Abstract
The rodent amygdaloid complex is composed of numerous subnuclei important for the sex specific regulation of sociosexual behavior. Although estrogen receptors (ERs) are critical for organizing functional and cytoarchitectural sex differences in these subnuclei, a detailed developmental profile of ER expression in the amygdaloid complex is not available. Moreover, the kisspeptin gene (Kiss1) was recently identified in the adult amygdala, but it remains unknown if it is expressed during development. To fill these data gaps, rat brains (5–7/group) were assessed on postnatal days (PNDs) 0, 2, 4, 7 and 19 for ER alpha (ERα; Esr1), beta (ERβ; Esr2) and Kiss1 expression using in situ hybridization. Expression was quantified in the posterodorsal portion of the medial amygdala MePD, lateral (PLCo) and medial (PMCo) components of the posterior cortical nucleus, and the amygdalohippocampal area (AHi). ERα expression was high throughout the amygdala at birth, but sexually dimorphic only in the AHi. ERα expression in the MePD and the PLCo showed a U-shaped expression pattern over time. In the PMCo, ERα expression decreased from PND 2 and remained low through PND 19. Sexually dimorphic expression of ERβ in the MePD was observed on PND 0, with higher levels in females, but reversed by PND 4 due to declining levels in females. No Kiss1 signal was observed in the postnatal amygdala, suggesting that expression arises after puberty. These data reveal that ER expression is region specific within the neonatal amygdala. These differences likely contribute to sex differences in sociosexual behavior across the lifespan.
Keywords: development, kisspeptin, sexually dimorphic, hypothalamus, neonatal
INTRODUCTION
The amygdaloid complex comprises several subnuclei (nomenclature adopted from (Watson et al., 2012)), each with distinct, steroid hormone sensitive, functions. For example, lesions to the medial amygdala (Me) or cortical amygdaloid nucleus (Co) impairs male sexual behavior (Kondo, 1992; Lehman et al., 1980), while lesioning of the posterior amygdaloid nucleus enhances maternal behavior (Fleming et al., 1980) and decreases the lordotic response in females (Masco and Carrer, 1980). Each subregion sends and receives projections to other subnuclei within the amygdaloid complex, as well as an array of other nuclei throughout the brain (Canteras et al., 1992; Cooke and Simerly, 2005; Maras and Petrulis, 2010; Simerly, 2002), including the bed nuclei of the stria terminalis (BNST) and the anterior hypothalamus (Coolen et al., 1997; Coolen and Wood, 1999; Manzo et al., 1999; Simerly, 2002). Collectively these networks play critical roles in modulating a variety of social and sexual behaviors by integrating environmental cues, such as odors and pheromones, with hormonal signals. Behaviors for which the amygdala is well recognized to play a central role include sexual behavior (Harris and Sachs, 1975; Newman, 1999), juvenile rough and tumble play (Meaney et al., 1981), aggression (Wang et al., 1997), memory (Cahill et al., 1996), and parental behavior (De Vries and Villalba, 1997; Fleming et al., 1980). Although it is well recognized that neonatal exposure to estrogen, aromatized from testicular androgens, participates in the orchestration of structural sex differences within the amygdaloid complex that ultimately confer behavioral sex differences, no detailed profile of ER expression in the amygdala during neonatal development is currently available. The present studies were undertaken to fill this knowledge gap with the hypothesis that ERs would be differentially and dynamically expressed in a sex specific manner across the subnuclei during neonatal maturation.
For the present studies, we focused on the posterodorsal portion of the medial amygdala (MePD), the cortical amygdaloid nucleus, posterolateral (PLCo) and the posteromedial (PMCo), and the amygdalo-hippocampal area (AHi). These regions were selected because they collectively represent three functionally distinct regions of the amygdaloid complex. The Me and PMCo, together with the nucleus of the accessory olfactory tract, belong to the vomeronasal amygdala and receive direct projections from the accessory olfactory bulb (Kevetter and Winans, 1981a). The PLCo is a component of the olfactory amygdala and receives afferents from the main olfactory bulb (Kevetter and Winans, 1981b). The AHi is a hipopallial nucleus (also called the posterior amygdale nucleus (PA)) (Canteras et al., 1992; Swanson and Petrovich, 1998) which receives input from the PLCo, PMCo and Me, and projects to the Me, PMCo, BNST and numerous hypothalamic nuclei. This region helps integrate odor and pheromonal cues with hormonal information to regulate of a wide range of sociosexual behaviors (Canteras et al., 1992; Simerly, 2002; Swanson and Petrovich, 1998). In adults, ER protein and mRNA have been identified throughout the amygdaloid complex, with ERα as the predominant subtype (Osterlund et al., 1998; Shughrue et al., 1997; Shughrue and Merchenthaler, 2001; Simerly et al., 1990). In the developing rat brain, a prior study identified ERα by immunoreactive histochemistry on the day of birth in the MePD and the posterior cortical amygdaloid nucleus (Yokosuka et al., 1997), and on PND 3 in the Me, Co, AHi, lateral amygdaloid nucleus, basolateral amygdala nucleus and amygdala piriform area (Perez et al., 2003). That study also observed ERβ in the MeA as early as PND 3 (Perez et al., 2003) but did not look earlier, and no sex differences were found. A study, published before the discovery of ERβ, and using an indirect 125I E2 binding assay, identified ER protein from PNDs 2 to 49 with levels higher in females than in males in the Me on PND 49 (Kuhnemann et al., 1994). The present study was designed to enhance the current literature by providing the most detailed expression map to date of the two primary ER subtypes, ERα and ERβ, in the developing MePD, PLCo, PMCo and AHi across neonatal life; revealing their temporal and sexually dimorphic expression from birth to pre-weaning. The data provided here will help elucidate the underlying mechanistic roles each ER subtype plays in the sex specific organization of the amygdaloid subnuclei and their respective roles in sociosexual behavior.
We also sought to characterize the ontogeny of kisspeptin (Kiss1) mRNA expression in the rat amygdaloid complex. It is now well established that kisspeptin (KP) neurons in the hypothalamus regulate positive and negative feedback on gonadotropin-releasing hormone (GnRH) neurons, but a recent study reported that Kiss1 is also expressed in the MePD of both rats and mice. This expression is sexually dimorphic with higher levels in males than diestrus females (Kim et al., 2011), and under the regulation of circulating sex steroids with lower levels in gonadectomized rodents of both sexes. Importantly, amplification of Kiss1 signal by steroid hormone administration appears to occur through estrogen receptor-dependent pathways (Kim et al., 2011) because nonaromatizable androgens do not modulate Me Kiss1 expression. These results indicate that Kiss1 in the MePD may play a role in modulating reproductive and/or non-reproductive behavior in rodents, but the specific functional role of these KP neurons remains unknown. There is no data regarding Kiss 1 expression in the neonatal rat brain, thus we sought to elucidate the expression profile during this critical period with the hypothesis that it would be sexually dimorphic.
MATERIALS AND METHODS
Animal care and tissue collection
Timed pregnant Long Evans (LE) rats (n = 7; Charles River, Raleigh, NC), were individually housed in a temperature and light controlled room (14:10 h light: dark cycle; lights on at 0700 h) at 23°C and 50% average relative humidity with food and water available ad libitum. To minimize exposure to endocrine disrupting compounds (Brown and Setchell, 2001; Degen et al., 2002; Patisaul, 2005), all dams were housed in thoroughly washed polysulfone (Bisphenol A (BPA) free) caging, with glass water bottles, and fed a semi-purified, phytoestrogen-free diet (AIN-93G, Test Diet, Richmond, IN). The animals were maintained at the Biological Resource Facility of North Carolina State University (NCSU) according to the applicable portions of the Animal Welfare Act and the U.S. Department of Health and Human Services Guide for the Care and use of Laboratory Animals. All procedures were approved by the Institutional Animal Care and Use Committee of NCSU.
The animals used for this study were also used for a prior study (Cao and Patisaul, 2011). Briefly, female and male pups (n = 5 to 7 per sex, per group) were born to timed pregnant dams and sacrificed by rapid decapitation on postnatal days 0 (PND 0 = the day of birth, defined as within 12 hr after littering took place), 2, 4, 7 and 19. The whole head from the PND 0, 2 and 4 animals, and the brains from PND 7 and 19 animals, were rapidly frozen on powdered dry ice, and stored at −80°C until cryosectioning. No more than 2 pups of each sex (to minimize litter effects), at each time point, were selected from the same litter. Thus pups of the same sex are from at least 3 different dams.
In situ hybridization histochemistry (ISHH)
Details regarding tissue processing, probe generation and ISHH procedures are described in our prior, related, publication (Cao and Patisaul, 2011). The autoradiographs generated to quantify ERα, ERβ and Kiss1 in the mediobasal hypothalamus in the prior study (Cao and Patisaul, 2011) were used here to quantify gene expression in MePD, PLCo, PMCo and AHi.
In brief, the brains were cut into three serial sets of 12 µm coronal sections on a cryostat (Leica CM1900, Nussloch, Germany), mounted onto Superfrost plus slides (Fisher Scientific, Pittsburgh, PA), and stored at −80°C until ISHH processing. For each gene of interest, all sections were processed as a large batch simultaneously to avoid batch effects. The templates for the antisense probes ERα, ERβ and Kiss1 were 345, 501 and 318-bp cDNA fragments respectively. They correspond to the sequence from 610bp to 1025bp (Genbank NM_012689) for rat estrogen receptor 1 (Esr1, also called ERα) mRNA, from 454 bp to 954bp (Genbank NM_012754) for estrogen receptor 2 (Esr2; also called ERβ) mRNA, and from 33 bp to 350bp (Genbank NM_181692) for KiSS-1 metastasis-suppressor (Kiss1) mRNA. The specificity of the probes, the procedure for probe synthesis and purification has previously been described (Cao and Patisaul, 2011; Cao et al., 2011). On the day of the ISHH, one set of cryosections, including all regions to be examined, were thawed and dried at room temperature and standard pre-hybridization washes were completed as described previously (Cao and Patisaul, 2011; Cao et al., 2011). After the sections were dried, the cRNA probes (~0.5× 106 cpm, labeled by 35S-UTP) were applied to each tissue section in 25 µl of hybridization buffer and then sections were covered with glass coverslips and incubated in humid chambers overnight at 50°C.
After hybridization, the slides were washed and then dehydrated in a series of increasing concentrations of ethanol as described previously (Cao and Patisaul, 2011; Cao et al., 2011). Dried slides were exposed to Kodak Biomax MR X-ray film (Eastman Kodak, Rochester, NY, USA) for 14 days for ERα, 25 days for ERβ and 24 days for Kiss1, along with an autoradiographic 14C microscales (Amersham Life Sciences, Arlington Heights, IL, USA) to generate optical density curves. After exposure, films were developed using a Konica SRX-101A film processor (Konica Corporation, Tokyo, Japan).
In order to visualize cell-specific silver grain labeled clusters, the slides were dipped in NTB3 emulsion (Kodak, Rochester NY). All slides were kept at 4°C for 42 days for ERα, 73 days for ERβ and 91 days for Kiss1, and then developed in Dektol developer and Kodak fixant (Kodak, Rochester NY) according to the user manual. Then, all slides were counterstained with Mayer’s Hematoxylin (Sigma) as described previously (Cao et al., 2012; Cao and Patisaul, 2011).
Landmark identification and image analysis
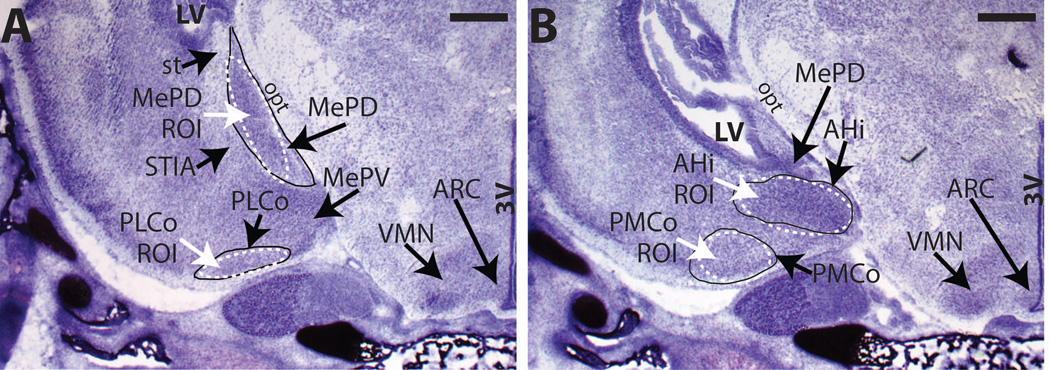
A rat brain atlas (Watson and Paxinos, 2007) and previously published studies (Canteras et al., 1992; Cooke and Simerly, 2005; Cooke et al., 2007; Osterlund et al., 1998; Simerly, 2002; Simerly et al., 1990; Vinader-Caerols et al., 1998) were used in conjunction with an in-house library of Nissl stained sections to identify each subregion across neonatal development, as well as the region of interest (ROI) for quantification (Figure 1; each subregion is encircled with a solid line, and the ROI for film quantification is encircled with a dashed line). Because these subnuclei become larger with age, the ROI for quantification also modified with age but was standardized for all animals at that age.
Fig. 1.
Representative NISSL-stained sections from PND 2 females depicting the anatomical landmarks used to identify each brain region of interest (circled with a solid black solid) and the corresponding sampling template created to define the area of interest (ROI, white dashed line) and quantify the autoradiographic signal within that brain area. An ROI was created for each age and brain area, and then used for all sections, regardless of sex, within that age group. (A) Representative sections containing a midlevel section of the MePD and PLCo, and (B) the AHi and PMCo were obtained from animals in our existing colony to help identify anatomical landmarks in the hybridized tissue used for the present studies. For abbreviations see list. Scale bar = 500 µm.
The MePD and PLCo (Figure 1A) were detected in the same coronal sections as the caudal portion of the VMNvl and ARC. The MePD was defined as the posterodorsal aspect of the Me (Figure 1A). The medial border of the MePD lies between the cell-dense region and the cell-sparse molecular layer immediately lateral to the optic tract. The dorsal portion of the subregion is a cell-sparse zone indicative of the stria terminalis (st). The lateral border is medial to a relatively cell-sparse region, the intra-amygdaloid division of the BNST (STIA), and typically appears as a chevron-shape and terminates ventrally at the molecular layer between the posteroventral Me (MePV) and the MePD (Cooke and Simerly, 2005; Cooke et al., 2007). The PLCo extends lengthwise rostrocaudally (Figure 1A) almost to the anterior cortical nucleus in the rostromedial border, and extends to the rostral portion of the PMCo. It has a well-defined trilaminar structure, a molecular layer, and a superficial cell layer made of tightly packed pyramidal neurons (Canteras et al., 1992). The PMCo, belonging to the Co, posterior portion, is a large mass of medium-sized cells (Figure 1B), which are densely packed in the rostral portion and become loosely arranged in the caudal portion (Canteras et al., 1992; Vinader-Caerols et al., 2000). The PMCo lies in the caudal third of the amygdaloid complex, medial to the PLCo, and ventral to the posteromedial AHi. The AHi (also known as posterior amygdala nucleus (PA)) (Canteras et al., 1992; Swanson and Petrovich, 1998) appears caudally to the MePD and is separated from the posterior MePD by a band of fibers related to intraamygdalar parts of the st (Canteras et al., 1992). In our coronal sections, the AHi emerges just as the VMNvl disappears and is present along with the most caudal aspect of the ARC (Figure 1B). Within each of these regions the cells are more densely packed than in the surrounding areas, except for the PMCo, which contains relatively loosely packed cells.
Autoradiograms depicting ERα, ERβ, and Kiss1 signals were used to quantify mRNA expression levels of each gene, at each age, using optical density (MCID Core Image software program; InterFocus Imaging Ltd, Cambridge, England) as described previously (Cao and Patisaul, 2011). All quantification was conducted by an investigator blinded to treatment groups. For each brain area, a sampling template encompassing the ROI was created and used for all sections from the same age in both sexes to standardize the area examined (Figure 1). ROI and background levels were measured unilaterally from anatomically matched sections. For optical density quantification of each gene, five anatomically matched sections for each region were used. The optical density reading from each brain section, after background subtraction, was averaged and used as the final measurement for that region of each animal. Optical densities were converted to nCi/g tissue by using a “best fit” curve (3rd degree polynomial) generated from the autoradiographic 14C microscale. In all cases, signal was within the limits of the curve.
The emulsion dipped and counterstained slides for each gene were used to confirm the labeling observed on the autoradiograms, viewed on a Leica 5000DM microscope and imaged using a Retiga 2000R color camera (QImaging, Surry, British Colombia, Canada). The presence of dense clusters of silver grains confined within discreetly counterstained nuclei was considered to be confirmation of both label specificity and identification of regions of interest. Silver grain density was not quantified.
Statistics
All datasets were first analyzed by two-way analysis of variance (ANOVA) with sex and exposure group as factors, followed by one way ANOVAs to determine the effect of age within each sex. If significant effects were found, the Dunnett’s Multiple Comparison post hoc test was used to compare each age group to PND 0 within the same sex group. Sex differences at each age were identified using t-tests. In all cases, the results were considered significant when P ≤ 0.05.
RESULTS
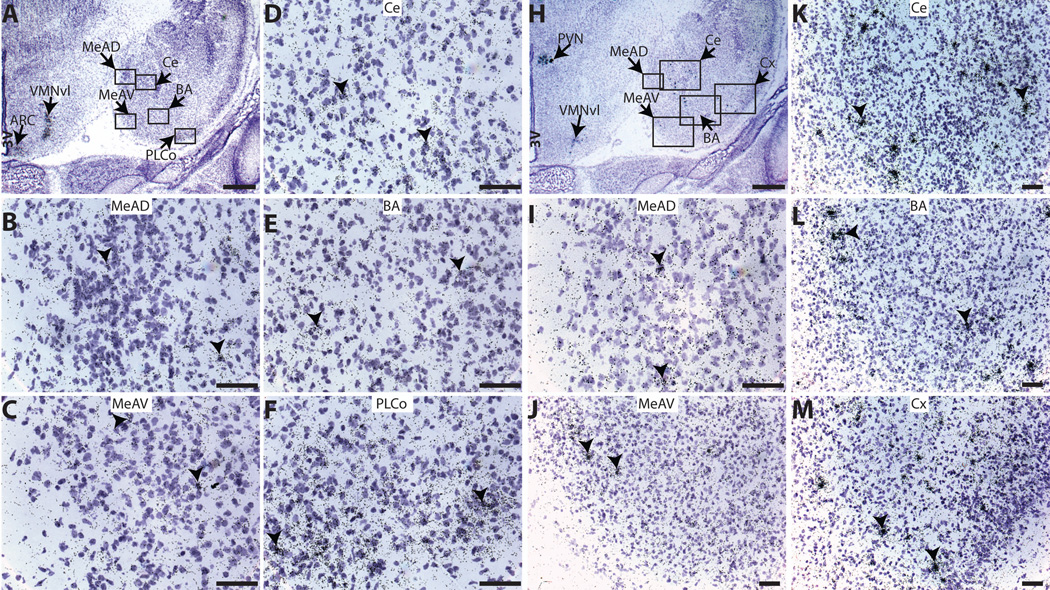
All results are summarized in Table 1. In both sexes (ages from PND 0 to 19), ERα expression was detected in most of the examined subregions of the amygdala. The intensity of ERα labeling was relatively weak in anterior amygdaloid nuclei (Figure 2A), such as the Me anteriordorsal (MeAD) (Figure 2B), the Me anteriorventral (MeAV) (Figure 2C), the central amygdaloid nucleus (Ce) (Figure 2D) and the basal amygdaloid nucleus (BA) (Figure 2E) with only loosely packed silver grain clusters visable. ERα signal was higher in the MePD and the PLCo (Figure 2F and 3), the PMCo and the AHi (Figure 4). ERβ mRNA was also observed in the anterior amygdaloid nuclei (Figure 2H), showing distinct and densely packed silver grain clusters compared with ERα in identical sections, including the MeAD (Figure 2I), the MeAV (Figure 2J), the Ce (Figure 2K), the BA (Figure 2L) and the cortex (Cx, Figure 2M). ERα and ERβ mRNA levels were not quantified in the anterior amygdaloid nuclei because section numbers were insufficient. In both sexes, ERα mRNA was robustly expressed in the four subregions selected for quantification (Figure 3 to 4). ERα mRNA expression levels differed over time in each examined area, but sexually dimorphic expression was only observed in the AHi on PND 0 (Figure 4). In the ROIs selected, ERβ signal was primarily confined to the MePD (Figure 5), and showed both temporal and sexually dimorphic patterns. No visible Kiss1 signal was observed in any examined areas in any age group (Figure 6).
Table 1.
Expression of ERα, ERβ and Kiss1 mRNA in the Postnatal Rat Amygdaloid complex
| Gene Name | Area Examined | PND0 | PND 2 | PND 4 | PND 7 | PND 19 |
|---|---|---|---|---|---|---|
| ERα | MePD | F=M | F=M | ⇓F=M↓ | ⇓F=M↓ | F=M |
| PLCo | F=M | ⇓F=M↓ | ⇓F=M↓ | F=M | F=M | |
| PMCo | F=M | ⇓F=M↓ | ⇓F=M↓ | ⇓F=M↓ | ⇓F=M↓ | |
| AHi | F<M | F=M↓ | F=M↓ | F=M↓ | F=M↓ | |
| ERβ | MePD | F>M | F>Ma | ⇓F<M | ⇓F<Mb | ⇓F<M |
| Kiss1 | MePD/PMCo/PLCo/AHi | ND | ND | ND | ND | ND |
ND=No Detectable Signal;
P = 0.12;
P = 0.16
“⇓” and “⇑” represent signal significantly decreased or increased, respectively, compared with PND 0 females.
“↓” and “↑” represent signal significantly decreased or increased, respectively, compared with PND 0 males.
Fig. 2.
Representative autoradiographic images from PND 0 female rats depicting ERα and ERβ mRNA signal in the anterior portion of the neonatal amygdala, and representative images of emulsion dipped slides depicting silver grain deposition in two consecutive sections labeled for ERα (Figure 2 A to F) and ERβ (Figure 2 H to M). ERα silver grain clusters were detected in the MeAD (B), MeAV (C), Ce (D), BA (E) and PLCo (F), but diffusely distributed. ERα signal was also robust in ARC and VMNvl in the same section confirming the specificity and quality of the labeling (A). ERβ signal (H) was readily detectable in the MeAD (I), MeAV (J), Ce (K), BA (L) and Cortex (Cx, M). ERβ mRNA silver grain clusters were densely packed compared to ERα labeling in the same subregions. ERβ signal was also detected in the PVN and VMNvl in the same section (H) confirming the specificity and quality of the labeling. For abbreviations, see list. Scale bar = 500 µm for A and H, and 50µm for B to F and I to M. Arrow heads indicate the silver grain clusters.
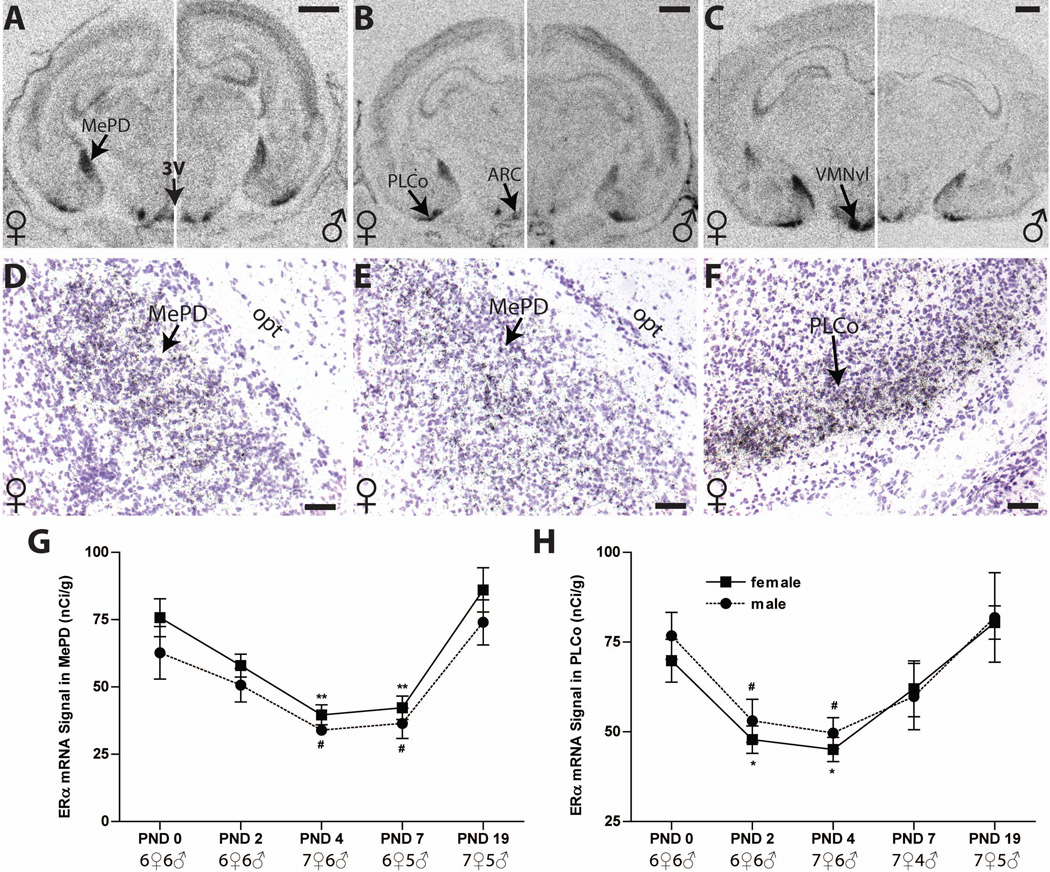
Fig. 3.
Representative autoradiographs depicting ERα mRNA signal in the postnatal rat MePD and PLCo of both sexes (A to C, females on the left side of each panel, males on the right) and silver grain deposits (D to F). Within the MePD and PLCo, labeling was intense in both sexes at birth (A), declined through PND 4 (B), and then returned to PND 0 levels by PND 19 (C) in both sexes (G-H). (D-E) Silver grain deposition confirmed the location of mRNA signal. ERα silver grain deposition is shown in the PND 0 female MePD (D), the PND 4 female MePD (E) and the PND 0 female CoAPL (F). Significant differences in expression compared with PND 0 levels are represented by *P ≤ 0.05 and **P ≤ 0.01 for the females, and #P ≤ 0.05 for the males. The sample size for each group is shown, and the data points represent mean ± SEM. For abbreviations, see list. Scale bar = 1000 µm for A to C, and 50µm for D to F.
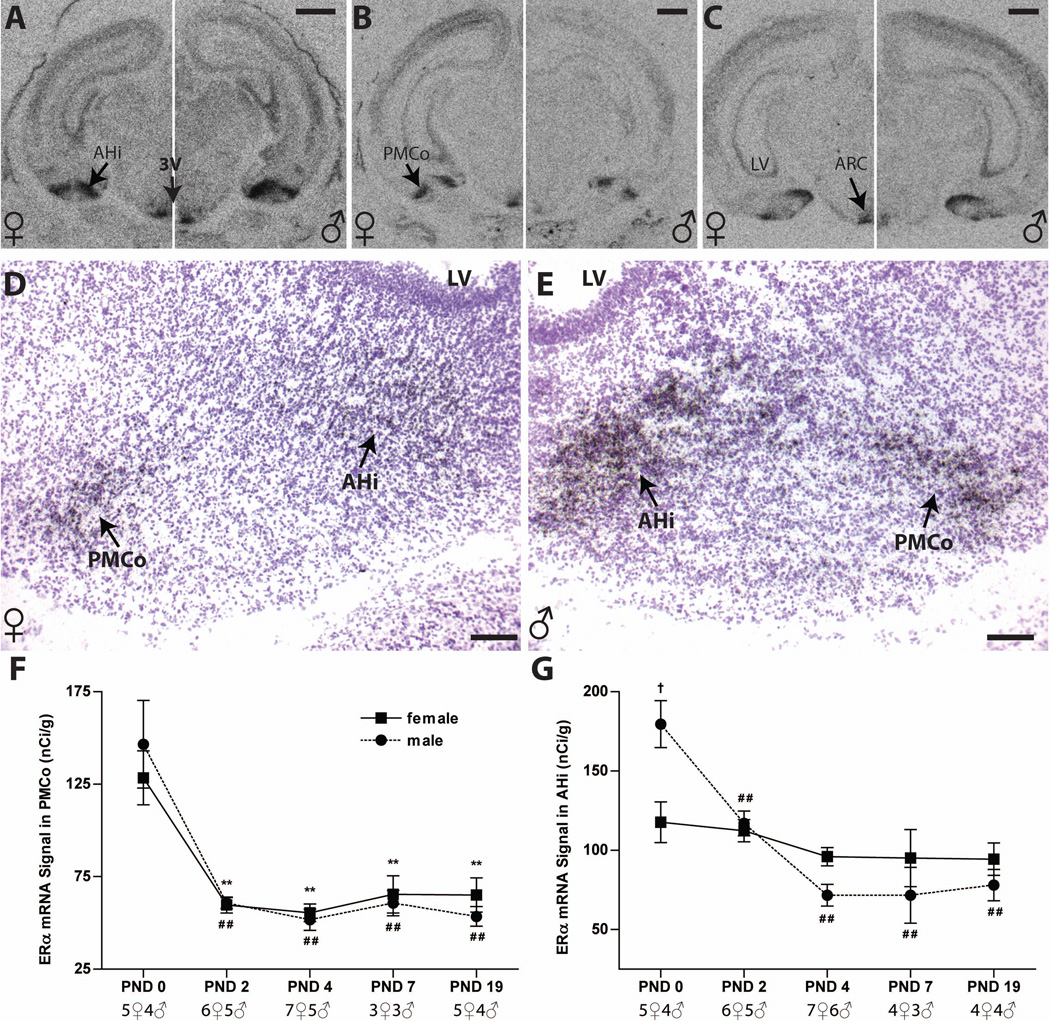
Fig. 4.
Autoradiographs depicting ERα mRNA labeling in the PMCo and AHi of postnatal male and female rats (A–C, females on the left side of each panel, males on the right) on PND 0 (A), PND 4 (B) and PND 7 (C). Representative hemotoxylin-counter stained sections depicting deposition of silver grain labeling for ERα in the PMCo and AHi of PND 0 females (D) and males (E). Expression of ERα mRNA in the PMCo (F) was not dimorphic and declined rapidly in both sexes between PNDs 0 and 2, then remained low through PND 19. (G) ERα expression in the AHi on PND 0 was sexually dimorphic with higher levels in males. Male levels then declined, thus eliminating the sex difference by PND 2. Significant differences in gene expression compared to PND 0 levels are represented by ##P ≤ 0.01 for the males. The significant sex difference is represented by †p ≤ 0.05. The graphs depict mean ± SEM and the sample size is provided for each age. For abbreviations, see list. Scale bar = 1000 µm for A to C, and 100µm for D and E.
Fig. 5.
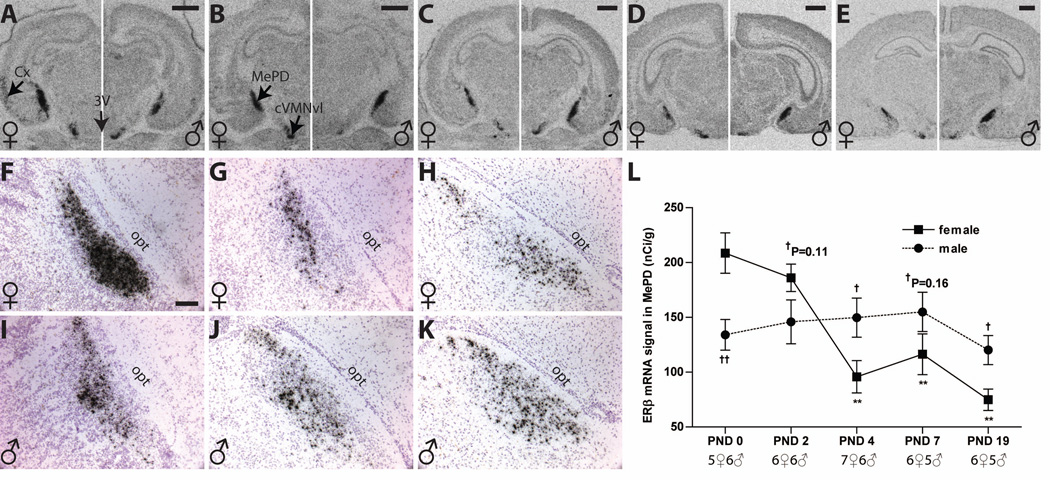
Autoradiographs showing ERβ mRNA expression in the postnatal MePD of both sexes (A–E, females on the left side of each panel, males on the right). The pattern of autoradiographic labeling was confirmed by observing silver grain deposition on the emulsion-dipped, counterstained slides (F-K), and the labeling appeared as tight clusters over the counterstained nuclei. ERβ expression was robust and sexually dimorphic at birth with levels higher in females (left panel of A, and F and L) than males (right panel of A, and I and L). This sex difference was lost on PND 2 with decreasing signal in females (B). By PND 4 the sex difference reemerged but was reversed with males having more intense ERβ signal than females (C and L). This difference persisted through PND 7 (D, G, J and L) and PND 19 (E, H, K and L). Temporal expression patterns were sex specific, with signal generally decreasing with age in females, but remaining levels in males (L). Significant differences in expression compared to PND 0 levels are represented by **P ≤ 0.001 for the females. Significant sex differences are represented by †P ≤ 0.05, and ††P ≤ 0.01.The sample size for each age is indicated, and the data points represent mean ± SEM. For abbreviations, see list. Scale bar = 1000 µm for A to E, and 100 µm for F to K.
Fig. 6.
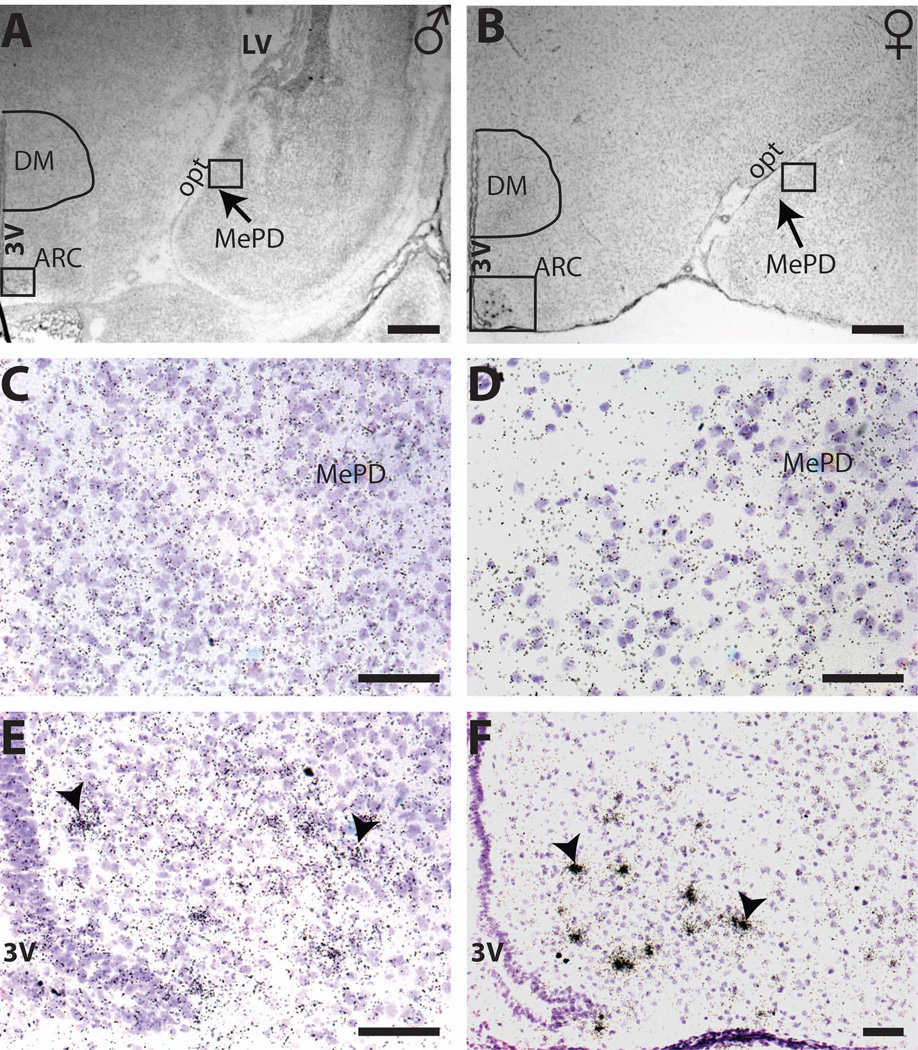
Representative micrographs showing the deposition of silver grain labeling for Kiss1mRNA in the postnatal mediobasal hypothalamus and amygdala of males (A, C, E) and females (B, D, F). No obvious silver grain clusters were found in the MePD at any age examined, including PND 0 (A and C) and PND 19 (B and D) Although some sections suggested the presence of faint clusters (C and D), their density did not rise above background. Distinct clusters of Kiss1 signal were observed in the ARC on all days examined, however, including PND0 (E) and PND 19 (F) within in the same coronal sections, confirming the success of the labeling. 3V = third ventricle; scale bar = 500 µm for A and B, 50 µm for C to F. Arrow head indicates a Kiss1 silver grain cluster.
Developmental expression of ERα mRNA in the postnatal MePD
Intense labeling for ERα was observed on PND 0 in the MePD of both sexes (Figure 3 A). Two way ANOVA identified a significant effect of sex (F (1, 50) = 4.729, P ≤ 0.05) and age (F (4, 50) = 17.17, P ≤ 0.0001), but no significant interaction. Follow-up one way ANOVA revealed a significant effect of age in females (F (4, 27) = 12.44, P ≤ 0.0001) and in males (F (4, 23) = 5.906, P ≤ 0.002). ERα mRNA levels in females (Figure 3A to C, left panels, D, E and G) were significantly decreased, compared to the day of birth, on PND 4 and remained low through 7 (Figure 3B left and G). By PND 19, levels approximated those seen at birth (Figure 3 G). Expression patterns over time were similar in males (Figure 3 A to C right panels and G), and no sexually dimorphic expression was observed at any of the examined time points (Figure 3G and Table 1). The localization of ERα mRNA signal was confirmed by examining silver grain deposition following emulsion dipping (Figure 3 D and E).
Developmental expression of ER mRNA in the postnatal PLCo
The autoradiographs revealed that the level of ERα mRNA within the PLCo transiently decreased in both sexes and showed a U-shape expression pattern similar to ERα mRNA in the MePD (Figure 3 A to C and H). Two-way ANOVA showed a significant effect of age (F (4, 50) = 9.841, P ≤ 0.0001), but not sex (F (1, 50) = 0.60, P = 0.44), nor was there an interaction effect (F (4, 50) = 0.15, P =0.96) (Figure 3H). One-way ANOVA confirmed a significant age effect in females (F (4, 28) = 7.667, P ≤ 0.0003) with significantly lower levels on PNDs 2 (P ≤ 0.05) and 4 (P ≤ 0.01; Figure 3H). In males (F (4, 22) = 3.576, P ≤ 0.022), significantly decreased signal was also observed on PNDs 2 (P ≤ 0.05), and 4 (P ≤ 0.05) (Figure 3H and Table 1). At PND 7 ERα signal began to increase and reached PND 0 levels by PND 19 (Figure 3 A to C and H). Observational analysis of the dipped slides confirmed this pattern of ERα expression in the PLCo (Figure 3F).
Developmental expression of ER mRNA in the postnatal PMCo
In the PMCo, the ERα mRNA signal was robust on the day of birth in both sexes (Figure 4A, D and E), then sharply decreased to a very low level on PND 2 and remained at this low level through PND 19 (Figure 4B, C and F). Two-way ANOVA revealed a significant effect of age (F (4, 37) = 24.84, P ≤ 0.0001), but not sex (P =0.98), and there was no significant interaction (P =0.65). A follow up one-way ANOVA showed significant effects of age in females (F (4, 21) = 12.37, P ≤ 0.0001) and males (F (4, 16) = 13.02, P ≤ 0.0001). Compared to PND 0, ERα expression at all other ages was significantly lower with P ≤ 0.01 in both sexes (Figure 4 F and Table 1). Silver grain deposition on PND 0 positively identified the anatomical borders of the PMCo and silver grain distribution within the area (Figure 4 D and E).
Developmental and sexually dimorphic expression of ERα mRNA in the postnatal AHi
ERα expression in the AHi was most robust on PND 0, and the expression pattern across time was unique compared to the other amygdalar subregions examined (Figure 4A to E, G). Two-way ANOVA revealed a significant effect of age (F (4, 38) = 14.25, P ≤ 0.0001), and an interaction with sex (F (4, 38) = 5.755, P = 0.001), but not a main effect of sex (F (1, 38) = 0.006, P = 0.94). In females (Figure 4 A to C left panels, D and G), expression levels remained relatively unchanged with age (F (4, 21) = 1.154, P = 0.36). ERα mRNA expression in males, however (F (4, 17) = 18.32, P ≤ 0.0001), sharply declined from PND 2 (P ≤ 0.01), reached their lowest levels by PND 4 (P ≤ 0.01), and remained low from PND 7 through 19 (P ≤ 0.01) (Figure 4 A to C right panels, E and G). Sexually dimorphic expression was observed on PND 0, with higher expression in males than in females (P ≤ 0.05), but this sex difference was lost with decreasing expression in males (Figure 4 G). Inspection of the deposited silver grains confirmed the overall expression patterns observed in the AHi on the autoradiographs (Figure 4 D and E).
Developmental and sexually dimorphic expression of ERβ mRNA in the postnatal MePD
ERβ mRNA signal in the MePD was robust, and expressed in both temporal and sex specific patterns (Figure 5). Two-way ANOVA revealed a significant main effect of age (F (4, 49) = 6.929, P ≤ 0.0002) a significant interaction between age and sex (F (4, 49) = 6.411, P ≤ 0.0003), but no main effect of sex (P = 0.74). In females (Figure 5 A to E left panels, F to H and L), ERβ mRNA levels were high on PND 0, then declined with time (F (4, 29) = 14.26, P ≤ 0.0001) reaching their lowest levels by PND 4 (P ≤ 0.01), and remaining at that level through PND 19 (P ≤ 0.01). There was no change in male expression across time (Figure 5 A to E right panels, I to K and L). Sexually dimorphic expression of ERβ in the MePD was found on PND 0 (Figure 5 A and L) with higher levels in females (P ≤ 0.01), but this sex difference disappeared after PND 2 with decreasing signal in females (Figure 5 B and L). A sex difference reemerged on PND 4 (Figure 5 C and L) but reversed levels now higher in males than in females (P ≤ 0.05). These levels remained consistent through PND 19 (P ≤ 0.05; Figure 5 D, E and L). Silver grain clusters were very densely packed, confirming the results obtained from the autoradiograms and revealing that numerous cells within the MePD strongly express ERβ mRNA (Figure 5 F to K).
No expression of Kiss1 mRNA in the postnatal amygdaloid complex was observed
No visible Kiss1 mRNA signal was observed in any subregion of the amygdala in the postnatal rat brain on the autoradiograms (Figure 6 A to B). On the emulsion dipped slides, a few faint cluster-like structures suggestive of Kiss1 neurons were apparent in the MePD (Figure 6 C to D), but these structures were also distributed in other areas, and their density was not significantly higher than background level signals. Kiss1 signal was clearly observed in the neighboring ARC (Figure 6 E and F) in the same coronal sections as the amygdala throughout neonatal development (Cao and Patisaul, 2011) demonstrating that the ISHH procedures effectively and robustly detect Kiss1 mRNA. Although a previous report clearly shows Kiss 1 expression in the adult MePD of both rats and mice (Clarkson et al., 2009; Kim et al., 2011), the absence of signal pre-weaning suggests that appreciable expression may not occur until peri-puberty.
DISCUSSION
The present study provides the most detailed and comprehensive profile to date of ER expression in the amygdaloid complex across neonatal development, and reveals that Kiss1 is not expressed in this region during this period. Collectively, we observed that ERα was more broadly expressed than ERβ, which is consistent with what has been reported previously in adult rats (Osterlund et al., 1998; Perez et al., 2003; Shughrue et al., 1997; Shughrue and Merchenthaler, 2001; Simerly et al., 1990). As expected, expression of the two ER subtypes overlaps in some regions, such as the Ce, BA and Me (Kuhnemann et al., 1994; Osterlund et al., 1998; Perez et al., 2003; Shughrue et al., 1997; Shughrue and Merchenthaler, 2001; Simerly et al., 1990; Yokosuka et al., 1997). Both ERs are robustly expressed on the day of birth and then diverge in temporal and sexually dimorphic patterns; observations which are consistent with and expand upon what is known about the developing rat amygdala (Kuhnemann et al., 1994; Perez et al., 2003; Yokosuka et al., 1997). The absence of Kiss1 expression in any of the examined subregions, including the MePD where it is normally expressed in a sexually dimorphic pattern in adulthood (Kim et al., 2011), suggests that its expression may require a mature hypothalamic-pituitary-gonadal (HPG) axis.
In rodents, the cytoarchitecture of several amygdaloid subregions is age dependent and sexually dimorphic. Our results provide insight into the relative roles the two nuclear ER subtypes play in the organization of these differences by showing that the temporal and/or sexually distinct patterns of ERα and ERβ are region specific. For example, the robust difference in ERα expression patterns between the PLCo and PMCo across postnatal life emphasizes the functional differences between these two subregions in adult life (Canteras et al., 1992; Simerly, 2002; Swanson and Petrovich, 1998). Sexually dimorphic expression of ERα was observed in the AHi on the day of birth, an observation which provides mechanistic support for the organization of behavioral sex differences attributed to this region, such as the need for greater salt intake by females (Canteras et al., 1992; Nitabach et al., 1989; Schulkin et al., 1989).
Although the focus of this project was ER expression, it is important to recognize that other processes and steroid hormone receptors play an organizational role in subregional sexual differentiation. In the MePD, for example, dimorphisms include total volume, neuronal soma size, dendrite length, and astrocyte number and complexity, with greater values in males in both adult and young rodents (Cooke et al., 2007; Cooke et al., 1999; Hines et al., 1992; Johnson et al., 2008; Morris et al., 2008b). These differences are well recognized to be under the control of circulating sex hormones (Cooke, 2006; Cooke et al., 2007; Cooke et al., 1999) and organized by sex hormones during the perinatal window of development (Morris et al., 2008a). Both ER and AR are expressed in the rat MePD (McAbee and DonCarlos, 1998; Simerly et al., 1990; Yokosuka et al., 1997). Prior studies where estradiol or the non-aromatizable androgen, dihydrotestosterone (DHT) were administered (Cooke et al., 2003), or using testicular feminization mutation (tfm) male rats with dysfunctional AR (Morris et al., 2005), collectively indicate that both ER and AR contribute to the masculinization of the rodent MePD. Importantly, AR appears to be required for full masculinization of the MePD (Johansen et al., 2004) indicating that both ERs and AR participate in the masculinization of the MePD.
In the present study, ERβ, but not ERα, expression was found to be sex specific in the newborn MePD; a result which was opposite to what was hypothesized. This observation could indicate that the androgen metabolite 5alpha-androstane-3beta, 17beta-diol (3β-diol), now recognized to be a potent endogenous ligand for ERβ, may have a masculinizing role in the neonatal MePD (Handa et al., 2009; Kuiper et al., 1998). Collectively, these results suggest that, in neonates, sex differences in ERα expression, ERβ expression, or the ratio of ERβ to ERα expression, is fundamental for estrogen dependent sexually dimorphic organization of amygdaloid subnuclei. Sex differences are also conferred and/or enhanced via other mechanisms including the direct action of androgens through AR and sex specific synaptogenesis, a process that is dynamic in the first two weeks of life in the amygdala (Morys et al., 1998).
Sex differences in ER expression in the brain have historically been considered a negative feedback response to localized neonatal estrogen, which is aromatized from testosterone (Amateau et al., 2004; Cao and Patisaul, 2011; DonCarlos et al., 1995; Ikeda et al., 2003; Kuhnemann et al., 1995; Lauber et al., 1997). Here we found that, in the male AHi, ERα expression is robust at birth then decreases from PND 2 (Figure 4), an effect which is likely a consequence of decreasing levels of circulating male sex hormones and, consequently, locally produced estrogen in the AHi (Amateau et al., 2004; Lauber et al., 1997). Female levels remained relatively flat across the neonatal period, a pattern that is consistent with the low levels of sex hormones present during this developmental period. These data support the hypothesis that negative feedback contributes to sex differences in ER expression levels.
In other subnuclei, however, the mechanisms contributing to sex specific ER expression appear to be more complex. For example, aromatase immunolabeled neurons are present in the developing Me as early as E16, and peak during the perinatal critical window, with higher levels in males than in females in late gestation (Tsuruo et al., 1994). This supports the hypothesis that males may generate higher local estrogen levels than females in the Me and other amygdaloid subnuclei. Consequently, it would be expected that these higher local estrogen levels would reduce ER expression levels via negative feedback, resulting in lower levels in males compared to females. Instead, however, we found no sex differences in ERα expression in the MePD at any age examined, and higher ERβ expression levels at birth in females. ERβ levels then decreased with age, an effect opposite of what was expected based on the negative feedback hypothesis. Similarly, no sex differences in the PMCo or the PLCo were identified at any age examined. Thus, a mechanism other than simple negative feedback regulation by local estrogens must be driving the region and sex specific expression of ERs and thus contributing to the sexually dimorphic organization of brain.
Epigenetic modification of the ER promoter is emerging as a novel mechanism underlying the differential regulation of ER expression and activity by the hormonal environment (reviewed in (McCarthy et al., 2009)). For example, in the Me of PND 1 rats, levels of DNA cytosine-5-methyltransferase (DNMT) 3a mRNA and protein are significantly higher in females, and levels of this enzyme are reduced to male-typical amounts by estradiol or DHT administration (Kolodkin and Auger, 2011). Similarly, the binding of histone deacetylase (HDAC) −4 to rat ERα promoters is sexually dimorphic in the perinatal rat brain (Matsuda et al., 2011). Inhibition of histone deacetylase (HDAC) −2 and −4 activity in newborn males reduces their sexual behavior in adult life, demonstrating that the degree of histone acetylation associated with the ERα promoter may impact the sex specific expression of ER and, by extention, gender appropriate sociosexual behavior. Another potential, but related, mechanism underlying differential ER expression is regional and/or sex differences in the levels and/or types of cofactors required for ER transcription. The nuclear receptor corepressor (NCoR), has sexually dimorphic expression in the neonatal MePD (Jessen et al., 2010). It can interact with methyl-binding proteins (Cukier et al., 2008; Kokura et al., 2001; Yoon et al., 2003) and associate with HDAC complexes (Jessen et al., 2010) to influence DNA transcription (McKenna et al., 1999; Tsai and O'Malley, 1994), or ER activity. In addition, cell specific recruitment of cofactors (Beekman et al., 1993; Horwitz et al., 1996), such as steroid receptor coactivator-1 (SRC-1) (Meijer et al., 2000; Mitev et al., 2003), may induce sex and regional differences in the activation of the ubiquitin-proteasome pathway (Wijayaratne and McDonnell, 2001), which affects ERα stability and indirectly regulates ER expression.
Finally, previous work has shown that Kiss1 is predominantly expressed in both the mouse and the rat MePD, and that this expression is sexually dimorphic with higher levels in males than in females in diestrus (Kim et al., 2011) suggesting that its expression may be organized in the neonatal critical period. Castration of animals decreases MePD Kiss1 expression in both sexes and sex hormone treatment amplifies it, demonstrating that, in adults, this action is under the regulation of circulating sex steroids via ER-dependent pathways (Kim et al., 2011). The MePD cooperates with the BNST and conducts the olfactory signal to hypothalamic nuclei, such as the AVPV and VMN (Cooke and Simerly, 2005; Simerly, 2002), thus Kiss1 in the MePD may play a role in modulating both reproductive and non-reproductive behavior in rodents. We did not detect Kiss1 signal in the neonate or in the early prepubertal MePD in rats. This suggests that the Kiss1 expression in the adult MePD may be organized during the pubertal window and possibly depends on a mature HPG axis, possibilities that will have to be addressed in future studies.
CONCLUSION
The detailed profile of neonatal ER mRNA levels provided here will help elucidate the relative roles each of the ERs play in the sex-specific, estrogen-dependent organization of the amygdaloid complex, and the sex specific social and sexual behaviors elicited in response to the pheromonal, hormonal, social and other environmental cues mediated by this brain region. Although sex differences in endogenous local estrogen levels likely contribute to the sex differences in ER expression observed in some regions during the early neonatal period through negative feedback, not all of the observations presented here are consistent with this mechanism. Other unknown mechanisms are likely involved in the regulation of the two ER subtypes and sexually dimorphic organization of the amygdala including epigenetic modifications and cofactor activity (Kolodkin and Auger, 2011; Matsuda et al., 2011; McCarthy et al., 2009; Menger et al., 2010; Nugent et al., 2011). Exploration of the specific molecular and cellular mechanisms by which ER activity organizes critical sex differences in the amygdaloid complex will help determine how sex differences in complex sociosexual behaviors arise and are modulated by the environment in early life.
Acknowledgements
This work was supported by NIEHS grant RO1 ES016001 to HBP. We thank Emily Sluzas and Meghan Radford for their critical reading of this manuscript and constructive edits.
Abbreviations
- 3V
third ventricle
- AHi
amygdalohippocampal area
- ANOVA
analysis of variance
- AR
androgen receptor
- ARC
arcuate hypothalamic nucleus
- BA
basal amygdaloid nucleus
- BNST
bed nuclei of the stria terminalis
- BPA
bisphenol A
- Ce
central amygdaloid nucleus
- Co
cortical amygdaloid nucleus
- Cx
cerebral cortex
- DM
dorsomedial hypothalamic nucleus
- DNMT
DNA cytosine-5-methyltransferases
- ER
estrogen receptor
- ERα
ER alpha
- ERβ
ER beta
- GnRH
gonadotropin-releasing hormone
- HDAC
histone deacetylase
- HPG
hypothalamic-pituitary-gonadal
- -ir
immunoreactivity
- ISHH
in situ hybridization histochemistry
- Kiss1
kisspeptin gene
- KP
kisspeptin
- LE
Long Evans
- Me
medial amygdaloid nucleus
- MeAD
Me anterodorsal
- MeAV
Me anteroventral
- MePD
Me posterodorsal
- MePV
Me posteroventral
- NCoR
nuclear receptor corepressor
- opt
optic track
- PA
posterior amygdala nucleus
- PLCo
posterolateral cortical amygdaloid nucleus
- PMCo
posteromedial cortical amygdaloid nucleus
- PND
postnatal day
- ROI
region of interest
- SRC-1
steroid receptor coactivator-1
- st
stria terminalis
- STIA
intra-amygdaloid division of the BNST
- VMNvl
ventrolateral division of the ventromedial hypothalamic nucleus
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest.
Author contributions: H.B.P. and J.C. designed the experiments, J.C. performed the research and analyzed the data, and J.C. wrote the manuscript with the assistance of H.B.P.
REFERENCES
- Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145(6):2906–2917. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- Beekman JM, Allan GF, Tsai SY, Tsai MJ, O'Malley BW. Transcriptional activation by the estrogen receptor requires a conformational change in the ligand binding domain. Molecular endocrinology (Baltimore, Md. 1993;7(10):1266–1274. doi: 10.1210/mend.7.10.8264659. [DOI] [PubMed] [Google Scholar]
- Brown NM, Setchell KD. Animal models impacted by phytoestrogens in commercial chow: implications for pathways influenced by hormones. Lab Invest. 2001;81(5):735–747. doi: 10.1038/labinvest.3780282. [DOI] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator D, Wu J, McGaugh JL. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(15):8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Connections of the posterior nucleus of the amygdala. The Journal of comparative neurology. 1992;324(2):143–179. doi: 10.1002/cne.903240203. [DOI] [PubMed] [Google Scholar]
- Cao J, Mickens JA, McCaffrey KA, Leyrer SM, Patisaul HB. Neonatal Bisphenol A exposure alters sexually dimorphic gene expression in the postnatal rat hypothalamus. Neurotoxicology. 2012;33(1):23–36. doi: 10.1016/j.neuro.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Patisaul HB. Sexually dimorphic expression of hypothalamic estrogen receptors alpha and beta and kiss1 in neonatal male and female rats. The Journal of comparative neurology Epub Ahead. 2011 doi: 10.1002/cne.22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Patisaul HB, Petersen SL. Aryl hydrocarbon receptor activation in lactotropes and gonadotropes interferes with estradiol-dependent and -independent preprolactin, glycoprotein alpha and luteinizing hormone beta gene expression. Molecular and cellular endocrinology. 2011;333(2):151–159. doi: 10.1016/j.mce.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, d'Anglemont de Tassigny X, Colledge WH, Caraty A, Herbison AE. Distribution of kisspeptin neurones in the adult female mouse brain. Journal of neuroendocrinology. 2009;21(8):673–682. doi: 10.1111/j.1365-2826.2009.01892.x. [DOI] [PubMed] [Google Scholar]
- Cooke BM. Steroid-dependent plasticity in the medial amygdala. Neuroscience. 2006;138(3):997–1005. doi: 10.1016/j.neuroscience.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Simerly RB. Ontogeny of bidirectional connections between the medial nucleus of the amygdala and the principal bed nucleus of the stria terminalis in the rat. The Journal of comparative neurology. 2005;489(1):42–58. doi: 10.1002/cne.20612. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Stokas MR, Woolley CS. Morphological sex differences and laterality in the prepubertal medial amygdala. The Journal of comparative neurology. 2007;501(6):904–915. doi: 10.1002/cne.21281. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Tabibnia G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(13):7538–7540. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen LM, Peters HJ, Veening JG. Distribution of Fos immunoreactivity following mating versus anogenital investigation in the male rat brain. Neuroscience. 1997;77(4):1151–1161. doi: 10.1016/s0306-4522(96)00542-8. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Wood RI. Testosterone stimulation of the medial preoptic area and medial amygdala in the control of male hamster sexual behavior: redundancy without amplification. Behavioural brain research. 1999;98(1):143–153. doi: 10.1016/s0166-4328(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Cukier HN, Perez AM, Collins AL, Zhou Z, Zoghbi HY, Botas J. Genetic modifiers of MeCP2 function in Drosophila. PLoS genetics. 2008;4(9):e1000179. doi: 10.1371/journal.pgen.1000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ, Villalba C. Brain sexual dimorphism and sex differences in parental and other social behaviors. Annals of the New York Academy of Sciences. 1997;807:273–286. doi: 10.1111/j.1749-6632.1997.tb51926.x. [DOI] [PubMed] [Google Scholar]
- Degen GH, Janning P, Diel P, Bolt HM. Estrogenic isoflavones in rodent diets. Toxicol Lett. 2002;128(1–3):145–157. doi: 10.1016/s0378-4274(02)00009-7. [DOI] [PubMed] [Google Scholar]
- DonCarlos LL, McAbee M, Ramer-Quinn DS, Stancik DM. Estrogen receptor mRNA levels in the preoptic area of neonatal rats are responsive to hormone manipulation. Brain research. 1995;84(2):253–260. doi: 10.1016/0165-3806(94)00179-4. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Vaccarino F, Luebke C. Amygdaloid inhibition of maternal behavior in the nulliparous female rat. Physiology & behavior. 1980;25(5):731–743. doi: 10.1016/0031-9384(80)90377-7. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Weiser MJ, Zuloaga DG. A role for the androgen metabolite, 5alpha-androstane-3beta,17beta-diol, in modulating oestrogen receptor beta-mediated regulation of hormonal stress reactivity. J Neuroendocrinol. 2009;21(4):351–358. doi: 10.1111/j.1365-2826.2009.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris VS, Sachs BD. Copulatory behavior in male rats following amygdaloid lesions. Brain Res. 1975;86(3):514–518. doi: 10.1016/0006-8993(75)90906-3. [DOI] [PubMed] [Google Scholar]
- Hines M, Allen LS, Gorski RA. Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Brain Res. 1992;579(2):321–326. doi: 10.1016/0006-8993(92)90068-k. [DOI] [PubMed] [Google Scholar]
- Horwitz KB, Jackson TA, Bain DL, Richer JK, Takimoto GS, Tung L. Nuclear receptor coactivators and corepressors. Molecular endocrinology (Baltimore, Md. 1996;10(10):1167–1177. doi: 10.1210/mend.10.10.9121485. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Nagai A, Ikeda MA, Hayashi S. Sexually dimorphic and estrogen-dependent expression of estrogen receptor beta in the ventromedial hypothalamus during rat postnatal development. Endocrinology. 2003;144(11):5098–5104. doi: 10.1210/en.2003-0267. [DOI] [PubMed] [Google Scholar]
- Jessen HM, Kolodkin MH, Bychowski ME, Auger CJ, Auger AP. The nuclear receptor corepressor has organizational effects within the developing amygdala on juvenile social play and anxiety-like behavior. Endocrinology. 2010;151(3):1212–1220. doi: 10.1210/en.2009-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JA, Jordan CL, Breedlove SM. Steroid hormone masculinization of neural structure in rats: a tale of two nuclei. Physiol Behav. 2004;83(2):271–277. doi: 10.1016/j.physbeh.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Johnson RT, Breedlove SM, Jordan CL. Sex differences and laterality in astrocyte number and complexity in the adult rat medial amygdala. The Journal of comparative neurology. 2008;511(5):599–609. doi: 10.1002/cne.21859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. I. Efferents of the "vomeronasal amygdale". The Journal of comparative neurology. 1981a;197(1):81–98. doi: 10.1002/cne.901970107. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. II. Efferents of the "olfactory amygdala". The Journal of comparative neurology. 1981b;197(1):99–111. doi: 10.1002/cne.901970108. [DOI] [PubMed] [Google Scholar]
- Kim J, Semaan SJ, Clifton DK, Steiner RA, Dhamija S, Kauffman AS. Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology. 2011;152(5):2020–2030. doi: 10.1210/en.2010-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokura K, Kaul SC, Wadhwa R, Nomura T, Khan MM, Shinagawa T, Yasukawa T, Colmenares C, Ishii S. The Ski protein family is required for MeCP2-mediated transcriptional repression. The Journal of biological chemistry. 2001;276(36):34115–34121. doi: 10.1074/jbc.M105747200. [DOI] [PubMed] [Google Scholar]
- Kolodkin MH, Auger AP. Sex difference in the expression of DNA methyltransferase 3a in the rat amygdala during development. Journal of neuroendocrinology. 2011;23(7):577–583. doi: 10.1111/j.1365-2826.2011.02147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y. Lesions of the medial amygdala produce severe impairment of copulatory behavior in sexually inexperienced male rats. Physiology & behavior. 1992;51(5):939–943. doi: 10.1016/0031-9384(92)90074-c. [DOI] [PubMed] [Google Scholar]
- Kuhnemann S, Brown TJ, Hochberg RB, MacLusky NJ. Sex differences in the development of estrogen receptors in the rat brain. Hormones and behavior. 1994;28(4):483–491. doi: 10.1006/hbeh.1994.1046. [DOI] [PubMed] [Google Scholar]
- Kuhnemann S, Brown TJ, Hochberg RB, MacLusky NJ. Sexual differentiation of estrogen receptor concentrations in the rat brain: effects of neonatal testosterone exposure. Brain Res. 1995;691(1–2):229–234. doi: 10.1016/0006-8993(95)00640-c. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139(10):4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Lauber ME, Sarasin A, Lichtensteiger W. Sex differences and androgen-dependent regulation of aromatase (CYP19) mRNA expression in the developing and adult rat brain. The Journal of steroid biochemistry and molecular biology. 1997;61(3–6):359–364. [PubMed] [Google Scholar]
- Lehman MN, Winans SS, Powers JB. Medial nucleus of the amygdala mediates chemosensory control of male hamster sexual behavior. Science (New York, NY. 1980;210(4469):557–560. doi: 10.1126/science.7423209. [DOI] [PubMed] [Google Scholar]
- Manzo J, Cruz MR, Hernandez ME, Pacheco P, Sachs BD. Regulation of noncontact erection in rats by gonadal steroids. Hormones and behavior. 1999;35(3):264–270. doi: 10.1006/hbeh.1999.1519. [DOI] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. Anatomical connections between the anterior and posterodorsal sub-regions of the medial amygdala: integration of odor and hormone signals. Neuroscience. 2010;170(2):610–622. doi: 10.1016/j.neuroscience.2010.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masco DH, Carrer HF. Sexual receptivity in female rats after lesion or stimulation in different amygdaloid nuclei. Physiology & behavior. 1980;24(6):1073–1080. doi: 10.1016/0031-9384(80)90050-5. [DOI] [PubMed] [Google Scholar]
- Matsuda KI, Mori H, Nugent BM, Pfaff DW, McCarthy MM, Kawata M. Histone deacetylation during brain development is essential for permanent masculinization of sexual behavior. Endocrinology. 2011;152(7):2760–2767. doi: 10.1210/en.2011-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAbee MD, DonCarlos LL. Ontogeny of region-specific sex differences in androgen receptor messenger ribonucleic acid expression in the rat forebrain. Endocrinology. 1998;139(4):1738–1745. doi: 10.1210/endo.139.4.5940. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, Murray EK, Nugent BM, Schwarz JM, Wilson ME. The epigenetics of sex differences in the brain. J Neurosci. 2009;29(41):12815–12823. doi: 10.1523/JNEUROSCI.3331-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna NJ, Lanz RB, O'Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocrine reviews. 1999;20(3):321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Dodge AM, Beatty WW. Sex-dependent effects of amygdaloid lesions on the social play of prepubertal rats. Physiology & behavior. 1981;26(3):467–472. doi: 10.1016/0031-9384(81)90175-x. [DOI] [PubMed] [Google Scholar]
- Meijer OC, Steenbergen PJ, De Kloet ER. Differential expression and regional distribution of steroid receptor coactivators SRC-1 and SRC-2 in brain and pituitary. Endocrinology. 2000;141(6):2192–2199. doi: 10.1210/endo.141.6.7489. [DOI] [PubMed] [Google Scholar]
- Menger Y, Bettscheider M, Murgatroyd C, Spengler D. Sex differences in brain epigenetics. Epigenomics. 2010;2(6):807–821. doi: 10.2217/epi.10.60. [DOI] [PubMed] [Google Scholar]
- Mitev YA, Wolf SS, Almeida OF, Patchev VK. Developmental expression profiles and distinct regional estrogen responsiveness suggest a novel role for the steroid receptor coactivator SRC-1 as discriminative amplifier of estrogen signaling in the rat brain. Faseb J. 2003;17(3):518–519. doi: 10.1096/fj.02-0513fje. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual dimorphism in neuronal number of the posterodorsal medial amygdala is independent of circulating androgens and regional volume in adult rats. The Journal of comparative neurology. 2008a;506(5):851–859. doi: 10.1002/cne.21536. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Dugger BN, Breedlove SM. Partial demasculinization of several brain regions in adult male (XY) rats with a dysfunctional androgen receptor gene. The Journal of comparative neurology. 2005;487(2):217–226. doi: 10.1002/cne.20558. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, King ZA, Northcutt KV, Breedlove SM. Sexual dimorphism and steroid responsiveness of the posterodorsal medial amygdala in adult mice. Brain Res. 2008b;1190:115–121. doi: 10.1016/j.brainres.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morys J, Berdel B, Kowianski P, Dziewiatkowski J. The pattern of synaptophysin changes during the maturation of the amygdaloid body and hippocampal hilus in the rat. Folia neuropathologica / Association of Polish Neuropathologists and Medical Research Centre, Polish Academy of Sciences. 1998;36(1):15–23. [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Annals of the New York Academy of Sciences. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Schulkin J, Epstein AN. The medial amygdala is part of a mineralocorticoid-sensitive circuit controlling NaCl intake in the rat. Behavioural brain research. 1989;35(2):127–134. doi: 10.1016/s0166-4328(89)80113-5. [DOI] [PubMed] [Google Scholar]
- Nugent BM, Schwarz JM, McCarthy MM. Hormonally mediated epigenetic changes to steroid receptors in the developing brain: implications for sexual differentiation. Hormones and behavior. 2011;59(3):338–344. doi: 10.1016/j.yhbeh.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund M, Kuiper GG, Gustafsson JA, Hurd YL. Differential distribution and regulation of estrogen receptor-alpha and -beta mRNA within the female rat brain. Brain Res Mol Brain Res. 1998;54(1):175–180. doi: 10.1016/s0169-328x(97)00351-3. [DOI] [PubMed] [Google Scholar]
- Patisaul HB. Phytoestrogen action in the adult and developing brain. Journal of neuroendocrinology. 2005;17(1):57–64. doi: 10.1111/j.1365-2826.2005.01268.x. [DOI] [PubMed] [Google Scholar]
- Perez SE, Chen EY, Mufson EJ. Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Brain research. 2003;145(1):117–139. doi: 10.1016/s0165-3806(03)00223-2. [DOI] [PubMed] [Google Scholar]
- Schulkin J, Marini J, Epstein AN. A role for the medial region of the amygdala in mineralocorticoid-induced salt hunger. Behavioral neuroscience. 1989;103(1):179–185. doi: 10.1037/0735-7044.103.1.178. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. The Journal of comparative neurology. 1997;388(4):507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. The Journal of comparative neurology. 2001;436(1):64–81. [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annual review of neuroscience. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. The Journal of comparative neurology. 1990;294(1):76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends in neurosciences. 1998;21(8):323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annual review of biochemistry. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Tsuruo Y, Ishimura K, Fujita H, Osawa Y. Immunocytochemical localization of aromatase-containing neurons in the rat brain during pre- and postnatal development. Cell and tissue research. 1994;278(1):29–39. doi: 10.1007/BF00305775. [DOI] [PubMed] [Google Scholar]
- Vinader-Caerols C, Collado P, Segovia S, Guillamon A. Sex differences in the posteromedial cortical nucleus of the amygdala in the rat. Neuroreport. 1998;9(11):2653–2656. doi: 10.1097/00001756-199808030-00042. [DOI] [PubMed] [Google Scholar]
- Vinader-Caerols C, Collado P, Segovia S, Guillamon A. Estradiol masculinizes the posteromedial cortical nucleus of the amygdala in the rat. Brain research bulletin. 2000;53(3):269–273. doi: 10.1016/s0361-9230(00)00332-4. [DOI] [PubMed] [Google Scholar]
- Wang Z, Hulihan TJ, Insel TR. Sexual and social experience is associated with different patterns of behavior and neural activation in male prairie voles. Brain Res. 1997;767(2):321–332. doi: 10.1016/s0006-8993(97)00617-3. [DOI] [PubMed] [Google Scholar]
- Watson C, Paxinos G. The rat brain in stereotaxic coordinates. London: Academic Press; 2007. [DOI] [PubMed] [Google Scholar]
- Watson C, Paxinos G, Puelles L. The Mouse Nervous System. Academic Press; 2012. p. 814. [Google Scholar]
- Wijayaratne AL, McDonnell DP. The human estrogen receptor-alpha is a ubiquitinated protein whose stability is affected differentially by agonists, antagonists, and selective estrogen receptor modulators. The Journal of biological chemistry. 2001;276(38):35684–35692. doi: 10.1074/jbc.M101097200. [DOI] [PubMed] [Google Scholar]
- Yokosuka M, Okamura H, Hayashi S. Postnatal development and sex difference in neurons containing estrogen receptor-alpha immunoreactivity in the preoptic brain, the diencephalon, and the amygdala in the rat. The Journal of comparative neurology. 1997;389(1):81–93. doi: 10.1002/(sici)1096-9861(19971208)389:1<81::aid-cne6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Yoon HG, Chan DW, Reynolds AB, Qin J, Wong J. N-CoR mediates DNA methylation-dependent repression through a methyl CpG binding protein Kaiso. Molecular cell. 2003;12(3):723–734. doi: 10.1016/j.molcel.2003.08.008. [DOI] [PubMed] [Google Scholar]