Abstract
Marijuana is the most widely used illicit drug in the U.S., and marijuana use by women is on the rise. Women have been found to be more susceptible to the development of cannabinoid abuse and dependence, have more severe withdrawal symptoms, and are more likely to relapse than men. The majority of research in humans suggests that women are more likely to be affected by cannabinoids than men, with reports of enhanced and decreased performance on various tasks. In rodents, females are more sensitive than males to effects of cannabinoids on tests of antinociception, motor activity, and reinforcing efficacy. Studies on effects of cannabinoid exposure during adolescence in both humans and rodents suggest that female adolescents are more likely than male adolescents to be deleteriously affected by cannabinoids. Sex differences in response to cannabinoids appear to be due to activational and perhaps organizational effects of gonadal hormones, with estradiol identified as the hormone that contributes most to the sexually dimorphic effects of cannabinoids in adults. Many, but not all sexually dimorphic effects of exogenous cannabinoids can be attributed to a sexually dimorphic endocannabinoid system in rodents, although the same has not yet been established firmly for humans. A greater understanding of the mechanisms underlying sexually dimorphic effects of cannabinoids will facilitate development of sex-specific approaches to treat marijuana dependence and to use cannabinoid-based medications therapeutically.
Keywords: adolescence, cannabinoids, endocannabinoids, estradiol, hormone, review, sex differences
Introduction
Marijuana is the most widely used illicit drug in the U.S. Although the number of men addicted to marijuana still outnumbers women, this gender gap has been steadily closing, as for most drugs of abuse, primarily because drug use in women has been increasing (Greenfield, et al., 2010). Epidemiological studies show that the patterns of drug use in women differ from those in men (Gunter, et al., 2006; Kelly, et al., 2006). For example, while women may initially take lower doses of an abused drug, they tend to become addicted faster and to relapse more frequently following a period of abstinence (Becker and Hu, 2008). Preliminary data suggest that the “telescoping” effect (faster trajectory from first use to dependence) initially observed in cocaine-using women may also occur for other abused drugs, including cannabis (Hernandez-Avila, et al., 2004). Additionally, recent studies report more withdrawal symptoms in women than men cannabis smokers who attempt to quit (Copersino, et al., 2010; Levin, et al., 2010). Sex differences also have been observed during other phases of the substance abuse process, including escalation and dysregulation (Carroll, et al., 2004). The purpose of the present review is to summarize the extant literature on sex differences in the CNS effects of exogenous cannabinoids and to describe how these differences may be related to underlying sexual dimorphism of the endocannabinoid system.
Sex Differences in the Effects of Exogenous Cannabinoids
Research in humans
A handful of studies has been conducted to compare the effects of cannabinoids in women vs. men. Smoked marijuana produced very similar effects on simulated driving performance in women and men (Anderson, et al., 2010), as well as on laboratory measures of impulsivity (McDonald, et al., 2003). In contrast, a dose of sublingual THC that did not affect spatial working memory performance in men significantly enhanced it in women (by decreasing between-search errors); this same dose of THC reduced errors in men but increased them in women engaged in a spatial span test (Makela, et al., 2006). Similarly, heavy marijuana-smoking women (tested while sober) were more impaired than light marijuana-smoking women on a visuospatial memory task, on which neither group of marijuana-smoking men showed impairment (Pope, et al., 1997). A single dose of oral dronabinol administered three times significantly slowed gastric emptying in women but not in men (Esfandyari, et al., 2006). Despite similar ratings of intoxication from THC and marijuana (and similar plasma THC levels), women reported more dizziness than did men (Mathew, et al., 2003). In contrast to these findings of greater cannabinoid effect in women, two studies report greater subjective THC effects (e.g., “high”) in men (Haney, 2007; Penetar, et al., 2005), with the latter study also reporting that women took longer than men to detect marijuana’s effects, experienced a shorter duration effect, and showed smaller increases in heart rate than did men. Cannabinoid effects on sexual functioning also vary between the sexes. Women report that low doses of marijuana facilitate arousal and desire whereas high doses decrease sexual motivation (Gorzalka, et al., 2010). Self-reports in men are more conflicting; however, in male rodents, cannabinoid exposure produces more consistently negative consequences on sexual functioning, including decreased motivation and erectile dysfunction (Gorzalka, et al., 2010).
Research in animals
A variety of cannabinoid effects have been compared in female vs. male animals, primarily rats. On tests of antinociception, several labs have reported that cannabinoids are more potent and occasionally more efficacious in female compared to male rats. For example, cannabinoids such as THC and CP55,940 were approximately twice as potent in female than male rats on warm water tail withdrawal and paw pressure tests (Craft, et al., 2012; Romero, et al., 2002; Tseng and Craft, 2001). Using a radiant heat tail flick test, THC was also more potent in female than male rats (Wiley, et al., 2007); although the sex difference in this study was not statistically significant, it was of the same magnitude reported by others, suggesting that the lack of significance may be due to the smaller sample sizes in this study.
Several studies also report that cannabinoid agonists are more potent in female than male adult rats in decreasing locomotor activity (Craft, et al., 2012; Tseng and Craft, 2001), as well as in producing catalepsy (Cohn, et al., 1972; Craft, et al., 2012; Wiley, et al., 2007). In mice, 1–10 mg/kg THC significantly increased locomotor activity in females but had no effect in males (Wiley, 2003). These studies indicate that female rodents are more sensitive to the biphasic effects of cannabinoids on locomotion -- i.e., increases at lower doses and decreases at higher doses. Sex differences in cannabinoid effects on motor activity are important not only as an assessment of cannabinoid “side-effects”, but also because effects like antinociception are typically measured as a lengthening of latency to respond – which may be caused by drug-induced suppression of motor activity. However, using peripheral THC administration against inflammatory pain, one study suggests that greater THC-induced antinociception is not secondary to greater THC-induced sedation in female compared to male rats (Craft and Kandasamy, unpublished data).
In animal models of marijuana abuse, female rodents may be more sensitive than males to the reinforcing and subjective effects of cannabinoids. For example, female rats acquired self-administration of WIN55,212-2 faster and maintained higher rates of responding than males (Fattore, et al., 2007), as well as being more sensitive to drug- and cue-induced reinstatement than males (Fattore, et al., 2010). Female mice acquired a THC discrimination in approximately 1/3 fewer sessions than did males (Wiley, et al., 2011b). Similar to some human studies of cannabinoid effects on cognition, cannabinoid-induced impairments in spatial learning were greater in female rats than males (Cha, et al., 2007). Cannabinoids are also commonly reported to enhance sexual behavior in female rodents but inhibit it in males (for a review, see Gorzalka, et al., 2010). Finally, female and male rats chronically treated with HU-210 showed similarly decreased immobility in the forced swim test (Morrish, et al., 2009), suggesting that antidepressant actions of cannabinoids are similar in females and males.
Several physiological effects of cannabinoids have been compared between the sexes. Gonadally intact female rats were more sensitive than males to cannabinoid-induced hypothermia (Borgen, et al., 1973; Wiley, et al., 2007), although the opposite sex difference was reported in gonadectomized guinea pigs (Diaz, et al., 2009). Both intact female rats and ovariectomized (OVX) female guinea pigs were less sensitive than their male counterparts to the hyperphagic effect of cannabinoid agonists (Diaz, et al., 2009; Miller, et al., 2004). Interestingly, exposure to high-fat diet profoundly decreased female rats’ sensitivity to the motoric and antinociceptive effects of THC, whereas it had little or no effect in males (Wiley, et al., 2011a). Taken together, much of the research on sex differences in exogenous cannabinoid effects indicates a greater sensitivity to cannabinoids in females than in males, with opposite sex differences in a few cases (e.g., for cannabinoid-induced hyperphagia).
Importance of adolescence
In mammals, vast changes in brain functioning occurring during adolescence are associated with patterns of behavior (e.g., increased risk taking, increased interactions with peers) that may, in humans, contribute to initiation of illicit drug use, including cannabinoids (Viveros, et al., 2011; Viveros, et al., 2012). Further, early marijuana use is associated with long-term effects, including impaired reaction times in a visual attention task (Ehrenreich, et al., 1999), lower levels of academic achievement (Brook, et al., 2008), and continued reductions in brain activation in prefrontal and cerebellar regions (Chang, et al., 2006). Although the self-selection of human users limits determination of underlying mechanisms, animal research suggests that the consequences of cannabinoid exposure on the brain and behavior of adolescent rodents differ from those seen in mature individuals, and some of these differences vary by sex. For example, adolescent female rats were less sensitive than adult females to several pharmacological effects of THC (e.g., antinociception and hypothermia), while male rats did not show this age-dependent difference (Wiley, et al., 2007). In a hole board test, female rats treated with cannabinoids during adolescence showed increased motor activity compared to control females. This cannabinoid-induced motor activation was not seen in males; however, cannabinoid-treated males showed decreased exploratory behavior compared to male controls, which was not evident in females (Biscaia, et al., 2003). Female rats chronically exposed to THC during adolescence also have been found to show depressive-like behavior, whereas the same was not true for males (Rubino, et al., 2008). Acute exposure to cannabinoids during adolescence also produced more anxiogenic responses in female than male rats (Marco, et al., 2006; Viveros, et al., 2005), and increased acquisition of cocaine self-administration in female but not male rats (Higuera-Matas, et al., 2008).
Several studies also report that adolescent rats repeatedly exposed to cannabinoids show greater memory impairment compared to rats exposed to cannabinoids as adults (O'Shea, et al., 2004; Schneider and Koch, 2003; Schneider, et al., 2008). Impairment was worse in male adolescent rats for some tasks, while other memory tasks resulted in more impairment in female adolescent rats (for reviews, see Viveros, et al., 2011; Viveros, et al., 2012). For example, adolescent male rats showed fewer THC-induced deficits in spatial learning in a Morris water maze task than did adolescent female rats (Cha, et al., 2007). In another study, chronic exposure to cannabinoids during adolescence increased the discrimination index on a spontaneous alternation task in male rats compared to control males, but this effect was not observed in females. In contrast, females exposed to cannabinoids during adolescence showed memory impairment in an object location task, an effect that was not seen in males (Mateos, et al., 2011). Stage of adolescence may also modulate sex differences in THC’s effects on memory. For example, one study found that chronic exposure to THC during early adolescence decreased retention in an active avoidance paradigm only in male rats, whereas chronic exposure to THC in late adolescence produced decreases only in female rats (Harte and Dow-Edwards, 2010).
In several instances, sex differences in neuroanatomy and/or neurophysiology have been found to accompany the sex differences in behavior observed following adolescent exposure to cannabinoids. After repeated injections with THC, greater CB1 receptor desensitization occurred in female adolescent rats compared to male adolescents, or to adult rats of either sex. This effect was not due to differences in downregulation of CB1 receptors, which was similar across groups (Burston, et al., 2010). Adolescent exposure to cannabinoids also disrupted the homeostatic relationship between glutamate and GABA transmission in female but not male rats (Higuera-Matas, et al., 2012). Finally, McQueeny et al. (2011) reported that adolescent human female marijuana users have increased right amygdala volume compared to controls, while marijuana use in males did not affect amygdala volume. Together, these studies of adolescent exposure to cannabinoids indicate that females tend to be more deleteriously affected; however, the sparse research is far from conclusive. In addition, hormonal factors strongly influence female response to cannabinoids, as explained in the following section.
Mechanisms Underlying Sex Differences in Exogenous Cannabinoid Effects
Hormonal influences
Sex differences in exogenous cannabinoid effects appear to be strongly influenced by ovarian hormones in adult animals. For example, THC-induced antinociception depends on females’ estrous stage, with females in late proestrus to estrus being the most sensitive to THC and thus the most different from males (Craft and Leitl, 2008; Wakley and Craft, 2011). Estradiol enhanced THC-induced antinociception (Craft and Leitl, 2008) and self-administration of WIN55,212-2 (Fattore, et al., 2010) in OVX female rats. Estradiol attenuated cannabinoid-induced hyperphagia and hypothermia in OVX guinea pigs (Kellert, et al., 2009), and cannabinoid-induced disruption of acquisition and performance of an operant task in OVX rats (Daniel, et al., 2002; Winsauer, et al., 2011). Thus, there is growing evidence for activational effects of ovarian hormones – particularly estradiol – underlying sexual dimorphism of cannabinoid sensitivity. Organizational hormone effects are largely unexamined to date; however, the fact that sex differences in cannabinoid sensitivity are still observed in guinea pigs gonadectomized as adults (Diaz, et al., 2009), and that a few sex differences in cannabinoid effects have been observed in adolescent rats (Romero, et al., 2002; Wiley, et al., 2007) suggests that cannabinoid systems may develop in a sexually dimorphic manner early in life.
Sexual dimorphism of the endocannabinoid system
Sex differences in, and estradiol modulation of, the behavioral effects of cannabinoids may be due to pharmacokinetic factors in rodents (for a review, see Craft, 2005), but are also likely linked to differential brain endocannabinoid systems. The endocannabinoid system is comprised of two receptors, endogenous cannabinoids, and their synthetic and metabolic enzymes. Of the two identified cannabinoid receptors, the CB1 receptor is the primary one found in the brain (Herkenham, et al., 1991), whereas the CB2 receptor is localized more prominently in the immune system (Galiegue, et al., 1995), although recent research suggests that functional CB2 receptors may also play a role in the brain (Van Sickle, et al., 2005; Xi, et al., 2011). The two best characterized endocannabinoids, anandamide and 2-arachidonoylglycerol (2-AG), are metabolized primarily by fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), respectively (Cravatt and Lichtman, 2002; Dinh, et al., 2002). Sex differences in this system may appear early in development. In the rat, CB1 receptors in the brain exhibit a progressive increase in number, but not in affinity, during the pre-weanling period [i.e., before postnatal day (PN) 21] and during early adolescence, with receptor pruning and a decline to adult levels during later adolescence (Belue, et al., 1995; McLaughlin, et al., 1994; Rodriguez de Fonseca, et al., 1993). Peak levels of CB1 receptors are reached earlier in females (PN 30) than in males (PN 40). Further, the endocannabinoid system may be involved in neuronal migration and brain development, processes that also exhibit sexual dimorphism (Berrendero, et al., 1998; Romero, et al., 1997). For example, although the amygdala in an adult male rat is larger than that in an adult female, female rats show greater cell proliferation in this brain area as early as PN 4 compared to newborn male rats (Krebs-Kraft, et al., 2010), suggesting that changes during later postnatal development are responsible for the observed adult differences. This greater proliferation in the amygdala of female newborns was prevented by administration of WIN 55,212-2, an effect that was associated with development of male-like patterns of social play in the treated females (Krebs-Kraft, et al., 2010). Notably, WIN55,212-2 did not affect cell proliferation in males. Subsequent experiments by this group showed that the anti-proliferation effects of WIN55,212-2 in females were mediated by its action on CB2 receptors. In addition to differences in the rate of cell proliferation, levels of endocannabinoids and their metabolic enzymes in the amygdala also differed between the sexes. Whereas the amygdala of males showed higher levels of 2-AG and anandamide, the amygdala of females contained greater concentrations of their primary metabolic enzymes, MAGL and FAAH, respectively (Krebs-Kraft, et al., 2010). Together, these results suggest that sex differences in endocannabinoid tone develop early and may set the stage for differential development of the system, with the likely consequence of later divergent responses to exogenous cannabinoids.
In adult rats, the endocannabinoid system is strongly influenced by hormonal factors, particularly circulating levels of estradiol (for review, see Lopez, 2010). Throughout most of the brain, endocannabinoid content is estrous cycle-dependent in females, with cycle-independent exceptions being sustained higher levels of 2-AG in the hypothalamus and pituitary and lower levels in the cerebellum, in comparison to levels in adult males (Bradshaw, et al., 2006). Compared to males and OVX females, intact females also exhibit lower levels of CB1 receptor binding in the hypothalamus and increased binding in the amygdala (Riebe, et al., 2010). Results across studies are conflicting for the hippocampus. A higher density of CB1 receptors in the hippocampus has been reported in males compared to females (Reich, et al., 2009), but also in OVX females compared both to intact females and to males (Riebe, et al., 2010). As has been reported for brain endocannabinoid levels, however, CB1 receptor numbers and mRNA transcripts in various brain areas are not static, but fluctuate across the estrous cycle in females (Gonzalez, et al., 2000; Rodriguez de Fonseca, et al., 1994), emphasizing the importance of this variable in mechanistic studies.
To date, only these relatively few published papers have focused specifically on sex differences in the endocannabinoid system. An additional smattering of studies has examined sex differences in behavioral effects of the endocannabinoids. In one of these studies, Basavarajappa et al. (2006) reported that female mice lacking the gene for producing FAAH (FAAH−/−) consumed more ethanol and were less sensitive to its hypothermic effects than were female wildtype mice or male mice of either genotype. The authors suggested that these findings resulted from an interaction between increased brain levels of anandamide and ovarian hormones. The results of a related study were only partly consistent, in that female, but also male, FAAH−/− mice exhibited increased ethanol consumption compared to their wildtype counterparts (Blednov, et al., 2007). Although a role for hormonal modulation of these effects was postulated, it was not specifically investigated.
While the extent to which these findings in animals model differences in humans is unknown as yet, preliminary evidence suggests that humans also exhibit sex differences in endocannabinoid system functioning. For example, higher activity of FAAH and the anandamide transporter has been observed in the platelets of female (vs. male) migraine patients (Cupini, et al., 2006). Further, individual variability in pain sensitivity in humans has been linked to a complex interaction of many factors. Among these variables are sex and loci in genes that code for transient receptor potential vanilloid type 1 (TRPV1) receptors (Kim, et al., 2004). [In addition to its effects on CB1 receptors, anandamide has been shown to activate TRPV1 receptors (Di Marzo, et al., 2002).] Finally, decreased serum levels of 2-AG were observed in female patients experiencing major depression, with longer duration depressive episodes associated with larger decreases in 2-AG (Hill, et al., 2008). Whether this effect would also occur in males is unknown since this study examined only female patients.
HPA axis-endocannabinoid interaction
In addition to the direct effects of the endocannabinoid system on the development of sexual dimorphism in the brain, indirect effects are also likely. A unique aspect of the endocannabinoid system is that endocannabinoids are released from neurons that are associated with release of many other neurotransmitters, including dopamine, GABA and glutamate (Howlett, et al., 2002). The predominant effect of endocannabinoids is inhibition of release of the primary neurotransmitter; however, because of the presynaptic localization of cannabinoid receptors on neurons that release both excitatory and inhibitory neurotransmitters, the end result of CB1 receptor activation may be either inhibitory [if release of an excitatory neurotransmitter is inhibited; i.e., depolarization-induced suppression of excitation (DSE)] or excitatory [if release of an inhibitory neurotransmitter is inhibited; i.e., depolarization-induced suppression of inhibition (DSI)] (Wilson and Nicoll, 2001). It is likely that endocannabinoids play a similar modulatory role within the hypothalamic-pituitary-gonadal (HPG) or -adrenal (HPA) axes. Further, while the predominant endocannabinoid effect within these systems is tonic inhibition, hormonal and endocannabinoid modulation of the systems is almost certainly bidirectional in nature. For example, a review of the interplay between sex steroids and the endocannabinoid system concluded that fluctuation in regional CB1 receptor densities and endocannabinoid levels across the estrous cycle was strongly influenced by estradiol (Lopez, 2010). A primary effect of increased estradiol (e.g., near ovulation) was to disinhibit tonic endocannabinoid suppression of hormone secretion by the HPG/ HPA axes (Lopez, 2010). In females, administration of cannabinoids mimicked this endocannabinoid inhibition of hormonal secretion, an effect that was reversed by estradiol. Interaction of the endocannabinoid system with the stress response and HPA functioning has also been noted. Under normal conditions, tonic activation of the endocannabinoid system inhibits HPA axis activity in rats of both sexes (Atkinson, et al., 2010; Lopez, 2010).
An elegant series of experiments (reviewed by Farhang, et al., 2009) demonstrated that administration of the cannabinoid agonist WIN55,212-2 modulated the tonic inhibition at proopiomelanocortin (POMC) synapses of the arcuate nucleus (ARC) of the guinea pig hypothalamus in a sexually divergent and hormone-dependent manner, with males exhibiting a testosterone-dependent increase in preprocorticotropin-releasing hormone and females showing an estradiol-dependent increase in POMC (Diaz, et al., 2009; Farhang, et al., 2009). In an electrophysiological preparation, both sexes show equal cannabinoid agonist-induced reduction in glutamatergic excitation in the anorexigenic ARC neurons, but male guinea pigs are less sensitive than females to agonist-induced attenuation of a GABAergic response (Farhang, et al., 2009). The net result is that CB1 activation in males produces a shift towards greater inhibition of the ARC neurons due to the concomitant effect of cannabinoid-induced inhibition of the excitatory effects of glutamate and resistance to cannabinoid-induced suppression of GABA (i.e., less excitation due to DSI). In contrast, more excitation is observed in females due to higher basal excitation and approximately equivalent inhibition of glutamate and GABA signaling. Behaviorally, these cellular effects are associated with greater sensitivity to agonist-induced hyperphagia (i.e., via inhibition of the anorexigenic effects of the ARC neurons) and hypothermia in male than in female gonadectomized guinea pigs (Farhang, et al., 2009). Stressful conditions can also alter functioning of the endocannabinoid system in a sexually differentiated manner. For example, under conditions of chronic mild stress, male rats showed a decrease in the density of hippocampal CB1 receptors whereas female rats showed an increase, effects that were not dependent upon adult hormonal status as they occurred in gonadectomized and intact adults (Reich, et al., 2009).
Conclusions
Although research on sex differences in cannabinoid pharmacology is increasing, knowledge of the mechanism(s) underlying sex differences in behavioral effects of cannabinoids remains limited. While activational hormonal factors have been shown to play a strong role in modulating endocannabinoid system functioning in adults, hormone-independent sex differences in this system have also been reported. Very little is known about the organizational effects of hormones during development of the endocannabinoid system. Understanding hormonal and non-hormonal mechanisms will facilitate development of sex-specific approaches to treat marijuana dependence and to use cannabinoid-based medications therapeutically – for example, in the treatment of pain and spasticity (Aggarwal, et al., 2009). In addition, elucidating endocrinological mechanisms that modulate the endocannabinoid system could ultimately enhance treatment of a wide variety of disorders in which dysregulation of the endocannabinoid system has been implicated, including obesity (Akhas, et al., 2009; Cota, et al., 2003), substance abuse (Beardsley, et al., 2009), and various neurological disorders (Williamson and Evans, 2000). Finally, there is a long-standing paucity of research on sex differences and in females of all species in nearly all biomedical fields, with pharmacology and neuroscience being among the weakest (Beery and Zucker, 2011). As noted in a recent editorial, “…the burden of proof is shifting from having to defend why sex-gender differences should be studied to having to defend why they should not” (Wetherington, 2007) [see also (Cahill, 2006; Mogil and Chanda, 2005)].
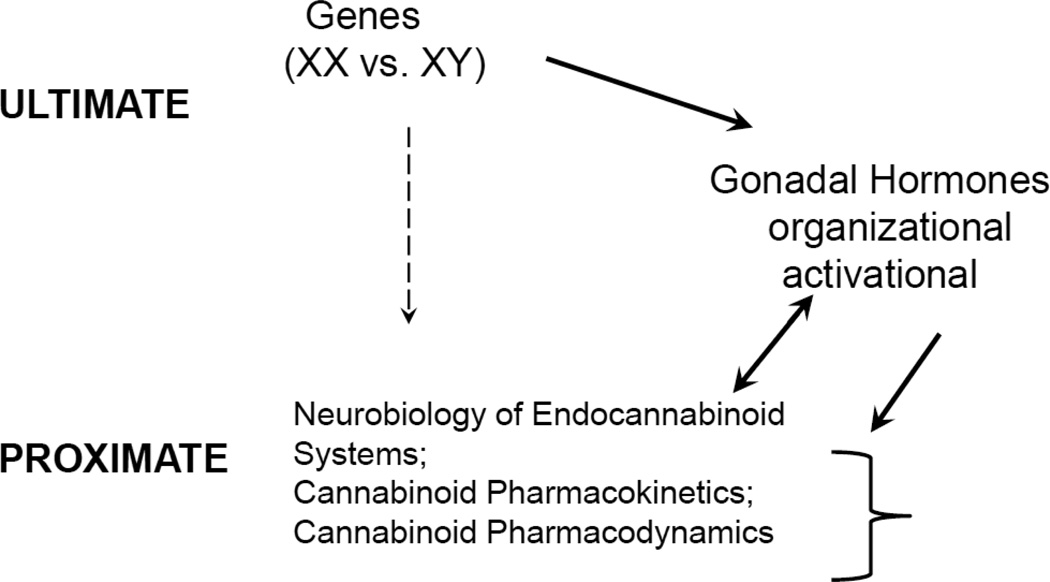
Figure 1. Sex differences in cannabinoid pharmacology: potential mechanisms.
Future Directions.
Given the paucity of studies in this area, considerable latitude exists for future research. Some of the major unanswered questions have been highlighted below:
Given the ubiquity of CB receptors at multiple levels of the neuraxis, localization of sex differences in cannabinoid function is crucial. For example, agonists can produce analgesia via supraspinal, spinal and peripheral CB receptor activation (Guindon and Hohmann, 2009) -- are all levels of the neuraxis sexually dimorphic in regard to cannabinoid function?
Do gonadal hormones act at genomic or membrane steroid receptors, or both, to influence endocannabinoid system activity? For example, in addition to its "slow" actions at genomic estrogen receptors (ER), estradiol also influences pain and the opioid system rapidly via membrane-bound ER (Eckersell, et al., 1998; Evrard and Balthazart, 2004). Does estradiol exert both types of effects on the cannabinoid system as well?
Studies in humans are needed to confirm or refute sex difference findings in rodents. Furthermore, the modulatory effects of estradiol need to be studied in two ways: cannabinoid effects in cycling women tested not only during follicular and luteal phases, but also during ovulation, when the greatest changes in sensitivity have been observed in rodents.
Acknowledgements
Preparation of this review was supported in part by National Institute on Drug Abuse grants DA-016644 and DA-03672; NIDA had no further role in the writing of the review or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal SK, Carter GT, Sullivan MD, ZumBrunnen C, Morrill R, Mayer JD. Medicinal use of cannabis in the United States: historical perspectives, current trends, and future directions. J Opioid Manag. 2009;5:153–168. doi: 10.5055/jom.2009.0016. [DOI] [PubMed] [Google Scholar]
- Akhas F, Gasteyger C, Sjodin A, Astrup A, Larsen TM. A critical review of the cannabinoid receptor as a drug target for obesity management. Obes Rev. 2009;10:58–67. doi: 10.1111/j.1467-789X.2008.00520.x. [DOI] [PubMed] [Google Scholar]
- Anderson BM, Rizzo M, Block RI, Pearlson GD, O'Leary DS. Sex differences in the effects of marijuana on simulated driving performance. J Psychoactive Drugs. 2010;42:19–30. doi: 10.1080/02791072.2010.10399782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson HC, Leggett JD, Wood SA, Castrique ES, Kershaw YM, Lightman SL. Regulation of the hypothalamic-pituitary-adrenal axis circadian rhythm by endocannabinoids is sexually diergic. Endocrinology. 2010;151:3720–3727. doi: 10.1210/en.2010-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa BS, Yalamanchili R, Cravatt BF, Cooper TB, Hungund BL. Increased ethanol consumption and preference and decreased ethanol sensitivity in female FAAH knockout mice. Neuropharmacology. 2006;50:834–844. doi: 10.1016/j.neuropharm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Thomas BF, McMahon LR. Cannabinoid CB1 receptor antagonists as potential pharmacotherapies for drug abuse disorders. Int Rev Psychiatry. 2009;21:134–142. doi: 10.1080/09540260902782786. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35:565–572. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belue RC, Howlett AC, Westlake TM, Hutchings DE. The ontogeny of cannabinoid receptors in the brain of postnatal and aging rats. Neurotoxicol Teratol. 1995;17:25–30. doi: 10.1016/0892-0362(94)00053-g. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Garcia-Gil L, Hernandez ML, Romero J, Cebeira M, de Miguel R, Ramos JA, Fernandez-Ruiz JJ. Localization of mRNA expression and activation of signal transduction mechanisms for cannabinoid receptor in rat brain during fetal development. Development. 1998;125:3179–3188. doi: 10.1242/dev.125.16.3179. [DOI] [PubMed] [Google Scholar]
- Biscaia M, Marin S, Fernandez B, Marco EM, Rubio M, Guaza C, Ambrosio E, Viveros MP. Chronic treatment with CP 55,940 during the peri-adolescent period differentially affects the behavioural responses of male and female rats in adulthood. Psychopharmacology (Berl) 2003;170:301–308. doi: 10.1007/s00213-003-1550-7. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Cravatt BF, Boehm SL, 2nd, Walker D, Harris RA. Role of endocannabinoids in alcohol consumption and intoxication: studies of mice lacking fatty acid amide hydrolase. Neuropsychopharmacology. 2007;32:1570–1582. doi: 10.1038/sj.npp.1301274. [DOI] [PubMed] [Google Scholar]
- Borgen LA, Lott GC, Davis WM. Cannabis-induced hypothermia: A dose-effect comparison of crude marihuana extract and synthetic D9-tetrahydrocannabinol in male and female rats. Res Comm Chem Path Pharmacol. 1973;5:621–626. [PubMed] [Google Scholar]
- Bradshaw HB, Rimmerman N, Krey JF, Walker JM. Sex and hormonal cycle differences in rat brain levels of pain-related cannabimimetic lipid mediators. Am J Physiol Regul Integr Comp Physiol. 2006;291:R349–R358. doi: 10.1152/ajpregu.00933.2005. [DOI] [PubMed] [Google Scholar]
- Brook JS, Stimmel MA, Zhang C, Brook DW. The association between earlier marijuana use and subsequent academic achievement and health problems: a longitudinal study. Am J Addict. 2008;17:155–160. doi: 10.1080/10550490701860930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burston JJ, Wiley JL, Craig AA, Selley DE, Sim-Selley LJ. Regional enhancement of CB1 receptor desensitization in female adolescent rats following repeated Δ9-tetrahydrocannabinol exposure. Br J Pharmacol. 2010;161:103–112. doi: 10.1111/j.1476-5381.2010.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Cha YM, Jones KH, Kuhn CM, Wilson WA, Swartzwelder HS. Sex differences in the effects of delta-9-tetrahydrocannabinol on spatial learning in adolescent and adult rats. Behav Pharmacol. 2007;18:563–569. doi: 10.1097/FBP.0b013e3282ee7b7e. [DOI] [PubMed] [Google Scholar]
- Chang L, Yakupov R, Cloak C, Ernst T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain. 2006;129:1096–1112. doi: 10.1093/brain/awl064. [DOI] [PubMed] [Google Scholar]
- Cohn RA, Barnes PR, Barratt E, Pirch JH. Sex differences in response to marijuana in the rat. In: Singh JM, Miller LH, Lal H, editors. Drug Addiction: Experimental Pharmacology. New York: Futura Publishing Company; 1972. pp. 227–234. [Google Scholar]
- Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, Simmons MS, Gorelick DA. Sociodemographic characteristics of cannabis smokers and the experience of cannabis withdrawal. Am J Drug Alcohol Abuse. 2010;36:311–319. doi: 10.3109/00952990.2010.503825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Lutz B, Vicennati V, Stalla GK, Pasquali R, Pagotto U. Endogenous cannabinoid system as a modulator of food intake. Int J Obes Relat Metab Disord. 2003;27:289–301. doi: 10.1038/sj.ijo.0802250. [DOI] [PubMed] [Google Scholar]
- Craft RM. Sex differences in behavioral effects of cannabinoids. Life Sci. 2005;77:2471–2478. doi: 10.1016/j.lfs.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Craft RM, Leitl MD. Gonadal hormone modulation of the behavioral effects of Delta9-tetrahydrocannabinol in male and female rats. Eur J Pharmacol. 2008;578:37–42. doi: 10.1016/j.ejphar.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Craft RM, Wakley AA, Tsutsui KT, Laggart JD. Sex differences in CB1 vs. CB2 receptor-selective antagonism of antinociception produced by delta-9-THC and CP55,490 in the rat. J Pharmacol Exp Ther. 2012 doi: 10.1124/jpet.111.188540. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Lichtman AH. The enzymatic inactivation of the fatty acid amide class of signaling lipids. Chem Phys Lipids. 2002;121:135–148. doi: 10.1016/s0009-3084(02)00147-0. [DOI] [PubMed] [Google Scholar]
- Cupini LM, Bari M, Battista N, Argiro G, Finazzi-Agro A, Calabresi P, Maccarrone M. Biochemical changes in endocannabinoid system are expressed in platelets of female but not male migraineurs. Cephalalgia. 2006;26:277–281. doi: 10.1111/j.1468-2982.2005.01031.x. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Winsauer PJ, Brauner IN, Moerschbaecher JM. Estrogen improves response accuracy and attenuates the disruptive effects of delta-9-THC in ovariectomized rats responding under a multiple schedule of repeated acquisition and performance. Behav Neurosci. 2002;116:989–998. [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L, Fezza F, Ligresti A, Bisogno T. Anandamide receptors. Prostaglandins Leukot Essen Fatty Acids. 2002;66:377–391. doi: 10.1054/plef.2001.0349. [DOI] [PubMed] [Google Scholar]
- Diaz S, Farhang B, Hoien J, Stahlman M, Adatia N, Cox JM, Wagner EJ. Sex differences in the cannabinoid modulation of appetite, body temperature and neurotransmission at POMC synapses. Neuroendocrinology. 2009;89:424–440. doi: 10.1159/000191646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of mu-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J Neurosci. 1998;18:3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, Gigerenzer G, Hoehe MR. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology (Berl) 1999;142:295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- Esfandyari T, Camilleri M, Ferber I, Burton D, Baxter K, Zinsmeister AR. Effects of a cannabinoid agonist on gastrointestinal transit and postprandial satiation in healthy human subjects: a randomized, placebo-controlled study. Neurogastroenterol Motil. 2006;18:831–838. doi: 10.1111/j.1365-2982.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- Evrard HC, Balthazart J. Rapid regulation of pain by estrogens synthesized in spinal dorsal horn neurons. J Neurosci. 2004;24:7225–7229. doi: 10.1523/JNEUROSCI.1638-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhang B, Diaz S, Tang SL, Wagner EJ. Sex differences in the cannabinoid regulation of energy homeostasis. Psychoneuroendocrinology. 2009 doi: 10.1016/j.psyneuen.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Fadda P, Fratta W. Drug- and cue-induced reinstatement of cannabinoid-seeking behaviour of male and female rats: influence of ovarian hormones. Br J Pharmacol. 2010;160:724–735. doi: 10.1111/j.1476-5381.2010.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W. Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. Br J Pharmacol. 2007;152:795–804. doi: 10.1038/sj.bjp.0707465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Boulaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Bisogno T, Wenger T, Manzanares J, Milone A, Berrendero F, Di Marzo V, Ramos JA, Fernandez-Ruiz JJ. Sex steroid influence on cannabinoid CB(1) receptor mRNA and endocannabinoid levels in the anterior pituitary gland. Biochem Biophys Res Commun. 2000;270:260–266. doi: 10.1006/bbrc.2000.2406. [DOI] [PubMed] [Google Scholar]
- Gorzalka BB, Hill MN, Chang SC. Male-female differences in the effects of cannabinoids on sexual behavior and gonadal hormone function. Horm Behav. 2010;58:91–99. doi: 10.1016/j.yhbeh.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Back SE, Lawson K, Brady KT. Substance abuse in women. Psychiatr Clin North Am. 2010;33:339–355. doi: 10.1016/j.psc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Hohmann AG. The endocannabinoid system and pain. CNS Neurol Disord Drug Targets. 2009;8:403–421. doi: 10.2174/187152709789824660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter TD, Arndt S, Wenman G. Characteristics of admissions for primary stimulant dependence during 2001. Subst Use Misuse. 2006;41:1277–1286. doi: 10.1080/10826080600754876. [DOI] [PubMed] [Google Scholar]
- Haney M. Opioid antagonism of cannabinoid effects: differences between marijuana smokers and nonmarijuana smokers. Neuropsychopharmacology. 2007;32:1391–1403. doi: 10.1038/sj.npp.1301243. [DOI] [PubMed] [Google Scholar]
- Harte LC, Dow-Edwards D. Sexually dimorphic alterations in locomotion and reversal learning after adolescent tetrahydrocannabinol exposure in the rat. Neurotoxicol Teratol. 2010;32:515–524. doi: 10.1016/j.ntt.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004;74:265–272. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Higuera-Matas A, Miguens M, Coria SM, Assis MA, Borcel E, Del Olmo N, Ambrosio E. Sex-specific disturbances of the glutamate/GABA balance in the hippocampus of adult rats subjected to adolescent cannabinoid exposure. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2011.12.028. [DOI] [PubMed] [Google Scholar]
- Higuera-Matas A, Soto-Montenegro ML, del Olmo N, Miguens M, Torres I, Vaquero JJ, Sanchez J, Garcia-Lecumberri C, Desco M, Ambrosio E. Augmented acquisition of cocaine self-administration and altered brain glucose metabolism in adult female but not male rats exposed to a cannabinoid agonist during adolescence. Neuropsychopharmacology. 2008;33:806–813. doi: 10.1038/sj.npp.1301467. [DOI] [PubMed] [Google Scholar]
- Hill MN, Miller GE, Ho WS, Gorzalka BB, Hillard CJ. Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry. 2008;41:48–53. doi: 10.1055/s-2007-993211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Kellert BA, Nguyen MC, Nguyen C, Nguyen QH, Wagner EJ. Estrogen rapidly attenuates cannabinoid-induced changes in energy homeostasis. Eur J Pharmacol. 2009;622:15–24. doi: 10.1016/j.ejphar.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly BC, Parsons JT, Wells BE. Prevalence and predictors of club drug use among club-going young adults in New York city. J Urban Health. 2006;83:884–895. doi: 10.1007/s11524-006-9057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Neubert JK, San Miguel A, Xu K, Krishnaraju RK, Iadarola MJ, Goldman D, Dionne RA. Genetic influence on variability in human acute experimental pain sensitivity associated with gender, ethnicity and psychological temperament. Pain. 2004;109:488–496. doi: 10.1016/j.pain.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Krebs-Kraft DL, Hill MN, Hillard CJ, McCarthy MM. Sex difference in cell proliferation in developing rat amygdala mediated by endocannabinoids has implications for social behavior. Proc Natl Acad Sci USA. 2010;107:20535–20540. doi: 10.1073/pnas.1005003107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin KH, Copersino ML, Heishman SJ, Liu F, Kelly DL, Boggs DL, Gorelick DA. Cannabis withdrawal symptoms in non-treatment-seeking adult cannabis smokers. Drug Alcohol Depend. 2010;111:120–127. doi: 10.1016/j.drugalcdep.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez HH. Cannabinoid-hormone interactions in the regulation of motivational processes. Horm Behav. 2010;58:100–110. doi: 10.1016/j.yhbeh.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Makela P, Wakeley J, Gijsman H, Robson PJ, Bhagwagar Z, Rogers RD. Low doses of delta-9 tetrahydrocannabinol (THC) have divergent effects on short-term spatial memory in young, healthy adults. Neuropsychopharmacology. 2006;31:462–470. doi: 10.1038/sj.npp.1300871. [DOI] [PubMed] [Google Scholar]
- Marco EM, Llorente R, Moreno E, Biscaia JM, Guaza C, Viveros MP. Adolescent exposure to nicotine modifies acute functional responses to cannabinoid agonists in rats. Behav Brain Res. 2006;172:46–53. doi: 10.1016/j.bbr.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Mateos B, Borcel E, Loriga R, Luesu W, Bini V, Llorente R, Castelli M, Viveros MP. Adolescent exposure to nicotine and/or the cannabinoid agonist CP 55,940 induces gender-dependent long-lasting memory impairments and changes in brain nicotinic and CB1 cannabinoid receptors. J Psychopharmacol. 2011;25:1676–1690. doi: 10.1177/0269881110370503. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Davis RJ. Postural syncope after marijuana: a transcranial Doppler study of the hemodynamics. Pharmacol Biochem Behav. 2003;75:309–318. doi: 10.1016/s0091-3057(03)00086-8. [DOI] [PubMed] [Google Scholar]
- McDonald J, Schleifer L, Richards JB, de Wit H. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology. 2003;28:1356–1365. doi: 10.1038/sj.npp.1300176. [DOI] [PubMed] [Google Scholar]
- McLaughlin CR, Martin BR, Compton DR, Abood ME. Cannabinoid receptors in developing rats: detection of mRNA and receptor binding. Drug Alcohol Depend. 1994;36:27–31. doi: 10.1016/0376-8716(94)90006-x. [DOI] [PubMed] [Google Scholar]
- McQueeny T, Padula CB, Price J, Medina KL, Logan P, Tapert SF. Gender effects on amygdala morphometry in adolescent marijuana users. Behav Brain Res. 2011;224:128–134. doi: 10.1016/j.bbr.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CC, Murray TF, Freeman KG, Edwards GL. Cannabinoid agonist, CP55,940, facilitates intake of palatable foods when injected into the hindbrain. Physio Behav. 2004;80:611–116. doi: 10.1016/j.physbeh.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Chanda ML. The case for the inclusion of female subjects in basic science studies of pain. Pain. 2005;117:1–5. doi: 10.1016/j.pain.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Morrish AC, Hill MN, Riebe CJ, Gorzalka BB. Protracted cannabinoid administration elicits antidepressant behavioral responses in rats: role of gender and noradrenergic transmission. Physiol Behav. 2009;98:118–124. doi: 10.1016/j.physbeh.2009.04.023. [DOI] [PubMed] [Google Scholar]
- O'Shea M, Singh ME, McGregor IS, Mallet PE. Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. J Psychopharmacol. 2004;18:502–508. doi: 10.1177/026988110401800407. [DOI] [PubMed] [Google Scholar]
- Penetar DM, Kouri EM, Gross MM, McCarthy EM, Rhee CK, Peters EN, Lukas SE. Transdermal nicotine alters some of marihuana’s effects in male and female volunteers. Drug Alcohol Depend. 2005;79:211–223. doi: 10.1016/j.drugalcdep.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Jacobs A, Mialet JP, Yurgelun-Todd D, Gruber S. Evidence for a sex-specific residual effect of cannabis on visuospatial memory. Psychother Psychosom. 1997;66:179–184. doi: 10.1159/000289132. [DOI] [PubMed] [Google Scholar]
- Reich CG, Taylor ME, McCarthy MM. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav Brain Res. 2009;203:264–269. doi: 10.1016/j.bbr.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebe CJ, Hill MN, Lee TT, Hillard CJ, Gorzalka BB. Estrogenic regulation of limbic cannabinoid receptor binding. Psychoneuroendocrinology. 2010;35:1265–1269. doi: 10.1016/j.psyneuen.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Ramos JA, Bonnin A, Fernandez RJJ. Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport. 1993;4:135–138. doi: 10.1097/00001756-199302000-00005. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Cebeira M, Ramos JA, Martin M, Fernandez-Ruiz JJ. Cannabinoid receptors in rat brain areas: sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci. 1994;54:159–170. doi: 10.1016/0024-3205(94)00585-0. [DOI] [PubMed] [Google Scholar]
- Romero EM, Fernandez B, Sagredo O, Gomez N, Uriguen L, Guaza C, De Miguel R, Ramos JA, Viveros MP. Antinociceptive, behavioural and neuroendocrine effects of CP55,940 in young rats. Devel Brain Res. 2002;136:85–92. doi: 10.1016/s0165-3806(02)00306-1. [DOI] [PubMed] [Google Scholar]
- Romero J, Garcia-Palomero E, Berrendero F, Garcia-Gil L, Hernandez ML, Ramos JA, Fernandez-Ruiz JJ. Atypical location of cannabinoid receptors in white matter areas during rat brain development. Synapse. 1997;26:317–323. doi: 10.1002/(SICI)1098-2396(199707)26:3<317::AID-SYN12>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Rubino T, Vigano D, Realini N, Guidali C, Braida D, Capurro V, Castiglioni C, Cherubino F, Romualdi P, Candeletti S, Sala M, Parolaro D. Chronic delta(9)-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: Behavioral and biochemical correlates. Neuropsychopharmacology. 2008;33:2760–2771. doi: 10.1038/sj.npp.1301664. [DOI] [PubMed] [Google Scholar]
- Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology. 2003;28:1760–1769. doi: 10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- Schneider M, Schomig E, Leweke FM. Acute and chronic cannabinoid treatment differentially affects recognition memory and social behavior in pubertal and adult rats. Addict Biol. 2008;13:345–357. doi: 10.1111/j.1369-1600.2008.00117.x. [DOI] [PubMed] [Google Scholar]
- Tseng AH, Craft RM. Sex differences in antinociceptive and motoric effects of cannabinoids. Eur J Pharmacol. 2001;430:41–47. doi: 10.1016/s0014-2999(01)01267-5. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Viveros MP, Llorente R, Moreno E, Marco EM. Behavioural and neuroendocrine effects of cannabinoids in critical developmental periods. Behav Pharmacol. 2005;16:353–362. doi: 10.1097/00008877-200509000-00007. [DOI] [PubMed] [Google Scholar]
- Viveros MP, Marco EM, Lopez-Gallardo M, Garcia-Segura LM, Wagner EJ. Framework for sex differences in adolescent neurobiology: a focus on cannabinoids. Neurosci Biobehav Rev. 2011;35:1740–1751. doi: 10.1016/j.neubiorev.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros MP, Llorente R, Suarez J, Llorente-Berzal A, Lopez-Gallardo M, Rodriguez de Fonseca F. The endocannabinoid system in critical neurodevelopmental periods: sex differences and neuropsychiatric implications. J Psychopharmacol. 2012;26:164–176. doi: 10.1177/0269881111408956. [DOI] [PubMed] [Google Scholar]
- Wakley AA, Craft RM. Antinociception and sedation following intracerebroventricular administration of Delta-tetrahydrocannabinol in female vs. male rats. Behav Brain Res. 2011;216:200–206. doi: 10.1016/j.bbr.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Wetherington CL. Sex-gender differences in drug abuse: a shift in the burden of proof? Exp Clin Psychopharmacol. 2007;15:411–417. doi: 10.1037/1064-1297.15.5.411. [DOI] [PubMed] [Google Scholar]
- Wiley JL. Sex-dependent effects of delta 9-tetrahydrocannabinol on locomotor activity in mice. Neurosci Lett. 2003;352:77–80. doi: 10.1016/j.neulet.2003.08.050. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Jones AR, Wright MJ., Jr Exposure to a high-fat diet decreases sensitivity to Delta(9)-tetrahydrocannabinol-induced motor effects in female rats. Neuropharmacology. 2011a;60:274–283. doi: 10.1016/j.neuropharm.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, O'Connell MM, Tokarz ME, Wright MJ., Jr Pharmacological effects of acute and repeated administration of delta-9-tetrahydrocannabinol in adolescent and adult rats. J Pharmacol Exp Ther. 2007;320:1097–1105. doi: 10.1124/jpet.106.108126. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Matthew Walentiny D, Vann RE, Baskfield CY. Dissimilar cannabinoid substitution patterns in mice trained to discriminate delta-9-tetrahydrocannabinol or methanandamide from vehicle. Behav Pharmacol. 2011b;22:480–488. doi: 10.1097/FBP.0b013e328348eced. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson EM, Evans FJ. Cannabinoids in clinical practice. Drugs. 2000;60:1303–1314. doi: 10.2165/00003495-200060060-00005. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, Daniel JM, Filipeanu CM, Leonard ST, Hulst JL, Rodgers SP, Lassen-Greene CL, Sutton JL. Long-term behavioral and pharmacodynamic effects of delta-9-tetrahydrocannabinol in female rats depend on ovarian hormone status. Addict Biol. 2011;16:64–81. doi: 10.1111/j.1369-1600.2010.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, Yang HJ, Bi GH, Li J, Gardner EL. Brain cannabinoid CB2 receptors modulate cocaine’s actions in mice. Nat Neurosci. 2011;14:1160–1166. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]