone resorption and the earliest time point assessed was 100 days after HCT. Since an increase in bone resorption has been observed as early as 21 days after HCT in adults (11), earlier changes may have been missed.
It is important to determine if bone resorption is increased in children after HCT in order to make appropriate treatment decisions for those at risk for bone loss. Therefore, the goal of this study was to prospectively examine changes in the markers of bone resorption in the first six months after pediatric HCT. We hypothesized that the levels of the markers of bone resorption will increase as early as 30 days after HCT.
Methods
Participants
Patients between the ages of 5 and 17 years meeting eligibility requirements for a first HCT for a malignancy or blood disorder between 2007 and 2010 at the University of Minnesota Amplatz Children’s Hospital were offered participation in the study. Children with previously diagnosed metabolic bone disease or metabolic storage disease, or previous HCT, were excluded from the study.
The study was approved by the University of Minnesota Institutional Review Board. Written informed consent was obtained from the participant’s parents/guardians (participants <18 years) and assent from children ≥ 7 years of age.
Laboratory tests and radiologic evaluation
Blood and urine were collected for biomarkers of bone resorption and 25-hydroxy vitamin D in the morning using the first or second morning urine after at least an 8 hour fast at baseline (prior to HCT), day +30 ± 5 days, day +100 ± 7 days, and day +180 ± 14 days. Laboratory assays were performed at the Advanced Research and Diagnostic Laboratory, University of Minnesota Medical Center. Deoxypyridinoline (DPD) was measured in urine using the MicroVue DPD enzyme immunoassay (EIA) kit from Quidel Corporation (San Diego, CA 92121). This kit measures DPD crosslinks by a competitive immunoassay. The inter-assay coefficient of variation (CV) was 0.9% at a mean concentration of 14.0 nmol/L, and 8.5% at a mean concentration of 106.7 nmol/L. Pyridinoline (PYD) was measured in urine using the Metra PYD EIA kit from Quidel Corporation (San Diego, CA 92121). This kit measures PYD crosslinks by a competitive immunoassay. The inter-assay CV was 8.6% at a mean concentration of 95.7 nmol/L, and 6.7% at a mean concentration of 426.6 nmol/L. The concentration of DPD and PYD was adjusted for variation in urine concentration by dividing the result by the creatinine concentration of the sample. N-telopeptide (NTX) was measured in plasma using the Osteomark NTx kit from Wampole Laboratories (Princeton, NJ 08540). This kit measures cross-linked N-telopeptides of type I collagen by a competitive-inhibition enzyme-linked immunosorbent assay. The inter-assay CV was 8.2% at a mean concentration of 28.0 nmBCE/L (nanomoles Bone Collagen Equivalents per liter). C-telopeptide (CTX) was measured in plasma using the UniQ ICTP EIA kit from Orion Diagnostica (Espoo, Finland). This kit measures C-terminal telopeptide of type I collagen by competitive immunoassay. The inter-assay CV was 8.9% at a mean concentration of 24.8 ug/L. 25-hydroxy vitamin D concentration was determined using liquid chromatography/tandem mass spectrometry. Bone age was assessed by the Greulich and Pyle method (13) before HCT and at day +180 ± 14 days after HCT.
Statistical methods
The analysis group included everyone with bone biomarker data at baseline and at +30 days. Baseline characteristics were evaluated for the overall and biomarker analysis groups. Mean change in bone biomarker concentrations over time were evaluated with confidence intervals and P-values based on t-tests for the whole group, then divided by gender, prepubertal vs. pubertal, and steroid vs. no steroid treatment. Due to missing data over time, the sample sizes were different for the change over different time frames. All analyses were conducted using R v12.13.1 (14). P-values less than 0.05 were considered statistically significant.
Results
Study Population
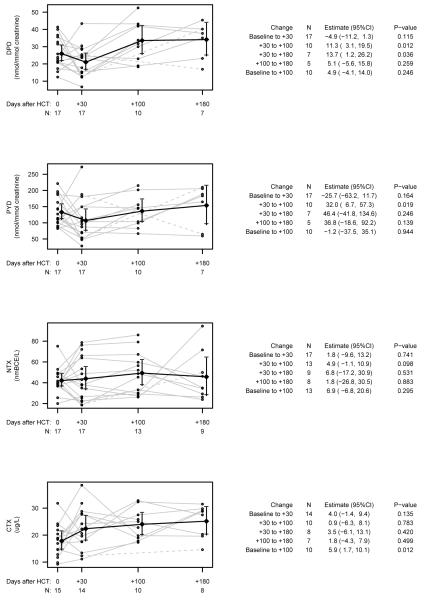
Twenty-six participants (8 females), age 10.9 ± 3.4 years (range 5.2-16.6 years) entered the study prior to HCT (Table 1). Of the 26 participants, 8 had acute myelogenous leukemia (AML), 2 acute lymphoblastic leukemia (ALL), 1 non-Hodgkin lymphoma, 1 myelodysplastic syndrome (MDS), 1 T-cell leukemia, 3 aplastic anemia and 10 Fanconi Anemia (FA). Over the course of the study, three participants died (day +54, +112 and +187) and 6 participants were either unable to return to our center or were critically ill. Thus, out of 26 participants, 17 competed day +30, 10-13 day +100, and 7-9 day +180 (Figure 1).
Table 1.
Baseline characteristics of the overall and biomarker analysis groups. Values presented are median, mean (SD) or n (%) as indicated.
| Covariate | Overall (N=26) |
Biomarker Analysis (N=17) |
|---|---|---|
| Age (yrs) | 10.5 10.9 (3.4) | 11.1 11.2 (2.9) |
| Female | 8 (30.8%) | 6 (35.3%) |
| Height Z-score | −0.7 −0. 9 (1.3) | −0.8 -1.1 (1.4) |
| Weight Z-score | −0.8 −0.6 (1.5) | −1.0 −0.6 (1.4) |
| BMI percent | 46.7 49.5 (31.0) | 46.8 52.9 (27.2) |
| Tanner stage | ||
| - Stage 1 | 11 (42.3%) | 9 (52.9%) |
| - Stage 2-3 | 5 (19.2%) | 4 (23.5%) |
| - Stage 4-5 | 5 (19.2%) | 1 (5.9%) |
| - Missing Stage | 5 (19.2%) | 3 (17.6%) |
| Bone age | 12.0 11.4 (4.2) | 11.0 11.1 (3.6) |
| Relative bone age | 1.0 1.0 (0.2) | 1.0 1.0 (0.2) |
| Missing bone age | 4 (15.4%) | 2 (11.8%) |
| Total body less head | ||
| - BMD (g/cm2) | 0.79 0.80 (0.16) | 0.79 0.78 (0.12) |
| - Missing BMD | 1 (3.9%) | 0 (0.0%) |
| - Z-score | −0.15 −0.02 (0.93) | −0.25 0.01 (1.01) |
| - Missing Z-score | 6 (23.1%) | 3 (17.7%) |
| Lumbar spine | ||
| - BMD (g/cm2) | 0.79 0.80 (0.18) | 0.79 0.78 (0.11) |
| - Missing BMD | 2 (7.7%) | 1 (5.9%) |
| - Z-score | −0.40 −0.50 (0.81) | −0.40 −0.48 (0.82) |
| - Missing Z-score | 7 (26.9%) | 4 (23.5%) |
| PYD (nmol/mmol creatinine) | 114.3 129.0 (42.1) | 114.3 133.0 (44.2) |
| DPD (nmol/mmol creatinine) | 22.7 25.7 (8.9) | 22.7 26.0 (8.7) |
| NTX (nmBCE/L) | 40.8 44.9 (13.7) | 39.5 42.1 (11.8) |
| CTX (μg/L) | 17.7 18.0 (6.3) | 18.4 17.8 (6.1) |
| 25-OH vitamin D | ||
| - <20 ng/ml | 1 (3.8%) | 0 (0.0%) |
| - 20-29 ng/ml | 11 (42.3%) | 7 (41.2%) |
| - >=30 ng/ml | 10 (38.5%) | 7 (41.2%) |
| - Missing vitamin D | 4 (15.4%) | 3 (17.6%) |
| Days to ANC >500* | 20.0 20.9 (9.6) | 23.0 22.0 (8.8) |
| Prep | ||
| - CY/CSA/ATG | 3 (11.5%) | 2 (11.8%) |
| - CY/FLU/TBI | 10 (38.5%) | 7 (41.2%) |
| - CY/FLU/TBI/ATG | 8 (30.8%) | 4 (23.5%) |
| - FLU/ATG/MELPH | 1 (3.8%) | 1 (5.9%) |
| - CY/FLU/ATG | 3 (11.5%) | 2 (11.8%) |
| - BU/CY | 1 (3.8%) | 1 (5.9%) |
| Total body irradiation | 18 (69.2%) | 11 (64.7%) |
| Steroid use | 16 (61.5%) | 9 (52.9%) |
| Acute graft versus host disease | 7 (26.9%) | 5 (29.4%) |
1 missing in Overall group
BMD = bone mineral density; PYD = urine pyridinoline; DPD = urine deoxypyridinoline; NTX = serum N-telopeptide; ANC = absolute neutrophil count (time to engraftment); CY = cyclophosphamide; CSA = cyclosporine; ATG = anti-thymoglobulin; FLU = fludarabine; MELPH = melphalan; BU = busulfan;
Fig. 1.
Changes in mean bone resorption for urine pyridinolines (PYD), urine deoxypyridinoline (DPD), serum N-telopeptide (NTX), and C-telopeptide (CTX). Data for each individual is plotted with individual trajectories in grey, dotted lines are used only when the value at day +100 was missing and points were connected between day +30 and +180, diamonds are means at each time point, which are connected with a black line and have bars representing 95% confidence intervals. The number of measurements at each time point is presented below the axis.
Four participants had short stature defined as a height SDS score < −2; baseline mean height Z-score was −0.89 (range −3.79 to 1.08). The majority of participants (81%) were treated with total body irradiation (TBI); 11 fractionated TBI (1320 cGy) and 7 single dose TBI (300-450 cGy, FA participants only). Two (8%) had grade 3 or higher acute GVHD. Only 1 out of 26 participants had vitamin D deficiency (25-OH vitamin D <20 ng/ml) at baseline; however this participant died before the day+30 visit and was not included in further analyses. Additional patients were identified as having vitamin D deficiency at later visits: 4 participants had vitamin D deficiency at day +30; 1 remained deficient at days +100 and +180, 1 died prior to day +100 and two were sufficient by day +100. Baseline vitamin D negatively correlated with baseline bone age (R = −0.60, p = 0.041).
Changes in bone resorption
There was considerable variability in change in bone biomarkers between each time point; on average, both DPD and PYD decreased over the first 30 days, increased between day +30 and day +100, then stabilized on average between day +100 and +180 (Figure 1). Changes between day +30 and +100 were statistically significant for both DPD and PYD (p=0.012 and p=0.019) and between days +30 and +180 for only DPD; although mean change between days +30 and +180 for PYD was greater than change between days +30 and +100 this change was not statistically significant likely due to a small sample size at day +180. CTX trended upward between baseline and day +30, and significantly increased from baseline to day +100 (p=0.012). There were no significant changes in serum NTX overall. Baseline bone age did not correlate with any markers of bone resorption.
We next evaluated whether there were differences in the change in biomarkers of bone resorption by gender, pubertal status, or steroid exposure. Males had a greater decrease in PYD from baseline to day +30 (estimate −12.6, 95%CI −36.3 to 11.2; p=0.032) and a lower increase in CTX from baseline to day +100 (estimate −9.7, 95%CI −17.9 to −1.5; p=0.026). There were no differences in change in biomarkers by pubertal status. Participants treated with steroids had a greater increase in DPD from day +30 to day +180 (estimate 23.0, 95%CI 5.2 to 40.7; p=0.021) and a greater increase in CTX from day +100 to day +180 (estimate 12.3, 95%CI 6.2 to 18.5; p=0.004). There were no other significant differences in change in biomarkers in males vs. females, prepubertal vs. pubertal participants, or in participants treated vs. not treated with steroids.
FA is a relatively uncommon indication for HCT where both the chemotherapy and radiation doses are reduced relative to other diagnoses. In order to evaluate the potential effects of this reduced exposure and because a significant proportion of our study patients had FA (n=10), we decided to perform a post hoc secondary analysis of the change in markers excluding the participants with FA. After excluding the FA patients, there were 12 participants at baseline, 12 at day +30, 8 at day +100, and 7-9 at day +180. We found a similar trend in change in all biomarkers except that without FA participants there was a significant decrease in both DPD (estimate −8.4 nmol/nmol creatinine, 95%CI: −15.6 to −1.3; p=0.025) and PYD (estimate −48.5 nmol/nmol creatinine, 95% CI: −87.9 to −9.1; p=0.020) from baseline to day +30; PYD trended down from baseline to day +100 as well (estimate −21.7 nmol/nmol creatinine, 95% CI: −45.1 to 1.8; p=0.065).
Discussion
Low BMD has been previously reported following pediatric HCT (4-6). The majority of bone loss occurs within the first 6 months after HCT in both children and adults, and is in part related to a decrease in bone formation (4). Several prospective studies in adult HCT recipients have demonstrated that a decrease in BMD is preceded by changes in the markers of bone turnover. Bone resorption (measured by type I collagen carboxyterminal telopeptide, ICTP) progressively increases and reaches a peak between 21 days and 100 days (15), followed by either return to the baseline level (4) or remaining above the normal range (16-22) at 12 months after HCT. The current study shows that, similar to adults, bone resorption (measured by urine DPD and PYD, and plasma CTX) increases over the first 100 days after pediatric HCT. In addition, after exclusion of participants with FA, there was a significant decrease in bone resorption between baseline and day +30. This transient decrease in bone resorption in non-FA patients may be due to inhibition/destruction of recipient’s osteoclasts after chemotherapy and TBI. This does not appear to be the case in FA patients who typically receive reduced intensity preparatory regimens. Osteoclasts are derived from the peripheral mononuclear cells and are thus donor in origin. It is interesting that the average time to engraftment was 21 days and that an increase in bone resorption followed engraftment, beginning at day +30.
Several mechanisms underlying this increase in osteoclast activity in HCT patients have been proposed, including the so called “cytokine storm” that occurs within the first 3 weeks after HCT, or an increase in the ratio of receptor activator of the nuclear factor-κB ligand (RANKL) to osteoprotegerin (OPG), which also reaches a peak at 3 weeks (22). It is currently unknown whether similar changes occur in children after HCT. Although we did not measure bone formation in this study, we previously evaluated this in a pediatric population over the first 12 months after HCT and found that bone formation (measured by osteocalcin) at day +100 predicted recovery of bone density by 12 months after HCT (4). Together these studies suggest that the uncoupling of bone resorption and formation within the first 30-100 days after HCT is involved in the pathophysiology of bone loss after pediatric HCT.
Bisphosphonates can improve BMD in adults with osteoporosis after HCT and prevent bone loss during HCT (16-19) by inducing osteoclast apoptosis and providing a physical barrier to osteoclast resorptive activity. One retrospective study has shown that empirical use of bisphosphonate therapy can improve BMD in pediatric HCT recipients as well (22). The annualized median change in BMD Z-score was significantly higher in those treated with bisphosphonates compared to those treated with calcium and vitamin D alone. However, biomarkers of bone resorption were not included.
This study is limited by a small sample size and missing data, particularly those completing the day+180 visit. If the missing data are not missing at random, then our estimates presented may be biased; however, we believe this is unlikely to be the case in the nine patients who died or were either unable to return to the center or critically ill. The withdrawal rate in the current study is similar to that in our previous study (4) and is expected given the significant burden of post transplant complications in this population. The small sample size limits our ability to evaluate the role of specific factors (i.e. preparatory regimen, days to engraftment) on changes in the bone resorption biomarkers. Although the sample size was small, we found consistent directionality of associations supporting our hypothesis that bone resorption is increased after pediatric HCT.
In summary, this study shows that biomarkers of bone resorption are increased over the first 100 days after pediatric HCT. However, a randomized controlled clinical trial of an anti-resorptive treatment in children after HCT is required before use in routine clinical care of children following treatment with HCT. Any intervention study in this population needs to take into account dynamic changes in bone homeostasis, and utilize changes in bone turnover as well as BMD to monitor treatment.
Acknowledgements
The authors wish to acknowledge Naomi Hanson, M.S. and Valerie Arends, M.S., Advanced Research and Diagnostic Laboratory, University of Minnesota, for performing biomarker assays, Todd Defor for database management, and the patients and families who participated in this study. This project was supported by the Children’s Cancer Research Fund (LEP), the National Institutes of Health Grant Number K23 AR057789 (LEP) as well as Grant Number 1UL1RR033183 from the National Center for Research Resources (NCRR) of the National Institutes of Health (NIH) to the University of Minnesota Clinical and Translational Science Institute (CTSI). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the CTSI or the NIH. The University of Minnesota CTSI is part of a national Clinical and Translational Science Award (CTSA) consortium created to accelerate laboratory discoveries into treatments for patients
Footnotes
Declaration
The author(s) declare that they have no competing interests.
Authors’ contributions
LEP and AP conceived of and designed the study, participated in interpretation of data, and writing of the manuscript. KR performed the statistical analysis, participated in interpretation of data and in revising the manuscript critically for important intellectual content. MD assisted with data collection. AS and KSB participated in revising the manuscript for important intellectual content. All authors read and approved the final manuscript.
References
- 1.Stern JM, Sullivan KM, Ott SM, et al. Bone density loss after allogeneic hematopoietic stem cell transplantation: a prospective study. Biol Blood Marrow Transplant. 2001;7:257–264. doi: 10.1053/bbmt.2001.v7.pm11400947. [DOI] [PubMed] [Google Scholar]
- 2.Nysom K, Holm K, Michaelsen KF, et al. Bone mass after allogeneic BMT for childhood leukaemia or lymphoma. Bone Marrow Transplant. 2000;25:191–196. doi: 10.1038/sj.bmt.1702131. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia S, Ramsay NK, Weisdorf D, Griffiths H, Robison LL. Bone mineral density in patients undergoing bone marrow transplantation for myeloid malignancies. Bone Marrow Transplant. 1998;22:87–90. doi: 10.1038/sj.bmt.1701275. [DOI] [PubMed] [Google Scholar]
- 4.Petryk A, Bergemann TL, Polga KM, et al. Prospective study of changes in bone mineral density and turnover in children after hematopoietic cell transplantation. J Clin Endocrinol Metab. 2006;91:899–905. doi: 10.1210/jc.2005-1927. [DOI] [PubMed] [Google Scholar]
- 5.Kaste SC, Shidler TJ, Tong X, et al. Bone mineral density and osteonecrosis in survivors of childhood allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004;33:435–441. doi: 10.1038/sj.bmt.1704360. [DOI] [PubMed] [Google Scholar]
- 6.Perkins JL, Kunin-Batson AS, Youngren NM, et al. Long-term follow-up of children who underwent hematopoeitic cell transplant (HCT) for AML or ALL at less than 3 years of age. Pediatr Blood Cancer. 2007;49:958–963. doi: 10.1002/pbc.21207. [DOI] [PubMed] [Google Scholar]
- 7.Daniels MW, Wilson DM, Paguntalan HG, Hoffman AR, Bachrach LK. Bone mineral density in pediatric transplant recipients. Transplantation. 2003;76:673–678. doi: 10.1097/01.TP.0000076627.70050.53. [DOI] [PubMed] [Google Scholar]
- 8.Goulding A, Jones IE, Taylor RW, Manning PJ, Williams SM. More broken bones: a 4-year double cohort study of young girls with and without distal forearm fractures. J Bone Miner Res. 2000;15:2011–2018. doi: 10.1359/jbmr.2000.15.10.2011. [DOI] [PubMed] [Google Scholar]
- 9.Clark EM, Ness AR, Bishop NJ, Tobias JH. Association between bone mass and fractures in children: a prospective cohort study. J Bone Miner Res. 2006;21:1489–1495. doi: 10.1359/jbmr.060601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachrach LK, Hastie T, Wang MC, Narasimhan B, Marcus R. Bone mineral acquisition in healthy Asian, Hispanic, black, and Caucasian youth: a longitudinal study. J Clin Endocrinol Metab. 1999;84:4702–4712. doi: 10.1210/jcem.84.12.6182. [DOI] [PubMed] [Google Scholar]
- 11.Kang MI, Lee WY, Oh KW, et al. The short-term changes of bone mineral metabolism following bone marrow transplantation. Bone. 2000;26:275–279. doi: 10.1016/s8756-3282(99)00265-3. [DOI] [PubMed] [Google Scholar]
- 12.Ebeling PR, Thomas DM, Erbas B, Hopper JL, Szer J, Grigg AP. Mechanisms of bone loss following allogeneic and autologous hemopoietic stem cell transplantation. J Bone Miner Res. 1999;14:342–350. doi: 10.1359/jbmr.1999.14.3.342. [DOI] [PubMed] [Google Scholar]
- 13.Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. Stanford University Press; Stanford, Calif.: 1959. [Google Scholar]
- 14.Team RDC . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna: 2011. [Google Scholar]
- 15.Valimaki MJ, Kinnunen K, Volin L, et al. A prospective study of bone loss and turnover after allogeneic bone marrow transplantation: effect of calcium supplementation with or without calcitonin. Bone Marrow Transplant. 1999;23:355–361. doi: 10.1038/sj.bmt.1701586. [DOI] [PubMed] [Google Scholar]
- 16.Kananen K, Volin L, Laitinen K, Alfthan H, Ruutu T, Valimaki MJ. Prevention of bone loss after allogeneic stem cell transplantation by calcium, vitamin D, and sex hormone replacement with or without pamidronate. J Clin Endocrinol Metab. 2005;90:3877–3885. doi: 10.1210/jc.2004-2161. [DOI] [PubMed] [Google Scholar]
- 17.Grigg AP, Shuttleworth P, Reynolds J, et al. Pamidronate reduces bone loss after allogeneic stem cell transplantation. J Clin Endocrinol Metab. 2006;91:3835–3843. doi: 10.1210/jc.2006-0684. [DOI] [PubMed] [Google Scholar]
- 18.Chae YS, Kim JG, Moon JH, et al. Pilot study on the use of zoledronic acid to prevent bone loss in allo-SCT recipients. Bone Marrow Transplant. 2009;44:35–41. doi: 10.1038/bmt.2008.414. [DOI] [PubMed] [Google Scholar]
- 19.D’Souza AB, Grigg AP, Szer J, Ebeling PR. Zoledronic acid prevents bone loss after allogeneic haemopoietic stem cell transplantation. Intern Med J. 2006;36:600–603. doi: 10.1111/j.1445-5994.2006.01154.x. [DOI] [PubMed] [Google Scholar]
- 20.Tauchmanova L, Ricci P, Serio B, et al. Short-term zoledronic acid treatment increases bone mineral density and marrow clonogenic fibroblast progenitors after allogeneic stem cell transplantation. J Clin Endocrinol Metab. 2005;90:627–634. doi: 10.1210/jc.2004-0509. [DOI] [PubMed] [Google Scholar]
- 21.Tauchmanova L, De Simone G, Musella T, et al. Effects of various antireabsorptive treatments on bone mineral density in hypogonadal young women after allogeneic stem cell transplantation. Bone Marrow Transplant. 2006;37:81–88. doi: 10.1038/sj.bmt.1705196. [DOI] [PubMed] [Google Scholar]
- 22.Carpenter PA, Hoffmeister P, Chesnut CH, 3rd, et al. Bisphosphonate therapy for reduced bone mineral density in children with chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13:683–690. doi: 10.1016/j.bbmt.2007.02.001. [DOI] [PubMed] [Google Scholar]