Abstract
Terbutaline, a β2-adrenoceptor agonist, is used off-label for long-term management of preterm labor; such use is associated with increased risk of neurodevelopmental disorders, including autism spectrum disorders. We explored the mechanisms underlying terbutaline’s effects on development of peripheral sympathetic projections in developing rats. Terbutaline administration on postnatal days 2–5 led to immediate and persistent deficiencies in cardiac norepinephrine levels, with greater effects in males than in females. The liver showed a lesser effect; we reasoned that the tissue differences could represent participation of retrograde trophic signaling from the postsynaptic site to the developing neuronal projection, since hepatic β2-adrenoceptors decline in the perinatal period. Accordingly, when we gave terbutaline earlier, on gestational days 17–20, we saw the same deficiencies in hepatic norepinephrine that had been seen in the heart with the later administration paradigm. Administration of isoproterenol, which stimulates both β1- and β2-subtypes, also had trophic effects that differed in direction and critical period from those elicited by terbutaline; methoxamine, which stimulates α1-adrenoceptors, was without effect. Thus, terbutaline, operating through trophic interactions with β2-adrenoceptors, impairs development of noradrenergic projections in a manner similar to that previously reported for its effects on the same neurotransmitter systems in the immature cerebellum. Our results point to the likelihood of autonomic dysfunction in individuals exposed prenatally to terbutaline; in light of the connection between terbutaline and autism, these results could also contribute to autonomic dysregulation seen in children with this disorder.
Keywords: Autism, β-Adrenergic agonists, Norepinephrine, Preterm delivery, Sympathetic nervous system, Terbutaline
INTRODUCTION
It is abundantly clear that “classic” neurotransmitters, such as norepinephrine, serotonin and acetylcholine, act as morphogens to direct the assembly of the mammalian brain (Bruel-Jungerman et al., 2011; Lauder, 1985; Whitaker-Azmitia, 1991), a function that likely evolved from their roles in invertebrate embryogenesis (Buznikov et al., 1996, 2007). Although less well studied, similar processes operate for development of peripheral innervation; in the sympathetic nervous system, for example, the development of cholinergic neurotransmission at the ganglion dictates the differentiation, neurotransmitter subtype and outgrowth of postganglionic neurons (Azevedo and Osswald, 1986; Black, 1980; Black et al., 1976). These types of neurotrophic relationships underlie the vulnerability of the immature nervous system to “misprogramming” resulting from exposure to neuroactive chemicals, leading ultimately to neurodevelopmental disorders (Grandjean and Landrigan, 2006; Lauder, 1985; Whitaker-Azmitia, 1991).
Considerable attention has been paid to the role of illicit drugs, and pesticides or other environmental neurotoxicants, but the potential impact of therapeutic agents has been less well explored. Recent attention has turned to the effects of β-adrenergic agonists and their use in the management of preterm labor, asthma and fetal heart block (Cheslack-Postava et al., 2007; Connors, 2008; Connors et al., 2005; Hadders-Algra et al., 1986; Kilburn et al., 2009; Pitzer et al., 2001; Robinson et al., 2001; Witter et al., 2009). Clinical and epidemiological studies show an increased risk of learning disabilities and autism spectrum disorders (ASD) resulting from use of these agents in pregnancy (Connors, 2008; Connors et al., 2005; Croen et al., 2011; Hadders-Algra et al., 1986; Kilburn et al., 2009; Pitzer et al., 2001; Robinson et al., 2001; Witter et al., 2009), and animal models indicate that such exposures lead to structural, neurochemical and behavioral damage (Feenstra, 1992; Garofolo et al., 2003; Rhodes et al., 2004; Slotkin et al., 2003; Witter et al., 2009; Zerrate et al., 2007). In part, this occurs because developing cells do not display β-adrenoceptor desensitization in response to excess stimulation, and instead actually show enhanced responsiveness (Slotkin et al., 2003; Slotkin and Seidler, 2006). Accordingly, receptor overstimulation leads to a positive feedback that further augments the cellular response to continued or subsequent stimulation, ultimately culminating in altered cell differentiation or even cell death (Connors, 2008; Fu et al., 2004; Yan et al., 2000). Such effects are particularly important because terbutaline, a β2-selective agonist, is still used “off label” for maintenance control of preterm labor (Goldenberg, 2002), notwithstanding its ineffectiveness for that purpose (Thornton, 2005), and despite the fact that the U.S. Food and Drug Administration specifically warns against its use (U.S. Food and Drug Administration, 2011). Prolonged terbutaline administration in the second to third trimester is associated with significantly increased incidence of ASD (Connors, 2008; Connors et al., 2005; Kilburn et al., 2009; Witter et al., 2009; Zerrate et al., 2007), a risk that is likely to be even greater in individuals with β-adrenoceptor polymorphisms that impair desensitization (Cheslack-Postava et al., 2007; Connors et al., 2005).
In our previous work, terbutaline administered to newborn rats on postnatal (PN) days 2–5, neurodevelopmentally equivalent to late second trimester human development, evoked structural, functional and behavioral anomalies (Garofolo et al., 2003; Rhodes et al., 2004; Slotkin et al., 1989, 1990; Zerrate et al., 2007). Some of the most notable changes were in the cerebellum and further, the structural alterations and pattern of neuroinflammation resembled those seen in postmortem samples of children and adults with ASD (Connors, 2008; Rhodes et al., 2004; Zerrate et al., 2007). At the neurotransmitter level, terbutaline appears to disrupt noradrenergic circuits in particular, reducing cerebellar synaptogenesis for this transmitter (Slotkin et al., 1989), while at the same enhancing the expression of both α1- and α2-adrenoceptors (Kreider et al., 2004; Slotkin et al., 1990); again, this likely reflects overstimulation that preempts the normal neurotrophic role of norepinephrine (Sanders et al., 2011). The question remains as to whether the effects of terbutaline reflect a specific role involving cerebellar noradrenergic projections (i.e. regional specification), or whether there is more widespread vulnerability of noradrenergic neurons that depends instead on a specific stage of neuronal differentiation. In the newborn rat, the window for the peak of cerebellar development corresponds also to the period in which peripheral noradrenergic projections develop (Rodier, 1988; Slotkin, 1986). This presents us with the opportunity to distinguish between the two possible mechanisms. If it is specifically the cerebellum that is targeted, then peripheral noradrenergic projections will not be affected similarly, but if it is a critical period of neurodifferentiation that is responsible for the defects, then the effects will be similar for sympathetic neuronal development. In the current study, we evaluated the effects of terbutaline (β2-agonist) on development of cardiac and hepatic noradrenergic innervation in contrast to the effects of isoproterenol (β1- and β2-agonist) and methoxamine (α1-agonist) during different developmental periods. We found evidence that β2-adrenoceptor stimulation during a critical developmental period leads to deficiencies in peripheral sympathetic noradrenergic innervation, providing a link to observations of autonomic dysfunction reported in ASD (Anderson et al., 2012; Fan et al., 2009; Witter et al., 2009).
METHODS
Animal treatments
All procedures utilized tissues that were archived from earlier studies and maintained frozen at −45° C, so that no additional animals were actually used for this study. Details of animal husbandry, institutional approvals, maternal and litter characteristics, and growth curves, have all been presented in earlier work from the original animal cohorts (Garofolo et al., 2003; Kreider et al., 2004; Slotkin et al., 1996; Thai et al., 1996). Timed-pregnant Sprague-Dawley rats were housed individually and given free access to food and water. For studies of gestational terbutaline treatment, dams received daily subcutaneous injections of 10 mg/kg terbutaline sulfate (Sigma Chemical Co., St. Louis, MO) by s.c. injection on gestational days (GD) 17–20, whereas controls received equivalent volumes (1 ml/kg) of isotonic saline vehicle. Postnatal treatments were conducted similarly with daily s.c. injections to the pups on PN2–5, PN21–24 (i.e. immediately after weaning) or for four consecutive days in adulthood (males only, body weight 250–300 g); treatment groups comprised 10 mg/kg terbutaline sulfate, 1.25 mg/kg l-isoproterenol HCl (Sigma) or 10 mg/kg methoxamine (Sigma), each with corresponding saline control groups. The PN21–24 and adult group were included to show that the effects seen with prenatal or early postnatal exposures were developmental, that is, they occur only with treatment in a critical window. Tissue samples were obtained 24 hr after the last injection, and additionally on PN60 for the cohort given terbutaline given on PN2–5. All groups comprised no more than one male and one female from a given litter.
The doses used in this study were selected so as to produce prolonged stimulation of the corresponding adrenergic receptors in heart and liver (β2 for terbutaline, β1 and β2 for isoproterenol, α1 for methoxamine), as evidenced by effects on tissue growth, receptor concentrations and receptor-mediated signal transduction (Garofolo et al., 2003; Kreider et al., 2004; Slotkin et al., 1996; Thai et al., 1996). It would be inappropriate to match the dose in newborn rats to terbutaline given to pregnant women because terbutaline is metabolized much more quickly in rats (Tegner et al., 1984); the drug is given to humans by continuous infusion or repeated oral dosing so as to maintain round-the-clock receptor stimulation (Lam et al., 2001), and we selected doses that, given once daily, achieve the same biologic effect in rats. For terbutaline, the treatment produces prolonged adenylyl cyclase activation, β2-receptor downregulation (Auman et al., 2001a, b), metabolic activation (Kudlacz et al., 1989; Morris and Slotkin, 1985), and brain neuroinflammation and structural changes resembling findings in ASD (Rhodes et al., 2004; Zerrate et al., 2007). The isoproterenol treatment elicits sustained, maximal elevation of heart rate (Hou et al., 1989b; Hou and Slotkin, 1989; Seidler and Slotkin, 1979) and metabolic activation in both heart (Bareis and Slotkin, 1978; Bartolome et al., 1977) and liver (Bartolome et al., 1985; Slotkin et al., 1986). The methoxamine regimen produces stimulation sufficient to downregulate α1-receptors (Thai et al., 1996).
Assays and data analysis
Tissues were thawed on ice and deproteinized by homogenization in 0.1 N perchloric acid containing 3,4-dihydroxybenzylamine (Sigma) as an internal standard. Homogenates were sedimented at 26,000 × g for 10 minutes, the supernatant solutions were decanted, and norepinephrine was then trace-enriched by alumina adsorption, separated by reverse-phase high performance liquid chromatography and quantitated by electrochemical detection (Seidler and Slotkin, 1981); values were corrected for recovery of the internal standard. Preliminary studies verified that measured norepinephrine levels were stable even after prolonged tissue storage at −45° C.
Data are presented as means and standard errors, with treatment differences established by ANOVA utilizing the factors of treatment, tissue, sex and age. Post-hoc tests for individual treatment effects were established with Fisher’s Protected Least Significant Difference Test. Significance was assumed at p < 0.05. Because each treatment paradigm involved a separate cohort of animals, treatment comparisons were made only to the matched control group from the same cohort.
RESULTS
As found in our earlier studies with these treatments, terbutaline given on GD17–20 had no effect on the number of fetuses and produced little or no change fetal body weight, heart weight of liver weight (Auman et al., 2001a; Slotkin et al., 2001), nor were any changes seen for the postnatal terbutaline regimens (Auman et al., 2001b; Slotkin et al., 2001). At all ages tested, neither isoproterenol nor methoxamine had any significant effects on body weights (Thai et al., 1996); liver weights were within 5% of normal, and were unchanged relative to body weight (Thai et al., 1996). However, isoproterenol produced significant cardiac hypertrophy when given on PN21–24 or in adulthood, but not earlier (Giannuzzi et al., 1995).
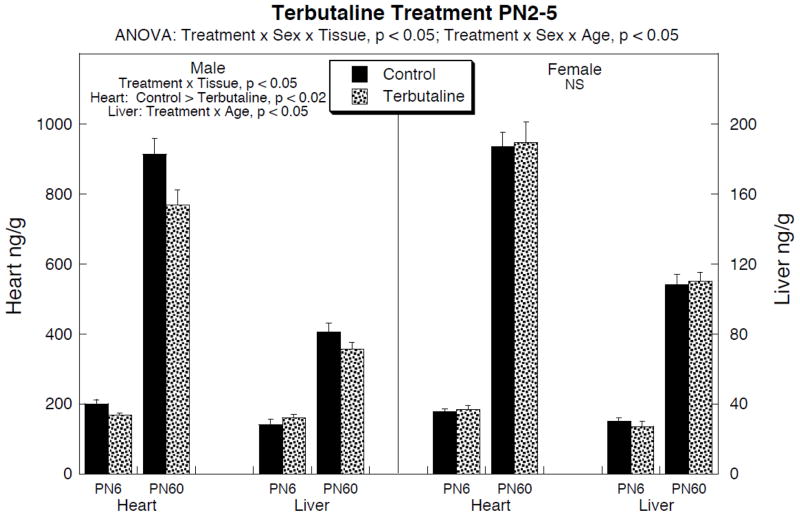
Terbutaline administration on PN2–5 elicited significant deficits in peripheral norepinephrine levels that were sex- and tissue-selective (Fig. 1). In males, cardiac norepinephrine showed significant decrements on PN6, persisting into young adulthood (PN60). In the liver, there was no initial deficit but values were subnormal on PN60; although this effect was individually nonsignificant, it was also indistinguishable from the significant deficit seen in the heart at the same age, and a comparison of treatment effects across the two tissues showed a significant main effect of terbutaline (p < 0.03) without a treatment × tissue interaction.
Figure 1.
Effects of terbutaline given on PN2–5 on norepinephrine levels in heart and liver (note different scales). Data represent means and standard errors obtained from 8–10 animals in each group for each age and sex. ANOVA appears at the top of the panel; lower-order tests for males and females were carried out because of the treatment interaction with sex, and these appear within the panel. For males, values were separated by tissue because of the treatment × tissue interaction. For the heart, there was no interaction of treatment × age, so only main treatment effects are reported, whereas tests at each age were carried out for the liver (significant treatment × age interaction) but were not significant; however, the decrement in both the heart and liver on PN60 were significant taken together (p < 0.03 for the main treatment effect, no interaction of treatment × tissue). No lower order tests were carried out for females because of the absence of significance for the overall ANOVA. NS = not significant.
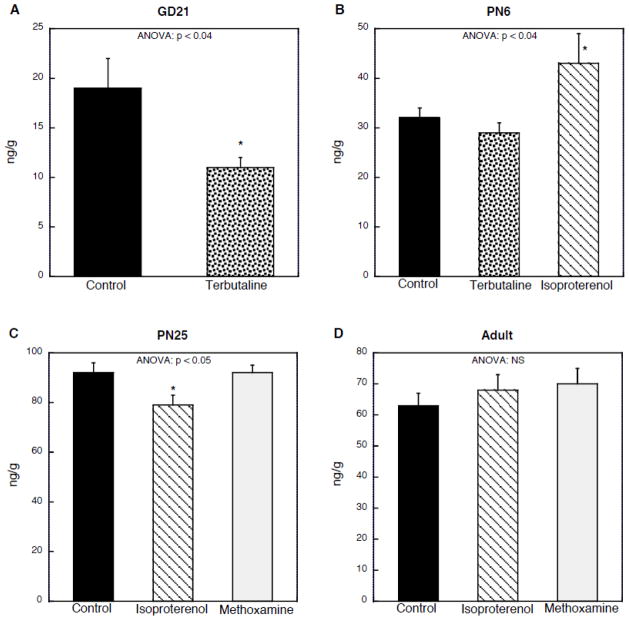
In our earlier work with terbutaline, we found that hepatic β-adrenoceptor downregulation was much larger than for the heart with either gestational or postnatal treatment (Auman et al., 2001a, b), reflecting the predominance of the β2-subtype in the liver as compared to the β1-subtype in the heart; also, unlike the heart, hepatic β2-receptors decline sharply after birth (McMillian et al., 1983). Accordingly, we explored whether the lack of immediate effect (PN6) on liver norepinephrine levels reflected an earlier critical period. Terbutaline given prenatally on GD17–20 elicited a large decrement in liver norepinephrine concentrations measured on GD21 (Fig. 2A), whereas the same treatment given postnatally on PN2–5 did not (Fig. 2B). Since terbutaline is a β2-selective agonist, we then investigated whether the relative insensitivity in the postnatal period was shared by the response to isoproterenol, which stimulates both the β1- and β2-subtypes; isoproterenol given on PN2–5 elicited a significant increase in hepatic norepinephrine (Fig. 2B). This effect also showed a critical period, since similar treatment on PN21–24 failed to show an increase and actually produced a significant decrease (Fig. 2C); when given in adulthood, there was no effect of isoproterenol (Fig. 2D). We also examined the consequences of α1-adrenergic receptor stimulation with methoxamine over the same developmental period in which the response to isoproterenol changed and disappeared. Methoxamine given on PN21–24 (Fig. 2C) or in adulthood (Fig. 2D) did not cause statistically significant changes in norepinephrine levels.
Figure 2.
Effects of adrenergic agonists on liver norepinephrine levels. The indicated agents were given for four days preceding the age at which evaluations were carried out, shown at the top of each panel: (A) terbutaline given on GD17–20, evaluated on GD21 (n=14); (B) terbutaline or isoproterenol given on PN2–5, evaluated on PN6 (control n=23, terbutaline n=17, isoproterenol n=6; (C) isoproterenol or methoxamine given on PN21–24, evaluated on PN25 (control n=11, isoproterenol n=12, methoxamine n=17); (D) isoproterenol or methoxamine given for four days in adulthood, evaluated on the fifth day (control n=12, isoproterenol n=17, methoxamine n=8). Values are shown without separation by sex: (A) sex was not determined in the fetuses; (B) and (C) there was no interaction of treatment × sex; (D) subjects were males only. ANOVA appears at the top of each panel and asterisks denote individual groups that differ from the corresponding control. Note different scales for each panel. NS = not significant.
DISCUSSION
Our results show that terbutaline impairs peripheral sympathetic neuronal development when exposure occurs during the same critical period in which it targets development of cerebellar noradrenergic projections, and further, that these effects are not shared by agonists that operate through adrenergic receptor subtypes other than the β2-adrenoceptor. The findings support a specific trophic role for the β2-adrenoceptor in neuronal development involving not only the central nervous system but also peripheral sympathetic projections.
Administration of terbutaline on PN2–5 elicited immediate and long-lasting deficits in cardiac norepinephrine levels, with the effect restricted to males. This parallels our earlier work showing greater adverse cerebellar effects of the same treatment in males (Rhodes et al., 2004). Notably, terbutaline given during this period also produces persistent β-adrenoceptor downregulation (Slotkin et al., 2005), which would further augment the functional consequences of deficient presynaptic norepinephrine levels. Interestingly, though, the effects were less notable in the liver, despite the fact that there are no substantial neurochemical disparities between these noradrenergic projections and those that innervate the heart; indeed, both pathways develop with a virtually identical time course (Slotkin et al., 1995). What is different, though, is the postsynaptic receptor population in the two tissues. The neonatal heart possesses high concentrations of β1-receptors and α1-receptors which are maintained into adulthood (Slotkin et al., 1995). In the liver, however, the predominant β-receptor subtype is the β2-adrenoceptor, which is extremely high in the fetus and newborn whereas α1-receptors are low (McMillian et al., 1983; Slotkin et al., 1995). Hepatic β2-adrenoceptors then decline and are replaced by the α1-subtype, which then assumes the same metabolic function (gluconeogenesis) previously controlled by β2-receptors (Exton, 1979; Katz et al., 1985; McMillian et al., 1983). If postsynaptic receptor populations are responsible for the difference in terbutaline’s effects on cardiac vs. hepatic noradrenergic projections, then it would imply that there is a retrograde signal from the end-organ that contributes to the development of these neurons. There is prior evidence for retrograde control of sympathetic neuronal development based on studies of end-organ removal (Dibner et al., 1977), but to our knowledge no one has looked at whether specific neurotransmitter receptors could trigger retrograde trophic signaling. Accordingly we focused on treatments targeting different receptor populations and different critical periods for their effects on hepatic norepinephrine.
Since hepatic β2-adrenoceptors decline sharply in the perinatal period (McMillian et al., 1983), we reasoned that terbutaline treatment earlier than PN2–5 might elicit a response more akin to that seen in the heart. This prediction was verified: when we administered terbutaline on GD17–20, we saw deficits in hepatic norepinephrine that paralleled the effect that had been seen in the heart with the later administration paradigm. We then explored whether other adrenergic receptor subtypes exerted similar trophic effects on hepatic noradrenergic projections. Administration of isoproterenol, which targets both β1- and β2-adrenoceptors, produced upregulation when given on PN2–5, the opposite effect from that obtained with terbutaline in the heart with the same regimen, or in the liver with the earlier regimen. It is thus evident that the β1-adrenoceptor also exerts trophic control over development of hepatic noradrenergic projections but in a direction opposite to that of the β2-receptor. There is ample precedent for these divergent trophic actions, since the two subtypes can have opposite effects on signaling pathways mediating apoptosis (Chesley et al., 2000; Shizukuda and Buttrick, 2002; Zaugg et al., 2000). The β1-dependent trophic component likewise displayed a critical period, since isoproterenol administration on PN21–25 decreased norepinephrine instead of increasing it; administration in adulthood had no impact, reinforcing the concept that these are indeed developmental effects. Finally, we examined the effects of methoxamine (α1-adrenoceptor agonist), administered during the period in which hepatic α1-adrenoceptors spike (McMillian et al., 1983) or in adulthood. There was no effect, reinforcing the unique trophic roles of β-adrenoceptors as distinct from the α1-subtype. It should be noted that the methoxamine regimen was sufficient to cause persistent overstimulation of the α1-receptors, as evidenced by downregulation of this receptor subtype (Thai et al., 1996), so the lack of effect on presynaptic norepinephrine did not reflect administration of a subeffective dose.
In the present study, we pursued the duration of the synaptic defects elicited by terbutaline in only one model (treatment on PN2–5) and found that the effect lasted into young adulthood. However, persistence is not required to produce lasting defects in sympathetic function. The perinatal stage is a critical period in which presynaptic stimulation of postsynaptic targets is required for proper development of end-organ function, so that early deficiencies result in permanently subnormal responses (Hou et al., 1989a, b; Hou and Slotkin, 1989; Navarro et al., 1991; Slotkin et al., 2003). Accordingly, later-emerging changes in receptor expression and tissue function are apparent after terbutaline exposure on GD17–20 or PN2–5 but not with exposure on PN11–14 (Slotkin et al., 2005).
In summary, our results point to a specific trophic role for β-adrenoceptors modulating the development of peripheral noradrenergic projections, likely through retrograde signaling via postsynaptic receptors located in the target tissues. This role is not shared by the α1-subtype. The results are of clinical relevance for two specific reasons. First, terbutaline is widely used in the long-term management of preterm labor, so that tens of thousands of newborns are exposed to this treatment each year in the U.S. alone (Goldenberg, 2002). Although there has been considerable work on increased risk of neurodevelopmental disorders resulting from such exposure (Cheslack-Postava et al., 2007; Connors, 2008; Connors et al., 2005; Hadders-Algra et al., 1986; Kilburn et al., 2009; Pitzer et al., 2001; Robinson et al., 2001; Witter et al., 2009), our results point to the likelihood of autonomic consequences as well, including cardiovascular and metabolic dysfunction. The second point is the potential relationship to ASD. Prolonged prenatal terbutaline exposure increases the risk of ASD, especially in association with β2-adrenoceptor polymorphisms that enhance responsiveness (Cheslack-Postava et al., 2007; Connors, 2008; Connors et al., 2005; Witter et al., 2009), and some of our morphological findings for cerebellar development parallel those in ASD (Rhodes et al., 2004; Vargas et al., 2004; Zerrate et al., 2007). ASD is also associated with autonomic dysfunction (Anderson et al., 2012; Fan et al., 2009; Witter et al., 2009) and there is a subset of ASD patients who show specific defects in sympathetic activation (Hirstein et al., 2001). The present work shows that terbutaline exposure during the relevant developmental period for its use in preterm labor, impairs development of peripheral sympathetic projections, with the same sex selectivity found for the incidence of ASD (male > female). Our earlier work detailed adverse effects of terbutaline on expression of postsynaptic adrenergic and cholinergic receptors in these same target tissues (Auman et al., 2001a, b; Slotkin et al., 2005), properties that would amplify presynaptic defects; and indeed, we identified parallel changes in both cardiac and hepatic function in response to sympathetic and parasympathetic stimuli (Auman et al., 2001a; Hou and Slotkin, 1989; Navarro et al., 1991; Slotkin et al., 2005). The present results are therefore relevant for outcomes of inappropriate terbutaline use in preterm labor, specifically pointing to the need to examine whether exposed children show autonomic dysfunction, as well as providing a contributory component to autonomic manifestations in ASD (Anderson et al., 2012; Fan et al., 2009; Hirstein et al., 2001; Witter et al., 2009).
Highlights.
Prenatal terbutaline exposure is associated with increased incidence of autism
Developing rats given terbutaline showed deficient peripheral noradrenergic development
Vulnerability depended on the concentration of β2-adrenoceptors in the target organs
Effects were not shared by stimulants acting at β1- or α1-adrenoceptors
Impaired noradrenergic development could contribute to autonomic dysfunction in autism or in offspring of women given terbutaline for preterm labor
Acknowledgments
Research support was provided by NIH ES10356.
Abbreviations
- ANOVA
analysis of variance
- ASD
Autism Spectrum Disorders
- GD
gestational day
- PN
postnatal day
Footnotes
Disclaimers: TAS has provided expert witness testimony in the past three years at the behest of the following law firms: The Calwell Practice (Charleston WV), Finnegan Henderson Farabow Garrett & Dunner (Washington DC), Carter Law (Peoria IL), Gutglass Erickson Bonville & Larson (Madison WI), The Killino Firm (Philadelphia PA), Alexander Hawes (San Jose, CA), Pardieck Law (Seymour, IN), Tummel & Casso (Edinburg, TX) and the Shanahan Law Group (Raleigh NC).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson CJ, Colombo J, Unruh KE. Pupil and salivary indicators of autonomic dysfunction in autism spectrum disorder. Dev, Psychobiol. 2012 doi: 10.1002/dev.21051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auman JT, Seidler FJ, Slotkin TA. Regulation of fetal cardiac and hepatic β-adrenoceptors and adenylyl cyclase signaling: terbutaline effects. Am J Physiol. 2001a;281:R1079–R89. doi: 10.1152/ajpregu.2001.281.4.R1079. [DOI] [PubMed] [Google Scholar]
- Auman JT, Seidler FJ, Tate CA, Slotkin TA. β-Adrenoceptor-mediated cell signaling in the neonatal heart and liver: responses to terbutaline. Am J Physiol. 2001b;281:R1895–R901. doi: 10.1152/ajpregu.2001.281.6.R1895. [DOI] [PubMed] [Google Scholar]
- Azevedo I, Osswald W. Trophic role of the sympathetic innervation. J Pharmacol. 1986;17:30–43. [PubMed] [Google Scholar]
- Bareis DL, Slotkin TA. Responses of heart ornithine decarboxylase and adrenal catecholamines to methadone and sympathetic stimulants in developing and adult rats. J Pharmacol Exp Ther. 1978;205:164–74. [PubMed] [Google Scholar]
- Bartolome J, Grignolo A, Bartolome M, Trepanier P, Lerea L, Weigel S, et al. Postnatal methyl mercury exposure: effects on ontogeny of renal and hepatic ornithine decarboxylase responses to trophic stimuli. Toxicol Appl Pharmacol. 1985;80:147–54. doi: 10.1016/0041-008x(85)90109-7. [DOI] [PubMed] [Google Scholar]
- Bartolome J, Lau C, Slotkin TA. Ornithine decarboxylase in developing rat heart and brain: role of sympathetic development for responses to autonomic stimulants and the effects of reserpine on maturation. J Pharmacol Exp Ther. 1977;202:510–8. [PubMed] [Google Scholar]
- Black IB. Developmental regulation of neurotransmitter phenotype. Curr Topics Dev Biol. 1980;15:27–40. doi: 10.1016/s0070-2153(08)60115-5. [DOI] [PubMed] [Google Scholar]
- Black IB, Bloom EM, Hamill RW. Central regulation of sympathetic neuron development. Proc Natl Acad Sci USA. 1976;73:3575–8. doi: 10.1073/pnas.73.10.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Lucassen PJ, Francis F. Cholinergic influences on cortical development and adult neurogenesis. Behav Brain Res. 2011;221:379–88. doi: 10.1016/j.bbr.2011.01.021. [DOI] [PubMed] [Google Scholar]
- Buznikov GA, Nikitina LA, Bezuglov VV, Francisco ME, Obispo-Peak IN, Peterson RA, et al. New perspectives on roles for neurotransmitters in early (“ pre-nervous ”) embryogenesis. In: Ruždijić S, Rakić L, editors. Neurobiological Studies — from Genes to Behaviour. Kerala, India: Signpost/Transworld Research Network; 2007. pp. 183–96. [Google Scholar]
- Buznikov GA, Shmukler YB, Lauder JM. From oocyte to neuron: do neurotransmitters function in the same way throughout development? Cell Mol Neurobiol. 1996;16:532–59. doi: 10.1007/BF02152056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheslack-Postava K, Fallin MD, Avramopoulos D, Connors SL, Zimmerman AW, Eberhart CG, et al. β2-Adrenergic receptor gene variants and risk for autism in the AGRE cohort. Mol Psychiat. 2007;12:283–91. doi: 10.1038/sj.mp.4001940. [DOI] [PubMed] [Google Scholar]
- Chesley A, Lundberg MS, Asai T, Xiao RP, Ohtani S, Lakatta EG, et al. The β2-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through Gi-dependent coupling to phosphatidylinositol 3′-kinase. Circ Res. 2000;87:1172–9. doi: 10.1161/01.res.87.12.1172. [DOI] [PubMed] [Google Scholar]
- Connors SL. Prenatal β2-adrenergic receptor signaling and autism: dysmaturation and retained fetal function. In: Zimmerman AW, editor. Autism: Current Theories and Evidence. Totowa NJ: Humana Press; 2008. pp. 147–82. [Google Scholar]
- Connors SL, Crowell DE, Eberhart CG, Copeland J, Newschaffer CJ, Spence SJ, et al. β2-Adrenergic receptor activation and genetic polymorphisms in autism: data from dizygotic twins. J Child Neurol. 2005;20:876–84. doi: 10.1177/08830738050200110401. [DOI] [PubMed] [Google Scholar]
- Croen LA, Connors SL, Matevia M, Qian Y, Newschaffer C, Zimmerman AW. Prenatal exposure to β2-adrenergic receptor agonists and risk of autism spectrum disorders. J Neurodev Disord. 2011;3:307–15. doi: 10.1007/s11689-011-9093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner MD, Mytilineou C, Black IB. Target organ regulation of sympathetic neuron development. Brain Res. 1977;123:301–10. doi: 10.1016/0006-8993(77)90481-4. [DOI] [PubMed] [Google Scholar]
- Exton JH. Mechanisms involved in α-adrenergic effects of catecholamines on liver metabolism. J Cyclic Nucleotide Res. 1979;5:277–87. [PubMed] [Google Scholar]
- Fan X, Miles JH, Takahashi N, Yao G. Abnormal transient pupillary light reflex in individuals with autism spectrum disorders. J Autism Dev Disord. 2009;39:1499–508. doi: 10.1007/s10803-009-0767-7. [DOI] [PubMed] [Google Scholar]
- Feenstra MGP. Functional neuroteratology of drugs acting on adrenergic receptors. Neurotoxicology. 1992;13:55–63. [PubMed] [Google Scholar]
- Fu YC, Chi CS, Yin SC, Hwang B, Chiu YT, Hsu SL. Norepinephrine induces apoptosis in neonatal rat endothelial cells via down-regulation of Bcl-2 and activation of beta-adrenergic and caspase-2 pathways. Cardiovasc Res. 2004;61:143–51. doi: 10.1016/j.cardiores.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Garofolo MC, Seidler FJ, Cousins MM, Tate CA, Qiao D, Slotkin TA. Developmental toxicity of terbutaline: critical periods for sex-selective effects on macromolecules and DNA synthesis in rat brain, heart, and liver. Brain Res Bull. 2003;59:319–29. doi: 10.1016/s0361-9230(02)00925-5. [DOI] [PubMed] [Google Scholar]
- Giannuzzi CE, Seidler FJ, Slotkin TA. β-Adrenoceptor control of cardiac adenylyl cyclase during development: agonist pretreatment in the neonate uniquely causes heterologous sensitization, not desensitization. Brain Res. 1995;694:271–8. doi: 10.1016/0006-8993(95)00781-k. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL. The management of preterm labor. Obstetrics and Gynecology. 2002;100:1020–37. doi: 10.1016/s0029-7844(02)02212-3. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–78. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Hadders-Algra M, Touwen BC, Huisjes HJ. Long-term follow-up of children prenatally exposed to ritodrine. Br J Obstet Gynæcol. 1986;93:156–61. doi: 10.1111/j.1471-0528.1986.tb07880.x. [DOI] [PubMed] [Google Scholar]
- Hirstein W, Iversen P, Ramachandran VS. Autonomic responses of autistic children to people and objects. Proc R Soc Lond B. 2001;268:1883–8. doi: 10.1098/rspb.2001.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q-C, Baker FE, Seidler FJ, Bartolome M, Bartolome J, Slotkin TA. Role of sympathetic neurons in development of β-adrenergic control of ornithine decarboxylase activity in peripheral tissues: effects of neonatal 6-hydroxydopamine treatment. J Dev Physiol. 1989a;11:139–46. [PubMed] [Google Scholar]
- Hou Q-C, Seidler FJ, Slotkin TA. Development of the linkage of β-adrenergic receptors to cardiac hypertrophy and heart rate control: neonatal sympathectomy with 6-hydroxydopamine. J Dev Physiol. 1989b;11:305–11. [PubMed] [Google Scholar]
- Hou Q-C, Slotkin TA. Effects of prenatal dexamethasone or terbutaline exposure on development of neural and intrinsic control of heart rate. Pediatr Res. 1989;26:554–7. doi: 10.1203/00006450-198912000-00005. [DOI] [PubMed] [Google Scholar]
- Katz MS, Boland SR, Schmidt SJ. Developmental changes of β-adrenergic receptor-linked adenylate cyclase of rat liver. Am J Physiol. 1985;248:E712–E8. doi: 10.1152/ajpendo.1985.248.6.E712. [DOI] [PubMed] [Google Scholar]
- Kilburn KH, Thrasher JD, Immers NB. Do terbutaline- and mold-associated impairments of the brain and lung relate to autism? Toxicol Industr Health. 2009;25:703–10. doi: 10.1177/0748233709348391. [DOI] [PubMed] [Google Scholar]
- Kreider ML, Seidler FJ, Slotkin TA. β-Adrenoceptor modulation of transiently-overexpressed α2-adrenoceptors in brain and peripheral tissues: mechanisms underlying the developmental toxicity of terbutaline. Brain Res Bull. 2004;62:305–14. doi: 10.1016/j.brainresbull.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Kudlacz EM, Navarro HA, Eylers JP, Lappi SE, Dobbins SS, Slotkin TA. Effects of prenatal terbutaline exposure on cellular development in lung and liver of neonatal rat: ornithine decarboxylase activity and macromolecules. Pediatr Res. 1989;25:617–22. doi: 10.1203/00006450-198906000-00013. [DOI] [PubMed] [Google Scholar]
- Lam F, Bergauer NK, Jacques D, Coleman SK, Stanziano GJ. Clinical and cost-effectiveness of continuous subcutaneous terbutaline versus oral tocolytics for treatment of recurrent preterm labor in twin gestations. J Perinatol. 2001;21:444–50. doi: 10.1038/sj.jp.7210553. [DOI] [PubMed] [Google Scholar]
- Lauder JM. Roles for neurotransmitters in development: possible interaction with drugs during the fetal and neonatal periods. In: Marois M, editor. Prevention of Physical and Mental Congenital Defects. New York: Alan R. Liss; 1985. pp. 375–80. [PubMed] [Google Scholar]
- McMillian MK, Schanberg SM, Kuhn CM. Ontogeny of rat hepatic adrenoceptors. J Pharmacol Exp Ther. 1983;227:181–6. [PubMed] [Google Scholar]
- Morris G, Slotkin TA. Beta-2 adrenergic control of ornithine decarboxylase activity in brain regions of the developing rat. J Pharmacol Exp Ther. 1985;233:141–7. [PubMed] [Google Scholar]
- Navarro HA, Kudlacz EM, Kavlock RJ, Slotkin TA. Prenatal terbutaline treatment: tissue-selective dissociation of perinatal changes in β-adrenergic receptor binding from regulation of adenylate cyclase activity. Life Sci. 1991;48:269–74. doi: 10.1016/0024-3205(91)90354-e. [DOI] [PubMed] [Google Scholar]
- Pitzer M, Schmidt MH, Esser G, Laucht M. Child development after maternal tocolysis with β-sympathomimetic drugs. Child Psychiat Hum Dev. 2001;31:165–82. doi: 10.1023/a:1026419720410. [DOI] [PubMed] [Google Scholar]
- Rhodes MC, Seidler FJ, Abdel-Rahman A, Tate CA, Nyska A, Rincavage HL, et al. Terbutaline is a developmental neurotoxicant: effects on neuroproteins and morphology in cerebellum, hippocampus and somatosensory cortex. J Pharmacol Exp Ther. 2004;308:529–37. doi: 10.1124/jpet.103.060095. [DOI] [PubMed] [Google Scholar]
- Robinson BV, Ettedgui JA, Sherman FS. Use of terbutaline in the treatment of complete heart block in the fetus. Cardiol Young. 2001;11:683–6. doi: 10.1017/s1047951101001123. [DOI] [PubMed] [Google Scholar]
- Rodier PM. Structural-functional relationships in experimentally induced brain damage. Prog Brain Res. 1988;73:335–48. doi: 10.1016/S0079-6123(08)60514-2. [DOI] [PubMed] [Google Scholar]
- Sanders JD, Happe HK, Bylund DB, Murrin LC. Changes in postnatal norepinephrine alter α2 adrenergic receptor development. Neuroscience. 2011;192:761–72. doi: 10.1016/j.neuroscience.2011.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler FJ, Slotkin TA. Presynaptic and postsynaptic contributions to ontogeny of sympathetic control of heart rate in the preweanling rat. Br J Pharmacol. 1979;65:431–4. doi: 10.1111/j.1476-5381.1979.tb07847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler FJ, Slotkin TA. Development of central control of norepinephrine turnover and release in the rat heart: responses to tyramine, 2-deoxyglucose and hydralazine. Neuroscience. 1981;6:2081–6. doi: 10.1016/0306-4522(81)90047-6. [DOI] [PubMed] [Google Scholar]
- Shizukuda Y, Buttrick PM. Subtype specific roles of β-adrenergic receptors in apoptosis of adult rat ventricular myocytes. J Mol Cell Cardiol. 2002;34:823–31. doi: 10.1006/jmcc.2002.2020. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Endocrine control of synaptic development in the sympathetic nervous system: the cardiac-sympathetic axis. In: Gootman PM, editor. Developmental Neurobiology of the Autonomic Nervous System. Clifton, NJ: Humana Press; 1986. pp. 97–133. [Google Scholar]
- Slotkin TA, Auman JT, Seidler FJ. Ontogenesis of β-adrenoceptor signaling: implications for perinatal physiology and for fetal effects of tocolytic drugs. J Pharmacol Exp Ther. 2003;306:1–7. doi: 10.1124/jpet.102.048421. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Baker FE, Dobbins SS, Eylers JP, Lappi SE, Seidler FJ. Prenatal terbutaline exposure in the rat: selective effects on development of noradrenergic projections to cerebellum. Brain Res Bull. 1989;23:263–5. doi: 10.1016/0361-9230(89)90206-2. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Kavlock RJ, Cowdery T, Orband L, Bartolome M, Gray JA, et al. Functional consequences of prenatal methylmercury exposure: effects on renal and hepatic responses to trophic stimuli and on renal excretory mechanisms. Toxicol Lett. 1986;34:231–45. doi: 10.1016/0378-4274(86)90215-8. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Kudlacz EM, Lappi SE, Tayyeb MI, Seidler FJ. Fetal terbutaline exposure causes selective postnatal increases in cerebellar α-adrenergic receptor binding. Life Sci. 1990;47:2051–7. doi: 10.1016/0024-3205(90)90440-3. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Lorber BA, McCook EC, Barnes GA, Seidler FJ. Neural input and the development of adrenergic intracellular signaling: neonatal denervation evokes neither receptor upregulation nor persistent supersensitivity of adenylate cyclase. Dev Brain Res. 1995;88:17–29. doi: 10.1016/0165-3806(95)00067-n. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Saleh JL, Zhang J, Seidler FJ. Ontogeny of β-adrenoceptor/adenylyl cyclase desensitization mechanisms: the role of neonatal innervation. Brain Res. 1996;742:317–28. doi: 10.1016/s0006-8993(96)00978-x. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Anomalous regulation of β-adrenoceptor signaling in brain regions of the newborn rat. Brain Res. 2006;1077:54–8. doi: 10.1016/j.brainres.2006.01.047. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Tate CA, Cousins MM, Seidler FJ. β-Adrenoceptor signaling in the developing brain: sensitization or desensitization in response to terbutaline. Dev Brain Res. 2001;131:113–25. doi: 10.1016/s0165-3806(01)00282-6. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Tate CA, Cousins MM, Seidler FJ. Imbalances emerge in cardiac autonomic cell signaling after neonatal exposure to terbutaline or chlorpyrifos, alone or in combination. Dev Brain Res. 2005;260:219–30. doi: 10.1016/j.devbrainres.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Tegner K, Nilsson HT, Persson CGA, Persson K, Ryrfeldt Å. Elimination pathways of terbutaline. Eur J Resp Dis. 1984;65 (Suppl 134):93–100. [PubMed] [Google Scholar]
- Thai L, Galluzzo JM, McCook EC, Seidler FJ, Slotkin TA. Atypical regulation of hepatic adenylyl cyclase and adrenergic receptors during a critical developmental period: agonists evoke supersensitivity accompanied by failure of receptor downregulation. Pediatr Res. 1996;39:697–707. doi: 10.1203/00006450-199604000-00023. [DOI] [PubMed] [Google Scholar]
- Thornton JG. Maintenance tocolysis. Br J Obstet Gynæacol. 2005;1:118–21. doi: 10.1111/j.1471-0528.2005.00599.x. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. [accessed 18 May 2012];FDA Drug Safety Communication: New warnings against use of terbutaline to treat preterm labor. 2011 http://www.fda.gov/drugs/drugsafety/ucm243539.htm.
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2004;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. Role of serotonin and other neurotransmitter receptors in brain development: basis for developmental pharmacology. Pharmacol Rev. 1991;43:553–61. [PubMed] [Google Scholar]
- Witter F, Zimmerman A, Reichmann J, Connors S. In utero β2 adrenergic agonist exposure and adverse neurophysiologic and behavioral outcomes. Am J Obstet Gynecol. 2009;201:553–9. doi: 10.1016/j.ajog.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Yan LZ, Herrmann V, Hofer JK, Insel PA. β-Adrenergic receptor/cAMP-mediated signaling and apoptosis of S49 lymphoma cells. Am J Physiol. 2000;279:C1665–C74. doi: 10.1152/ajpcell.2000.279.5.C1665. [DOI] [PubMed] [Google Scholar]
- Zaugg M, Xu WM, Lucchinetti E, Shafiq SA, Jamali NZ, Siddiqui MAQ. β-Adrenergic receptor subtypes differentially affect apoptosis in adult rat ventricular myocytes. Circulation. 2000;102:344–50. doi: 10.1161/01.cir.102.3.344. [DOI] [PubMed] [Google Scholar]
- Zerrate MC, Pletnikov M, Connors SL, Vargas DL, Seidler FJ, Zimmerman AW, et al. Neuroinflammation and behavioral abnormalities after neonatal terbutaline treatment in rats: implications for autism. J Pharmacol Exp Ther. 2007;322:16–22. doi: 10.1124/jpet.107.121483. [DOI] [PubMed] [Google Scholar]