Abstract
Leishmania parasites are intracellular protozoans capable of salvaging and remodeling lipids from the host. To understand the role of lipid metabolism in Leishmania virulence, it is necessary to characterize the enzymes involved in the uptake and turnover of phospholipids. This study focuses on a putative phospholipase A2 (PLA2)/platelet-activating factor acetylhydrolase (PAF-AH) in L. major. In mammals, PAF-AH is a subgroup of PLA2 catalyzing the hydrolysis/inactivation of platelet-activating factor (PAF), a potent mediator of many leukocyte functions. By immunofluorescence microscopy, L. major PLA2/PAF-AH is predominantly localized in the ER. While wild type L. major parasites are able to hydrolyze PAF, this activity is completely absent in the PLA2/PAF-AH-null mutants. Meanwhile, deletion of PLA2/PAF-AH had no significant effect on the turnover of common glycerophospholipids such as phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, and phosphatidylglycerol. PLA2/PAF-AH is not required for the growth of L. major parasites in culture, or the production of GPI-anchored virulence factors. Nonetheless, it does play a key role in the mammalian host as the PLA2/PAF-AH null mutants exhibit attenuated virulence in BALB/c mice. In conclusion, these data suggest that Leishmania parasites possess a functional PAF-AH and the degradation of PAF or PAF-like lipids is an important step in infection.
Keywords: Phospholipase, virulence, Leishmania, platelet-activating factor, PAF-AH, macrophage
1. Introduction
Leishmania parasites are Trypanosomatid protozoans which cause a range of diseases from self-limiting cutaneous skin lesion to lethal, visceral infection that damages the spleen, liver, and bone marrow [1]. It is estimated that 350 million people in 88 countries are at risk and 12 million people are already infected by Leishmania [2]. Current prevention and treatment strategies are often ineffective with harmful side effects [3, 4]. To develop new drugs and vaccines, it is necessary to understand the molecular interaction between host cells and parasites.
In the vector host (sandfly), Leishmania parasites proliferate extracellularly as flagellated promastigotes. In the mammalian host, they grow and replicate inside the parasitophorous vacuole of macrophages as non-flagellated amastigotes [5]. The parasitophorous vacuole is formed after phagocytosis and constitutes a permissive environment for Leishmania to gain nutrients and interact with the host [6]. It is known that Leishmania amastigotes can salvage and remodel host lipids [7–9]. Recently, we characterized an inositol phosphosphingolipid phospholipase C-like protein (ISCL) in L. major which is responsible for the degradation of both sphingomyelin (a prevalent sphingolipid in mammals) and inositol phosphorylceramide (the dominant sphingolipid in Leishmania) [10]. Deletion of ISCL led to a mild defect in morphology during the promastigote phase [10]. Importantly, these ISCL-null mutants were hypersensitive to acidic pH [11] and lacked virulence in mice [10]. Infectivity was restored when ISCL-null mutants were complemented with a functional sphingomyelinase [10, 12]. These findings indicate that the turnover of host sphingolipids is essential for Leishmania survival in mammals.
In addition to sphingomyelin, other types of recycled lipids, such as phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), and phosphatidylglycerol (PG) should also be plentiful in the parasitophorous vacuole. Given the importance of ISCL in Leishmania virulence, it is of interest to investigate the enzymes responsible for the salvage and turnover of these lipid molecules. In most organisms, a diverse family of phospholipases including phospholipase A1 (PLA1), phospholipase A2 (PLA2), phospholipase C, and phospholipase D are involved in the degradation and remodeling of glycerophospholipids. These enzymes have a broad range of functions from membrane repair to generation of signaling molecules. In mammals, PLA1 (EC 3.1.1.32) and PLA2 (EC 3.1.1.4) can produce bioactive lipids such as lysophospholipids and arachidonic acid [13–17]. PLA1 hydrolyzes the sn-1 acyl esters from glycerophospholipids and has been detected in many cell types. PLA2 specifically catalyzes the release of fatty acid at sn-2 position to generate a free fatty acid and a lysophospholipid. There are four major types of PLA2: secreted PLA2, cytosolic PLA2, calcium-independent PLA2, and platelet-activating factor acetylhydrolase (PAF-AH) [18]. Secreted PLA2s are characterized by a low molecular weight (13–19 KDa) and a large number of disulfide bonds [13]. Intracellular and calcium-independent PLA2s (85–88 KDa) are responsible for cell signaling by generating arachidonic acid, a precursor of eicosanoids (including prostaglandins and leukotrienes) which regulate inflammation and immunity [19, 20].
Another type of PLA2 is PAF-AH (EC 3.1.1.47), which removes the sn-2 acetyl group from platelet-activating factor (PAF) to generate lysophosphocholine and acetate. In animals, PAF (1-alkyl-2-acetyl-sn-glycero-3-phosphocholine) [21] is a bioactive molecule produced by activated platelets, leukocytes, and endothelial cells [22, 23]. In addition to promoting platelet aggregation, PAF is a powerful pro-inflammatory signal involved in many leukocyte functions [22, 23]. By removing the sn-2 acetyl group, PAF-AH eliminates the biological activity of PAF and therefore regulates the homeostasis of PAF [24].
Genes encoding putative phospholipases are present in the L. major genome with syntenic homologues in other Leishmania species and related Trypanosomatids such as Trypanosoma brucei and Trypanosoma cruzi (Table 1). In T. brucei, a PLA1 has been cloned and biochemically characterized as a cytosolic protein (35 KDa) that prefers PC as a substrate and is crucial for the production of lysophosphatidylcholine [25–27]. PLA2 activity has also been described in T. brucei which can be stimulated by Ca2+ and regulates the release of arachidonic acid [28, 29]. In T. cruzi, PLA activities have been detected in whole cell lysate and potential substrates include phosphatidylinositol and inositol phosphorylceramide [30]. In Toxoplasma gondii, an Apicomplexan parasite, a secreted PLA2 is involved in the host cell invasion process [31–33]. Reports on the role of PLA (both endogenous and exogenous) in Leishmania species have also begun to emerge [34–36].
Table 1.
Predicted phospholipase genes in L. major
| Annotation | Tritryp DB ID | References |
|---|---|---|
| PLA1, putative | LmjF.31.2460 | [34]; [25]; [26] |
| PLA2/PAF-AH | LmjF.35.3020 | This study |
| Phospholipase C (PLC)-like protein | LmjF.30.2950 | None |
| Phosphatidylinositol-specific PLC-like protein | LmjF.35.0040 | None |
| Phosphoinositide-specific PLC, putative | LmjF.22.1680 | None |
| Lysophospholipase, putative | LmjF.24.1840 | None |
Syntenic orthologues for these putative phospholipase genes are present in L. braziliensis, L. infantum, T. brucei, and T. cruzi.
In this study, we focused on a putative PLA2 from L. major which shows significant homology to the cytosolic PAF-AH in mammals. We tested whether this PLA2/PAF-AH possessed any activity against PAF or common phospholipids (such as 1,2-diacyl-glycerophospholipids with C16–18 in fatty acyl chain length). Previous reports suggest that PAF may inhibit Leishmania survival in macrophages by activating nitric oxide (NO) synthesis [37, 38]. The leishmanial PLA2/PAF-AH may thus serve as the degradative enzyme to inactivate PAF. Alternatively, this enzyme may be responsible for the degradation and/or remodeling of glycerophospholipids which are important membrane components and anchors for surface glycoconjugates. Results from this study should provide new insight into the role of lipid metabolism in Leishmania-host interaction.
2. Materials and methods
2.1 Materials
1-O-hexadecyl-2-acetyl-3H[N]-sn-glycero-3-phosphocholine ([3H]-PAF, 10–30 Ci/mmol) was purchased from Perkin Elmer Inc. Purified bovine pancreas PLA2 was purchased from Sigma-Aldrich (P 8913). The following fluorescently labeled lipids were purchased from Avanti Polar Lipids: 1-oleoyl-2-[12-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]lauroyl]-sn-glycero-3-phosphoethanolamine (NBD-PE); 1-oleoyl-2-[12-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]lauroyl]-sn-glycero-3-phosphocholine (NBD-PC); 1-oleoyl-2-[12-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]lauroyl]-sn-glycero-3-[phospho-rac-(1-glycerol)] ammonium salt (NBD-PG); 1-oleoyl-2-[12-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]lauroyl]-sn-glycero-3-phosphoserine ammonium salt (NBD-PS); and 12-N-methyl-(7-nitrobenz-2-oxa-1,3-diazo)aminostearic acid (NBD-fatty acid or NBD-FA). These NBD-labeled lipids were used as substrates or TLC standards in the PLA2 assay described in 2.7. All other chemicals were purchased from Fisher Scientifics unless specified otherwise.
2.2 Molecular cloning
The entire open reading frame of PLA2/PAF-AH (1362 bp) was PCR amplified from L. major genomic DNA using primers #37 (GTATCGAGGATCCACCATGCACCCAATCTTCGACTAC) and #38 (ATTCACGGATCCTTACCCCCTCAGCGCAGCGGC). The resulting DNA fragment was digested with BamHI and cloned into the expression site of pXG vector [39] to generate pXG-PLA2 (B118). To generate knockout constructs, the 5’- and 3’-untranslated regions of PLA2/PAF-AH were PCR amplified and cloned into pUC18 vector. Primers #39 (TACGCAGAATTCCTTGTGACGCATGGCCTCTAC) and #40 (TCGATCGGGATCCGTGCAATCTAGATGTGCGCGATGCGTGTGTGC) were used to produce the 5’-flanking region (~0.6 Kb). Primers #41 (ATGCTCGGATCCAGGTTGTTTGCCACTTTAC) and #42 (CATCGTAAGCTTGGCGACGGCGTCTCTGTCGCC) were used to produce the 3’-flanking region (~1.0 Kb). Subsequently, genes conferring resistance to puromycin (PAC) and blasticidin (BSD) were cloned between the 5’- and 3’-flanking regions to generate pUC18-KO-PLA2:PAC (B120) and pUC18-KO-PLA2:BSD (B121), respectively.
For localization studies, PLA2/PAF-AH was fused in frame with a green fluorescence protein (GFP) gene at the C-terminus (to preserve the predicted N-terminal signal peptide sequence) (Fig. 1) to generate PLA2-GFP. To do so, a modified form of PLA2/PAF-AH without stop codon was PCR amplified using primers #37 and #259 (GATCATGGATCCCCCCCTCAGCGCAGCGGCGAGCGC) and cloned into pXG-‘GFP+ [39] to generate pXG-PLA2-GFP (B271). All molecular constructs were confirmed by restriction enzyme digestion and DNA sequencing.
Figure 1. Sequence alignment of PLA2/PAF-AH.
Predicted amino acid sequences of putative PLA2/PAF-AH from T. brucei gambiense (Tbg972.9.7760), T. cruzi (Tc00.1047053510743.60), L. major (LmjF.35.3020), and human PAF-AH2 (NP_000428.2) were aligned using the Clustal Omega (1.0.3) algorithm. Shade area in LmjF.35.3020 represents predicted signal peptide and transmembrane helix. The lipase consensus motif (GXSXG) is underlined. Asterisks (*): fully conserved residues; colons (:): highly similar residues; periods (.): weakly similar residues.
2.3 Leishmania promastigote culture and transfection
L. major LV39 clone 5 (Rho/Su/59/P) promastigotes were cultured at 26 °C in M199 media with 10% heat-inactivated fetal bovine serum and other supplements as previously described [40]. To generate PLA2/PAF-AH-null mutants, DNA knockout constructs (B120 and B121) were linearized by restriction enzymes to sequentially replace the PLA2/PAF-AH alleles in wild type (WT) L. major promastigotes [41]. The resulting pla2− cells (ΔPLA2∷PAC/ΔPLA2∷BSD) were selected based on resistance to puromycin and blasticidin. To confirm the loss of PLA2/PAF-AH, genomic DNA from promastigotes was digested with BglII and HindIII, separated on a 0.7% agarose gel, transferred to a nitrocellulose membrane, and hybridized with a [32P]-labeled DNA probe that hybridized to a ~ 400-bp upstream flanking region of PLA2/PAF-AH. To complement the mutant, pXG-PLA2 or pXG-PLA2-GFP was transfected into pla2− by electroporation and the resulting cells were referred to as pla2−/+PLA2 (ΔPLA2∷PAC/ΔPLA2∷BSD/+pXG-PLA2) or pla2−/+PLA2-GFP (ΔPLA2∷PAC/ΔPLA2∷BSD/+pXG- PLA2-GFP). To prevent the loss of virulence during in vitro culture and genetic manipulation, stationary phase promastigotes of WT, pla2− and pla2−/+PLA2 were passed through BALB/c mice at 1–5 × 107 cells/mouse and recovered one month later [42]. These parasites were then converted back to promastigotes and used in the virulence study described in 2.6.
To determine in vitro growth rates, promastigotes of WT, pla2−, and pla2−/+PLA2 were inoculated in M199 medium at 1.0 × 105 cells/ml and cell densities were measured every 24 hours using a hemacytometer. To measure the percentage of dead cells during stationary phase, parasites were stained with 5.0 µg/ml of propidium iodide and analyzed by flow cytometry [43]. Infective metacyclics were isolated from stationary phase promastigotes using the lectin agglutination method [44] or density centrifugation method [45].
2.4 Immunofluorescence microscopy
In general, immunofluorescence microscopy was performed as previously described [46]. To determine the localization of PLA2-GFP in log phase promastigotes, pla2−/+PLA2-GFP parasites were permeabilized with 100% ethanol on ice and labeled with a rabbit anti-T. brucei BIP polyclonal antibody (1:10,000)[47], followed by a goat anti-rabbit IgG-Texas Red labeled secondary antibody (Life Technologies, 1:2000). Cells were then stained with Hoescht 33342 (2.5 µg/ml) and visualized using an Olympus BX51 Upright Fluorescence Microscope.
The distribution of PLA2-GFP was also examined in amastigotes. Briefly, bone marrow cells were isolated from the femur of female BALB/c mice and differentiated into macrophages (MΦs) using 30 ng/ml of macrophage colony stimulating factor [11]. Stationary phase promastigotes of pla2−/+PLA2-GFP were opsonized with 4% C57BL6 mouse serum and applied to MΦs at a ratio of 10 parasites per MΦ. After incubation at 33 °C for 48 hours, MΦs were fixed with 3.7% formaldehyde and permeabilized with 100% ethanol on ice. PLA2-GFP was then detected using a rabbit anti-GFP serum (Life Technologies, 1:1000) followed by a goat anti-rabbit IgG-Texas Red labeled secondary antibody (Life Technologies, 1:2000).
To label mitochondrion, log phase pla2−/+PLA2-GFP promastigotes were incubated in 250 nM of Mitotracker Red 580 (Life Technologies) in DMEM for 30 minutes at 26 °C and fixed in 3.7% formaldehyde. Parasites were then washed with phosphate buffered saline (PBS), attached to polylysine-coated coverslips, and washed again with 50% cold ethanol (prepared in PBS). Cells were then stained with Hoescht 33342 and visualized as described above.
To determine the localization of LPG or GP63, log phase promastigotes were probed with monoclonal antibodies against LPG (WIC79.3) [48] or GP63 (Cedarlane labs clone 96/26) as previously described [43]. In all immunofluorescence microscopy experiments, we included negative controls which were cells stained by secondary antibody only (to ensure a low background).
2.5 Western blotting
Leishmania promastigotes were inoculated at 1.0 × 105 cells/ml and cultured to log phase (<1.0 × 107 cells/ml) or stationary phase (2.5–3.5 × 107 cells/ml); Cells were harvested by centrifugation (a minimum of 1 × 108 cells were collected for log phase parasites and 4 × 108 cells were collected for stationary phase parasites); culture supernatants were then transferred to new tubes and boiled in 2 × SDS sample buffer (100 mM Tris–HCl, pH 6.8, 4% SDS, 0.2% bromophenol blue, 20% glycerol, and 10% 2-mercaptoethanol); and cells were boiled in 1 × SDS sample buffer (50 mM Tris–HCl, pH 6.8, 2% SDS, 0.1% bromophenol blue, 10% glycerol, and 5% 2-mercaptoethanol) at 5.0 × 107 cells/ml. Boiled cell lysates or culture supernatants were then separated by SDS-PAGE (10% polyacrylamide gel) and transferred to PVDF membranes, followed by blocking with 5% dry milk. To detect PLA2-GFP, the membrane was probed with a rabbit anti-GFP antiserum (Life Technologies, 1:1000), followed by a horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG secondary antibody (Life Technologies, 1:2000). To examine the expression level of GPI-anchored molecules, the membrane was probed with antibodies against LPG or GP63 as previously described [49]. To ensure equal loading, the membrane was also probed with a monoclonal antibody against α-tubulin (Sigma-Aldrich) (1:5000), followed by a HRP-conjugated goat anti-mouse IgG secondary antibody (1:2000). Western blot signals were detected using autoradiography or a FluorChem E imager (Protein Simple).
2.6 Use of mice in virulence study and ethics statement
BALB/c mice (female, 7–8 weeks old) were purchased from Charles River Laboratories International. The use of mice in this study was approved by the Animal Care and Use Committee at Texas Tech University (US PHS Approved Animal Welfare Assurance NO. A3629-01). Mice were housed and cared for in the facility operated by the Animal Care and Resources Center at Texas Tech University. The facility was inspected monthly and animals were monitored daily by staff members. A complete range of clinical veterinary services was available on a 24-hour basis and includes consultation, diagnostic work-up and clinical care. To minimize the pain and distress on animals without compromising the quality of research, mice were under anesthesia (through the peritoneal injection of ketamine hydrochloride/xylazine) during recurring procedures including the injection of Leishmania parasites into footpads, the recovery of parasites from infected mice, and the measurement of lesion size using a caliper. Usually, no more than one procedure was performed on any mouse within a week. Mice were monitored carefully (twice a week for appearance, size, movement, and general health condition) and euthanized by carbon dioxide asphyxiation when footpad lesions became over 2.5 mm or when secondary infections occurred. Mice were also euthanized prior to the isolation of femur cells and limiting dilution assay (to determine parasite load in infected tissue).
Virulence study of pla2− mutants was performed using the mouse footpad infection model as previously described [50]. Parasites that were recently recovered from mice were cultured in vitro for 3 passages and metacyclics were purified from late stationary phase promastigotes using the density centrifugation method [45]. Metacyclics were then resuspened in DMEM and injected into the footpads of 8-week old, female BALB/c mice (1.0 × 105 metacyclics/mouse, 5 mice per group). Footpad thickness was measured weekly using a Vernier caliper. Parasite numbers in infected footpads were determined by limiting dilution assay [50]. Proper restraining and injection techniques were employed by trained lab personnel to reduce pain and distress of animals.
2.7 PAF-AH assay and PLA2 assay
Promastigotes were cultured to the second day in stationary phase and collected by centrifugation. Cells were washed once in PBS and lysed at 4.0 × 108 cells/ml in a PBS-based lysis buffer containing 5 mM of MgCl2, 0.1 % Triton X-100, and 5 mM of dithiothreitol (5 min on ice with sonication). Protein concentrations in parasite lysates were determined using a BCA Protein Assay Kit-Reducing Agent Compatible (Thermo Scientific). The PAF-AH assay was performed as previously described with minor modifications [51]. Briefly, 50 µl of parasite lysate was combined with 60,000 cpm of [3H]-PAF to a final volume of 250 µl containing 50 mM of Tris-HCl and 5 mM of EDTA (pH 7.5). After one hour incubation at 37 °C, the reaction was terminated by adding 250 µl of water and 2.5 ml of chloroform:methanol (4:1, v/v). [3H]-acetate was collected in the aqueous phase after extraction and analyzed by scintillation counting. The amounts of [3H]-acetate (product), [3H]-PAF (substrate), and protein were used to calculate the PAF-AH activity in each sample (pmol/[mg × hour]). Negative control: 50 µl of boiled WT lysate (100 °C for 10 minutes). Positive Control: 10 µl BALB/c mouse serum diluted in lysis buffer to a final volume of 50 µl.
The PLA2 assay on glycerophospholipids was adopted from a previous report on the Ca2+-dependent cytosolic PLA2 from zebrafish [52]. Briefly, 50 µl of parasite lysate was combined with 0.5 nmole of NBD-labeled phospholipid substrate (PE, PC, PS, or PG; stocks for phospholipids were prepared in methanol) to a final volume of 250 µl containing 13.7 mM of NaCl, 0.537 mM of KCl, 0.025 mM of Na2HPO4, 0.044 mM of KH2PO4, 1.30 mM of CaCl2, 1 mM of MgCl2, and 4.2 mM of NaHCO3 (pH 7.2). After one hour incubation at 37 °C, the reaction was stopped by the addition of 250 µl of water and 2.5 ml of chloroform:methanol (4:1, v/v), followed by vortex for 30 seconds. The aqueous phase was then removed after centrifugation (1500 g for 10 minutes). The organic phase was dried under a steady stream of air and dissolved in 15 µl of chloroform: methanol (2:1, v/v). Lipids in the organic phase were then spotted onto a thin layer chromatography (TLC) plate (silica gel 60) and separated using toluene:diethyl ether:ethanol:acetic acid (50:40:2:0.2, v/v/v/v) as solvent. Fluorescent lipids on TLC plates were detected and quantified using a Storm 860 phosphoimager. The ratio of NBD-fatty acid/(NBD-FA + NBD-phospholipid) was used to calculate the amount of NBD-FA in each reaction. The apparent PLA2 activity was then calculated based on the amount of NBD-FA and total protein in each sample (nmol/[mg × hour]). Negative control: 50 µl of boiled WT lysate (100 °C for 10 minutes) or lysis buffer only. Positive control: 1.0 unit of purified bovine PLA2 diluted in lysis buffer to a final volume of 50 µl.
2.8 Statistical analysis
One-way ANOVA and pair-wise comparisons using the Holm-Sidak correction were used to compare PAF-AH activity levels.
3. Results
3.1 Identification and targeted deletion of a putative PLA2/PAF-AH in L. major
In addition to the previously described ISCL [10], Leishmania parasites possess other phospholipases. Mining the L. major genome revealed ortholog genes for two phospholipase C, one lysophospholipase, one PLA1, and one PLA2/PAF-AH (Table 1). As the focus of this study, L. major PLA2/PAF-AH (LmjF35.3020) is a 453-amino acid protein with 27–29% identity and 43–49% similarity to the cytoplasmic isoform 2 of mammalian PAF-AH (Fig. 1). Its N-terminus has a predicted signal peptide sequence followed by a transmembrane helix (the shaded portion in Fig. 1). Along with its syntenic orthologs in T. brucei and T. cruzi, L. major PLA2/PAF-AH possesses a GXSXG motif (underlined in Fig. 1) that is characteristic of serine-dependent neutral lipases [20] including the human intracellular PAF-AH2 (Fig. 1) [53].
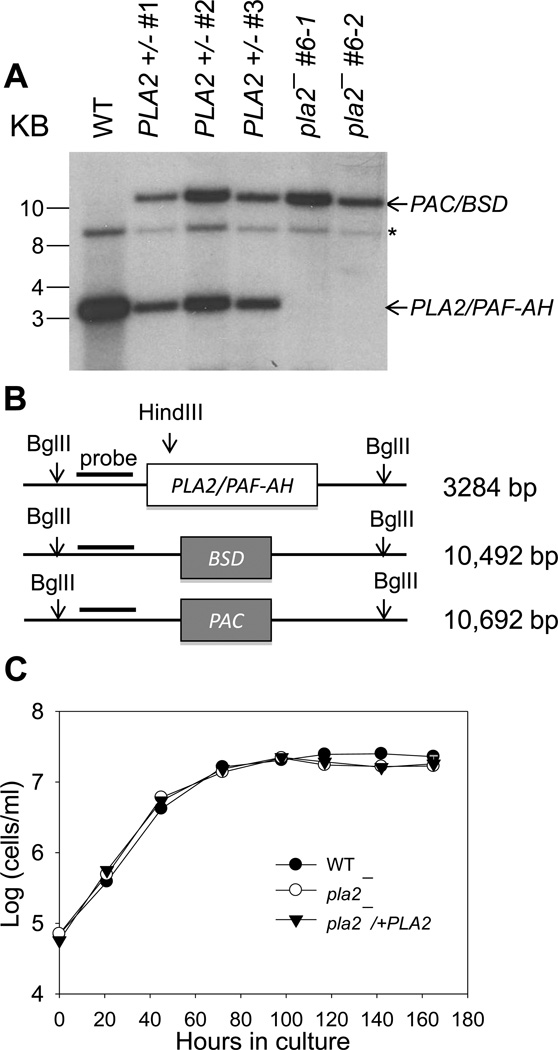
To evaluate its role, we generated the PLA2/PAF-AH-knockout mutant in L. major (referred to as pla2−) through targeted gene deletion. Southern blot analysis confirmed the loss of PLA2/PAF-AH alleles in the homozygote mutants (Fig. 2A–B). Two independent knockout clones (pla2− #6-1 and pla2− #6-2, Fig. 2A) exhibited very similar phenotypes and the data for pla2− #6-1 and its complemented control (referred to as pla2−/+PLA2) are presented here. In culture, pla2− and pla2−/+PLA2 parasites were fully viable and grew at a nearly identical rate as WT L. major (Fig. 2C). We also measured the differentiation of pla2− parasites into infective metacyclics during stationary phase using the peanut aggluntinin method [54] and results showed no significant difference from WT promastigotes (data not shown). Therefore, PLA2/PAF-AH is not essential for the survival, replication, or differentiation of L. major in culture.
Figure 2. L. major pla2− mutants grow normally as promastigotes in culture.
(A–B) Southern blot showing the replacement of PLA2/PAF-AH alleles with marker genes (PAC/BSD) in pla2−. Genomic DNAs from WT, PLA2+/− (heterologous parasites in which one of the two PLA2/PAF AH alleles has been deleted), and pla2− promastigotes were digested with BglII and HindIII and resolved on an agarose gel. Following transfer to a PVDF membrane, a radiolabeled DNA probe was used to detect the PLA2/PAF-AH allele (~3.3 Kb) and the PAC/BSD replacement alleles (10.5–10.7 Kb). (A) Result from autoradiography. The asterisk represents a non-specific band recognized by the probe. For pla2−, BSD and PAC replacements appear as one band due to the similarity in size. (B) Schematic representation of the Southern blot. (C) Promastigotes were inoculated in M199 medium and culture density was determined daily using a hemacytometer. Error bars represent standard deviations from 2 experiments.
3.2 PLA2/PAF-AH is not required for the degradation of glycerophospholipids
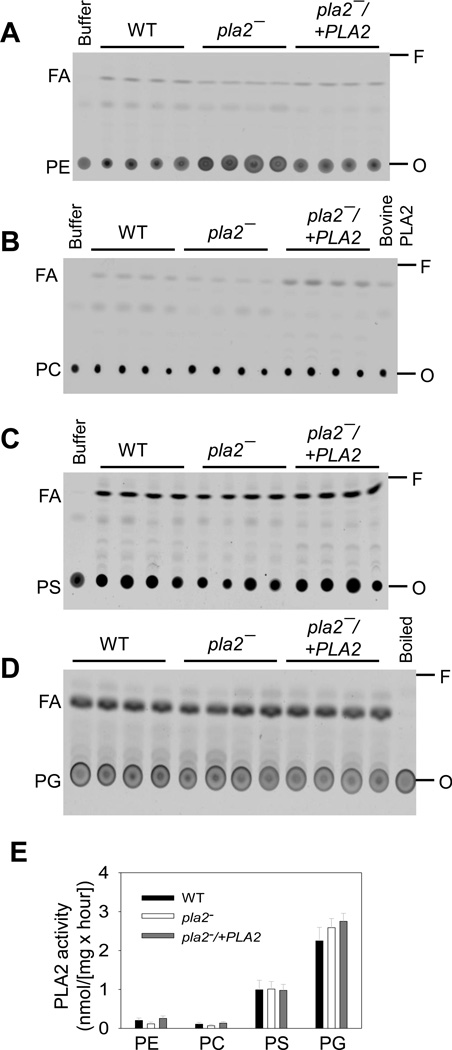
Because L. major PLA2/P AF-AH is annotated as a putative phospholipase, we examined whether it was involved in the degradation of glycerophospholipids such as PE, PC, PG and PS. To validate our PLA2 assay, a purified bovine PLA2 was incubated with a fluorescent PC (the fatty acyl chain at the sn-2 position was conjugated to NBD) to generate an unlabeled lyso-PC and a NBD-labeled fatty acid. Thin layer chromatography (TLC) was then used to separate the NBD-labeled PC (substrate) from the NBD-labeled fatty acid (product) because PC migrated much slower than fatty acid (the right 2 lanes in Fig. S1). Clearly, the purified bovine PLA2 caused the release of fatty acid from the sn-2 position of PC whereas reaction buffer alone did not (the left 2 lanes in Fig. S1), indicating that this assay could detect PLA2 activity.
Next, whole cell lysates from WT, pla2− and pla2−/+PLA2 promastigotes were assessed for PLA2 activity against NBD-labeled PE, PC, PS, or PG under the same condition as described for the purified PLA2. As shown in Fig. 3A–D, L. major parasites possessed a detectable level of PLA2 activity against PE, PC, PS, and PG. As expected, negative controls using lysis buffer only in Fig. 3A–C) or boiled WT lysate (in Fig. 3D) failed to show any PLA2 activity. The apparent PLA2 activity in Leishmania cell lysates against various phospholipid substrates was calculated based on the amount of fatty acid produced and the amount of protein in each reaction (Fig. 3E). Importantly, no significant difference in PLA2 activity was found among WT, pla2− and pla2−/+PLA2 parasites (Fig. 3E), suggesting that L. major PLA2/PAF-AH is not involved in the degradation of regular glycerophospholipids. Therefore, other enzyme(s) must be responsible for these activities. Meanwhile, parasite lysates exhibited a higher level of PLA2 activity towards PS and PG than PE and PC (Fig. 3E). It is not clear whether this is due to the substrate specificity or expression level of specific leishmanial lipases.
Figure 3. L. major parasites possess PLA2 activity against glycerophospholipids.
Whole cell lysates of WT, pla2−, and pla2−/+PLA2 parasites were incubated with NBD-PE (A), NBD-PC (B), NBD-PS (C), or NBD-PG (D). Lipid products were separated from the substrates by TLC under the condition described in Materials and Methods (2.7). In A–D, four replicates are shown for each Leishmania sample. Negative controls: buffer only (A–C) or boiled WT lysate (D). Positive control: 1.0 unit of bovine PLA2 (B). Arrows indicate the direction of migration on TLC. FA: fatty acid. O: origin. F: solvent front. (E) Apparent PLA2 activity was quantified based on the amount of NBD-labeled fatty acid produced from each reaction and the amount of protein in each sample. Error bars represent standard deviations from 2 experiments with 4 samples each time.
3.3 PLA2/PAF-AH is responsible for the PAF-AH activity in L. major
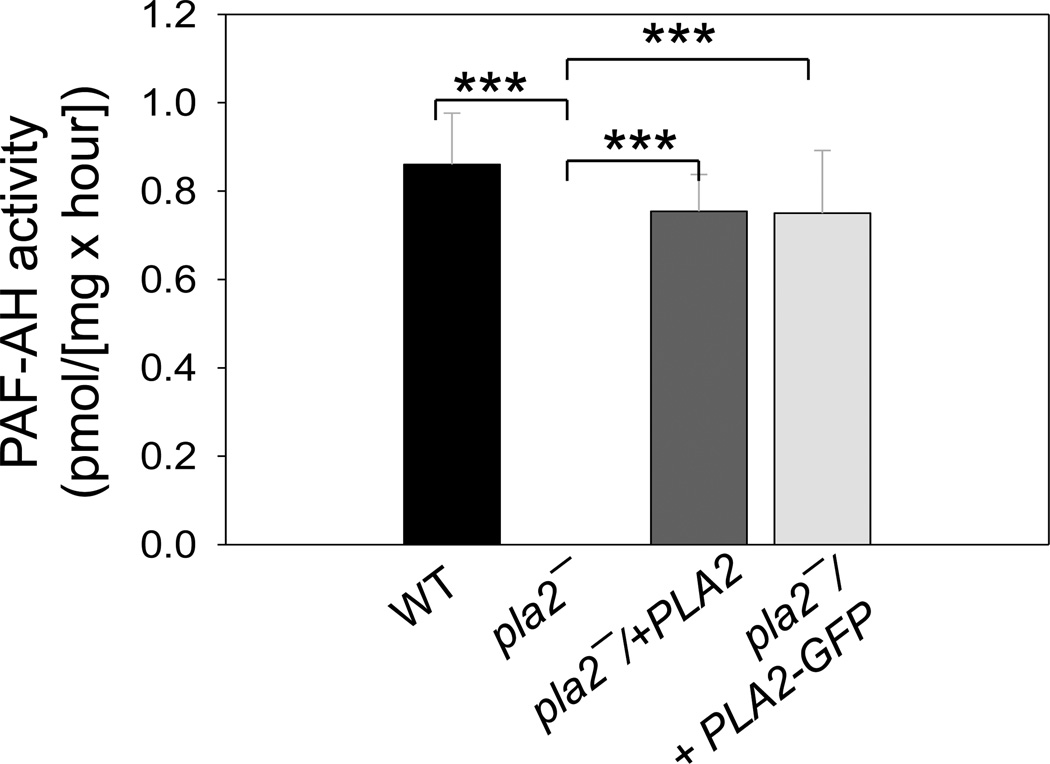
Because L. major PLA2/PAF-AH is similar to the PAF-AH2 in mammals (Fig. 1), we examined whether it can hydrolyze PAF, a special type of phospholipid (1-alkyl-2-acetyl-sn-glycero-3-phosphocholine) synthesized by immune cells to activate inflammation and platelet aggregation. To measure PAF-AH activity, Leishmania whole cell lysates were incubated with radiolabeled PAF (the acetyl-group at the sn-2 position was labeled with [3H]). After extraction, the amount of radioactive acetate in the aqueous phase was determined in each sample. As summarized in Fig. 4 and Table S1, WT and pla2−/+PLA2 parasites exhibited detectable levels of PAF-AH activity, which was absent in pla2− or boiled WT cells. These results suggest that PLA2/PAF-AH is responsible for the degradation of PAF in L. major.
Figure 4. PLA2/PAF-AH is responsible for the PAF-AH activity in L. major.
Promastigote lysates were incubated with radiolabeled PAF and the release of acetate was measured as described in Materials and Methods (2.7). Results were calculated after subtracting the value from boiled WT lysate (raw data were summarized in Table S1). Error bars represent standard deviations from 4 repeats. ***: p<0.001 (compared to pla2−).
3.4 PLA2/PAF-AH is important for L. major infection in BALB/c mice
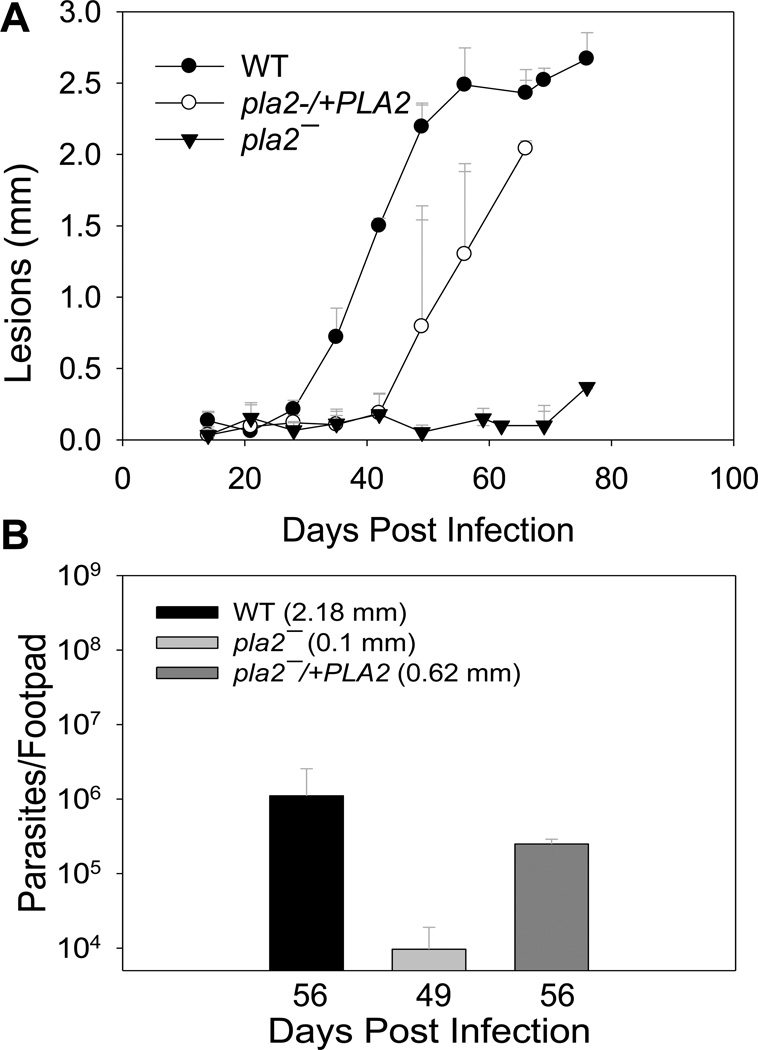
The lack of pleiotropic defects from pla2− promastigotes allowed us to examine whether PLA2/PAF-AH was required for parasite proliferation and pathogenesis in the mammalian host. To do so, metacyclic parasites of WT, pla2−, or pla2−/+PLA2 were inoculated into the footpads of BALB/c mice, which are susceptible to L. major infection. As shown in Fig. 5A, mice infected by WT parasites developed lesions that grew rapidly in size. In comparison, mice infected by pla2− parasites showed a significant delay in lesion development (Fig. 5A). With restored PLA2/PAF-AH expression, pla2−/+PLA2 parasites exhibited much improved virulence than pla2− (Fig. 5A). We also examined the number of parasites in the footpads of mice infected by WT, pla2−, or pla2−/+PLA2. As illustrated in Fig. 5B, lesion sizes showed a positive correlation with parasite numbers in infected footpads. Together, these data indicate that PLA2/PAF-AH is important for L. major to efficiently establish infection and proliferate in mice.
Figure 5. Pla2− parasites exhibit attenuated virulence.
BALB/c mice were infected with purified metacyclic parasites (105/mouse) in the left footpads. (A) Lesion sizes were measured weekly to monitor the progression of pathology. (B) Limiting dilution assay was conducted at the indicated times to determine parasite numbers in infected footpads (lesion sizes are indicated). Error bars represent standard deviations.
3.5 PLA2/PAF-AH is not required for the synthesis and targeting of GPI-anchored molecules
In Leishmania promastigotes, the dominant surface virulence factor is a heterogeneous glycoconjugate called lipophosphoglycan or LPG [55, 56]. Because LPG has a 1-alkyl-2-lyso-phosphatidylinositol anchor which could be generated from the activity of a PLA2, we tested whether the cellular abundance of LPG was altered in pla2− or pla2−/+PLA2 parasites by western blot using a LPG-specific monoclonal antibody [48]. As illustrated in Fig. S2 (results from two experiments were shown), deletion of PLA2/PAF-AH had no obvious effect on the cellular level of LPG or GP63, a GPI-anchored metalloprotease with 1-alkyl-2-acyl phosphatidylinositol anchor [57, 58]. We also examined the localization of LPG and GP63 by immunofluorescence microscopy. In WT promastigotes, LPG was predominantly found on the plasma membrane while GP63 was detected both on the plasma membrane and at intracellular locations which probably represent the Golgi (Fig. S3A–B). Very similar results were observed in pla2− or pla2−/+PLA2 parasites (Fig. S3A–B). Therefore, PLA2/PAF-AH is probably not required for the synthesis or targeting of GPI-anchored virulence factors.
3.6 Localization of PLA2/PAF-AH in promastigotes and amastigotes
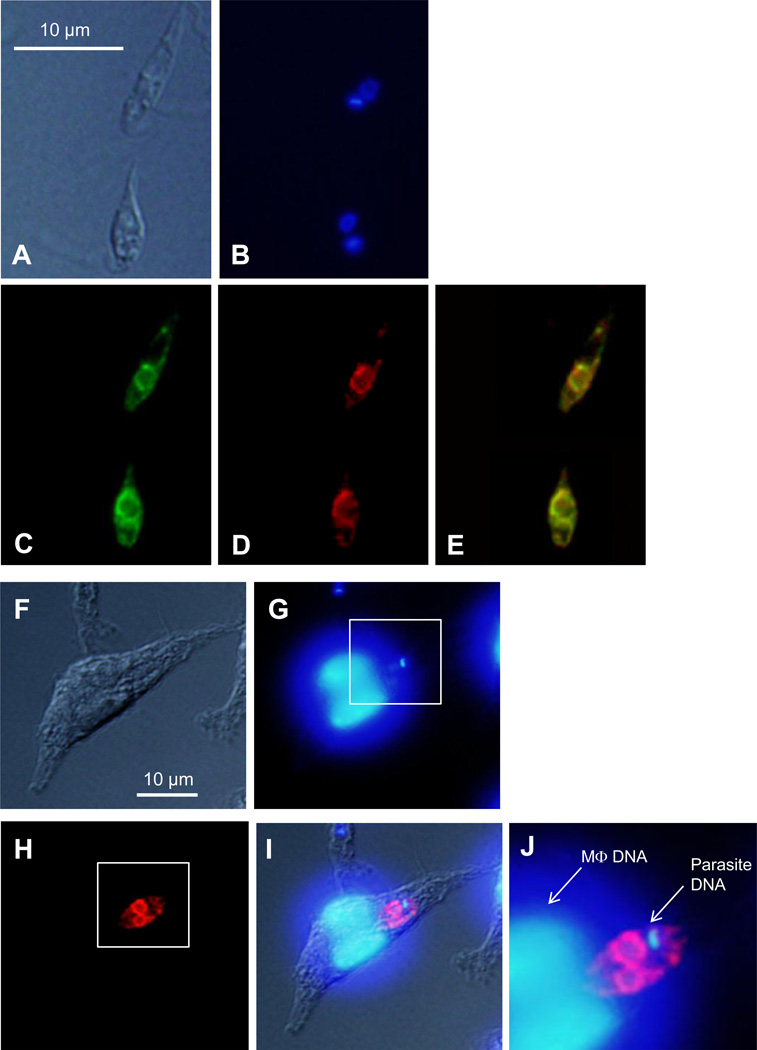
To determine the localization of PLA2/PAF-AH, we introduced a PLA2-GFP chimera into pla2− mutants. Western blot (using an anti-GFP antibody) revealed a ~69 KDa protein in the whole cell lysate but not the culture supernatant of pla2−/+PLA2-GFP promastigotes (Fig. S4), suggesting that PLA2-GFP is not secreted. As shown in Fig. 4 and Table S1, pla2−/+PLA2-GFP parasites had a near WT-level of PAF-AH activity, indicating that the PLA2-GFP fusion protein was functional. In promastigotes, we observed an uneven, intracellular distribution of PLA2-GFP extending from the peri-nuclear region (Fig. 6A–C). Co-staining with an anti-T. brucei BIP antibody (an endoplasmic reticulum or ER marker) [47] revealed significant overlap (Fig. 6D–E), suggesting that PLA2-GFP is primarily located at the ER. We also labeled promastigotes with Mitotracker Red 580 but did not detect any significant overlap between PLA2-GFP and mitochondrion (Fig. S5).
Figure 6. Localization of PLA2/PAF-AH in L. major.
(A–E) Log phase promastigotes of pla2−/+PLA2-GFP were subjected to immunofluorescence microscopy as described in Materials and Methods (2.4). (A) DIC image. (B) DNA staining with Hoechst 33342. (C) PLA2-GFP epifluorescence. (D) Immuno-staining with anti-BIP antibody to label ER. (E) Merge of C and D. (F–J) BM-MΦs were infected with pla2−/+PLA2-GFP parasites for 48 hours and subjected to immunofluorescence microscopy as described in Materials and Methods (2.4). (F) DIC image of an infected MΦ. (G) DNA staining with Hoescht 33342. (H) Immuno-staining with rabbit anti- GFP serum followed by goat anti-rabbit IgG conjugated with Texas Red. (I) Merge of F, G, and H. (J) Merged and magnified image of the boxed areas in G and H.
Finally, we examined the localization of PLA2/PAF-AH in the mammalian stage of L. major (Fig. 6F–J). Bone marrow-derived MΦs (from BALB/c mice) were infected with pla2−/+PLA2-GFP parasites for 48 hours. The distribution of PLA2-GFP in intracellular amastigotes was then determined by immunofluorescence microscopy using an anti-GFP antibody. As illustrated in Fig. 6H-J, the pattern of anti-GFP staining was quite similar to what was observed in pla2−/+PLA2-GFP promastigotes (Fig. 6A–C). Together, our results suggest that L. major PLA2/PAF-AH is an intracellular enzyme that was mainly targeted to the ER.
4. Discussion
Recent studies have highlighted the essentiality of ISCL, a developmentally regulated neutral sphingomyelinase, in L. major virulence [10, 12]. Based on these reports, it may be of interest to characterize other enzymes involved in the degradation of phospholipids synthesized by the host or Leishmania. In light of this, a group of putative phospholipases were identified from the L. major genome and among them is a PLA2/PAF-AH. Despite the lack of a canonical ER retention sequence, PLA2/PAF-AH is primarily found in the ER of promastigotes and amastigotes (Figs. 1 and 6), suggesting that this enzyme may utilize a novel signal. To characterize PLA2/PAF-AH, we first examined the PLA2 activity in L. major cell lysates on several glycerophospholipids using an assay previously developed for the intracellular PLA2 in zebra fish [52]. Results clearly showed that L. major parasites can degrade PC, PE, PS and PG by releasing fatty acids from the sn-2 position, indicating that they possess at least one functional PLA2 (Fig. 3). Since our assay was performed using whole cell lysate and diluted phospholipid substrates in an aqueous environment which might limit the performance of certain phospholipases, the actual PLA2 activity in L. major may be significantly higher than the observed value. Nonetheless, deletion of PLA2/PAF-AH has little impact on this activity (Fig. 3), suggesting that other lipases are responsible for the degradation and remodeling of glycerophospholipids. In addition, pla2− promastigotes exhibit no apparent defects in the synthesis or targeting of GPI-anchored virulence factors such as LPG (Figs. S2–3). One possibility is that PLA2/PAF-AH is not required for the generation of the 1-alkyl-2-lyso phosphatidylinositol anchor of LPG, i.e. phosphatidylinositol is not a substrate of PLA2/PAF AH. Alternatively, the LPG anchor in pla2− parasites may be somewhat altered which may be determined by mass spectrometry-based analysis.
Besides glycerophospholipids, Leishmania parasites ca n also hydrolyze PAF and this activity is clearly dependent on PLA2/PAF-AH (Fig. 4). In culture, pla2− mutants grow and differentiate normally (Fig. 2C) so PAF degradation is not essential for the promastigote stage of L. major. Effects of PAF on Leishmania infection of MΦs (their definitive host cells) have been investigated by several groups. When MΦs were treated with PAF, L. amazonensis infection was inhibited (by ~3 folds) [37, 38]. Mechanism of PAF’s action is not well understood. It may induce NO production from MΦs and therefore inhibit Leishmania infection and intracellular multiplication [37, 38]. The reduced virulence of pla2− mutants in BALB/c mice (Fig. 5) is consistent with previous reports on the anti-Leishmania effect of PAF [37, 38]. It is possible that PLA2/PAF-AH can regulate the level of PAF and/or lyso-PC during Leishmania infection to facilitate parasite survival by reducing inflammatory responses.
In addition to its effect on PAF, PLA2/PAF-AH may possess other unidentified functions that contribute to Leishmania infection. It is known that mammalian PAF-AHs (including the cytoplasmic isoform II that shares significant homology to PLA2/PAF-AH; Fig. 1) can hydrolyze substrates with longer chains (>2) at the sn-2 position including some oxidized phospholipids [53, 59, 60]. Some of these PAF-like lipids can induce inflammation and may act via the PAF receptor [61, 62]. In this study, the release of PLA2-GFP by intact promastigotes is undetectable (Fig. S4), raising the question of how an intracellular enzyme may gain access to host PAF or PAF-like lipids. One possibility is that endocytosed host lipids may interact with PLA2/PAF-AH via the endosome-ER network [63]. Alternatively, PLA2/PAF-AH released from lysed parasites may facilitate the survival of viable parasites in mammals. Another possibility is that this enzyme has endogenous targets. It is not known whether Leishmania parasites can synthesize PAF or PAF-like lipids, although it has been reported that promastigotes pre-treated with PAF infect macrophages more efficiently than untreated promastigotes [64, 65]. The mechanism of such an infection-enhancing effect (which is contrary to the effect of PAF treatment on macrophages) is not clear, although PAF could stimulate the activity of protein kinases secreted by Leishmania [64].
In summary, we have shown that L. major possesses a functional PAF-AH which is important for virulence in mammals. Future study may include the characterization of active, recombinant enzyme and identification of its endogenous target. Since homologues of PLA2/PAF-AH are present in other trypanosomatids, a better understanding of its function will likely shed new light into the role of lipid metabolism in this group of medically important pathogens.
Supplementary Material
0.4 unit of purified PLA2 (from bovine pancreas) was incubated with NBD-PC for 1 hour and the release of NBD-labeled fatty acid (FA) was detected by TLC as described in Materials and Methods (2.7). NBD-FA (FA standard) and NBD-PC (PC standard) were included in the TLC to illustrate the migration of FA and PC. The arrow indicates the direction of TLC. O: origin. F: solvent front.
Whole cell lysates from stationary phase day 1 (A) and day 3 (B) promastigotes were separated on SDS-PAGE (5 × 105 cells per lane) and probed with antibodies against LPG, GP63, or α-tubulin (to control loading). The numbers indicate relative signal intensity acquired using a FluorChem E imager.
WT, pla2−, and pla2−/+PLA2 promastigotes were labeled with monoclonal antibodies against LPG (A) or GP63 (B), followed by incubation with FITC-labeled goat anti-mouse IgG secondary antibody. Scale bars = 10 µm.
Whole cell lysates (1 × 106 cells per lane) and culture supernatant (10 µl per lane) from log phase promastigotes were subjected to western blot analysis using an anti-GFP antibody. The PLA2-GFP fusion protein was detected in the whole cell lysate of pla2−/+PLA2-GFP at the predicted molecular weight (~69 KDa) but not in the culture supernatant.
Log phase promastigotes of pla2−/+PLA2-GFP were stained with Mitotracker 580 and visualized by microscopy as described in Materials and Methods (2.7). (A) DIC image; (B) DNA staining with Hoechst 33342; (C) PLA2-GFP epifluorescence; (D) Staining result with Mitotracker; (E) Merge of C and D.
Research highlights.
L. major parasites possess a functional PAF-AH.
PAF-AH is not required for the degradation of common glycerophospholipids.
PAF-AH is not required for parasite growth or differentiation in culture.
Deletion of PAF-AH leads to attenuated virulence in mice.
Acknowledgements
We thank Drs. K.P. Chang (Rosalind Franklin University of Medicine and Science) and Jay Bangs (University of Wisconsin Madison) for providing us the rabbit anti-Leishmania GP63 antiserum and the rabbit anti-T. brucei BIP antiserum, respectively. This work was funded by US Public Health Service grants 1R15AI076909 (KZ), 1R56AI081781 (KZ) and 5R03AI076662 (KZ) from the National Institute of Allergy and Infectious Diseases.
Abbreviations
- PLA2
phospholipase A2
- PAF-AH
platelet-activating factor acetylhydrolase
- PAF
platelet-activating factor
- ISCL
inositol phosphosphingolipid phospholipase C-like protein
- NBD
7-nitro-2-1,3-benzoxadiazol-4-yl
- PAC
puromycin resistance gene
- BSD
blasticidin resistance gene
- TLC
thin layer chromatography
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PS
phosphatidylserine
- PG
phosphatidylglycerol
- LPG
lipophosphoglycan
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Note: Supplementary data associated with this article
References
- 1.Desjeux P. Leishmaniasis. Nat Rev Microbiol. 2004;2(9):692. doi: 10.1038/nrmicro981. [DOI] [PubMed] [Google Scholar]
- 2.Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27(5):305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Croft SL, Coombs GH. Leishmaniasis--current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 2003;19(11):502–508. doi: 10.1016/j.pt.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. Clin Microbiol Rev. 2006;19(1):111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Courret N, et al. Biogenesis of Leishmania-harbouring parasitophorous vacuoles following phagocytosis of the metacyclic promastigote or amastigote stages of the parasites. J Cell Sci. 2002;115(Pt 11):2303–2316. doi: 10.1242/jcs.115.11.2303. [DOI] [PubMed] [Google Scholar]
- 6.Antoine JC, et al. The biogenesis and properties of the parasitophorous vacuoles that harbour Leishmania in murine macrophages. Trends Microbiol. 1998;6(10):392–401. doi: 10.1016/s0966-842x(98)01324-9. [DOI] [PubMed] [Google Scholar]
- 7.Zhang K, et al. Leishmania salvage and remodelling of host sphingolipids in amastigote survival and acidocalcisome biogenesis. Mol Microbiol. 2005;55(5):1566–1578. doi: 10.1111/j.1365-2958.2005.04493.x. PMC Journal – In Process. PMID: 15720561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winter G, et al. Surface antigens of Leishmania mexicana amastigotes: characterization of glycoinositol phospholipids and a macrophage-derived glycosphingolipid. J Cell Sci. 1994;107(Pt 9):2471–2482. doi: 10.1242/jcs.107.9.2471. [DOI] [PubMed] [Google Scholar]
- 9.Schneider P, et al. Characterization of glycoinositol phospholipids in the amastigote stage of the protozoan parasite Leishmania major. Biochem J. 1993;295(Pt 2):555–564. doi: 10.1042/bj2950555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang O, et al. Degradation of host sphingomyelin is essential for Leishmania virulence. PLoS Pathog. 2009;5(12) doi: 10.1371/journal.ppat.1000692. p. e1000692. PMCID: 2784226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu W, et al. Sphingolipid degradation by Leishmania is required for its resistance to acidic pH in the mammalian host. Infection and Immunity. 2011;79(8):3377–3387. doi: 10.1128/IAI.00037-11. PMCID: 3147570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang O, Xu W, Pillai A, Zhang K. Developmentally Regulated Sphingolipid Degradation in Leishmania major. PLoS One. 2012;7(1):e31059. doi: 10.1371/journal.pone.0031059. p. PMCID: 3267774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res. 2009;50(Suppl):S237–S242. doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aoki J, et al. Structure and function of phosphatidylserine-specific phospholipase A1. Biochim Biophys Acta. 2002;1582(1–3):26–32. doi: 10.1016/s1388-1981(02)00134-8. [DOI] [PubMed] [Google Scholar]
- 15.Dennis EA. The growing phospholipase A2 superfamily of signal transduction enzymes. Trends Biochem Sci. 1997;22(1):1–2. doi: 10.1016/s0968-0004(96)20031-3. [DOI] [PubMed] [Google Scholar]
- 16.Brown WJ, Chambers K, Doody A. Phospholipase A2 (PLA2) enzymes in membrane trafficking: mediators of membrane shape and function. Traffic. 2003;4(4):214–221. doi: 10.1034/j.1600-0854.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- 17.Murakami M, et al. Regulatory functions of phospholipase A2. Crit Rev Immunol. 1997;17(3–4):225–283. doi: 10.1615/critrevimmunol.v17.i3-4.10. [DOI] [PubMed] [Google Scholar]
- 18.Murakami M, Kudo I. Phospholipase A2. J Biochem. 2002;131(3):285–292. doi: 10.1093/oxfordjournals.jbchem.a003101. [DOI] [PubMed] [Google Scholar]
- 19.Calder PC. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2006;75(3):197–202. doi: 10.1016/j.plefa.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Winstead MV, Balsinde J, Dennis EA. Calcium-independent phospholipase A(2): structure and function. Biochim Biophys Acta. 2000;1488(1–2):28–39. doi: 10.1016/s1388-1981(00)00107-4. [DOI] [PubMed] [Google Scholar]
- 21.Demopoulos CA, Pinckard RN, Hanahan DJ. Platelet-activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators) J Biol Chem. 1979;254(19):9355–9358. [PubMed] [Google Scholar]
- 22.Prescott SM, et al. Platelet-activating factor and related lipid mediators. Annu Rev Biochem. 2000;69:419–445. doi: 10.1146/annurev.biochem.69.1.419. [DOI] [PubMed] [Google Scholar]
- 23.Venable ME, et al. Platelet-activating factor: a phospholipid autacoid with diverse actions. J Lipid Res. 1993;34(5):691–702. [PubMed] [Google Scholar]
- 24.Arai H, et al. Platelet-activating factor acetylhydrolase (PAF-AH) J Biochem. 2002;131(5):635–640. doi: 10.1093/oxfordjournals.jbchem.a003145. [DOI] [PubMed] [Google Scholar]
- 25.Richmond GS, Smith TK. The role and characterization of phospholipase A1 in mediating lysophosphatidylcholine synthesis in Trypanosoma brucei. Biochem J. 2007;405(2):319–329. doi: 10.1042/BJ20070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richmond GS, Smith TK. A novel phospholipase from Trypanosoma brucei. Mol Microbiol. 2007;63(4):1078–1095. doi: 10.1111/j.1365-2958.2006.05582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Opperdoes FR, van Roy J. The phospholipases of Trypanosoma brucei bloodstream forms and cultured procyclics. Mol Biochem Parasitol. 1982;5(5):309–319. doi: 10.1016/0166-6851(82)90038-x. [DOI] [PubMed] [Google Scholar]
- 28.Eintracht J, et al. Calcium entry in Trypanosoma brucei is regulated by phospholipase A2 and arachidonic acid. Biochem J. 1998;336(Pt 3):659–666. doi: 10.1042/bj3360659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ridgley EL, Ruben L. Phospholipase from Trypanosoma brucei releases arachidonic acid by sequential sn-1, sn-2 deacylation of phospholipids. Mol Biochem Parasitol. 2001;114(1):29–40. doi: 10.1016/s0166-6851(01)00234-1. [DOI] [PubMed] [Google Scholar]
- 30.Bertello LE, et al. Evidence for phospholipases from Trypanosoma cruzi active on phosphatidylinositol and inositolphosphoceramide. Biochem J. 2000;345(Pt 1):77–84. [PMC free article] [PubMed] [Google Scholar]
- 31.Bonhomme A, et al. Signaling during the invasion of host cells by Toxoplasma gondii. FEMS Microbiol Rev. 1999;23(5):551–561. doi: 10.1111/j.1574-6976.1999.tb00413.x. [DOI] [PubMed] [Google Scholar]
- 32.Cassaing S, et al. Toxoplasma gondii secretes a calcium-independent phospholipase A(2) Int J Parasitol. 2000;30(11):1137–1142. doi: 10.1016/s0020-7519(00)00101-6. [DOI] [PubMed] [Google Scholar]
- 33.Gomez Marin JE, et al. Role of interferon-gamma against invasion by Toxoplasma gondii in a human monocytic cell line (THP1-involvement of the parasite's secretory phospholipase A2. Cell Immunol. 1996;169(2):218–225. doi: 10.1006/cimm.1996.0112. [DOI] [PubMed] [Google Scholar]
- 34.Belaunzaran ML, Lammel EM, de Isola EL. Phospholipases a in trypanosomatids. Enzyme Res. 2011;2011 doi: 10.4061/2011/392082. p. 392082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belaunzarán ML, Lammel EM VA, et al. Identification, cloning and expression of a novel Phospholipase A from Leishmania braziliensis. Biocell. 2010;34:91. [Google Scholar]
- 36.Passero LF, et al. The effect of phospholipase A2 from Crotalus durissus collilineatus on Leishmania (Leishmania) amazonensis infection. Parasitol Res. 2008;102(5):1025–1033. doi: 10.1007/s00436-007-0871-6. [DOI] [PubMed] [Google Scholar]
- 37.Lonardoni MV, Russo M, Jancar S. Essential role of platelet-activating factor in control of Leishmania (Leishmania) amazonensis infection. Infect Immun. 2000;68(11):6355–6361. doi: 10.1128/iai.68.11.6355-6361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santiago HC, et al. Platelet activating factor receptor-deficient mice present delayed interferon-gamma upregulation and high susceptibility to Leishmania amazonensis infection. Microbes Infect. 2006;8(11):2569–2577. doi: 10.1016/j.micinf.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 39.Ha DS, et al. Use of the green fluorescent protein as a marker in transfected Leishmania. Mol Biochem Parasitol. 1996;77(1):57–64. doi: 10.1016/0166-6851(96)02580-7. [DOI] [PubMed] [Google Scholar]
- 40.Kapler GM, Coburn CM, Beverley SM. Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol Cell Biol. 1990;10(3):1084–1094. doi: 10.1128/mcb.10.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beverley SM. Protozomics: trypanosomatid parasite genetics comes of age. Nat Rev Genet. 2003;4(1):11–19. doi: 10.1038/nrg980. [DOI] [PubMed] [Google Scholar]
- 42.Titus RG, et al. Exacerbation of experimental murine cutaneous leishmaniasis with CD4+ Leishmania major-specific T cell lines or clones which secrete interferon-gamma and mediate parasite-specific delayed-type hypersensitivity. Eur J Immunol. 1991;21(3):559–567. doi: 10.1002/eji.1830210305. [DOI] [PubMed] [Google Scholar]
- 43.Zhang K, et al. Sphingolipids are essential for differentiation but not growth in Leishmania. EMBO J. 2003;22(22):6016–6026. doi: 10.1093/emboj/cdg584. PMCID: 275442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sacks DL, Hieny S, Sher A. Identification of cell surface carbohydrate and antigenic changes between noninfective and infective developmental stages of Leishmania major promastigotes. J Immunol. 1985;135(1):564–569. [PubMed] [Google Scholar]
- 45.Spath GF, Beverley SM. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp Parasitol. 2001;99(2):97–103. doi: 10.1006/expr.2001.4656. [DOI] [PubMed] [Google Scholar]
- 46.Descoteaux A, et al. Leishmania LPG3 encodes a GRP94 homolog required for phosphoglycan synthesis implicated in parasite virulence but not viability. Embo J. 2002;21(17):4458–4469. doi: 10.1093/emboj/cdf447. PMCID: 126187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bangs JD, et al. Molecular cloning and cellular localization of a BiP homologue in Trypanosoma brucei. Divergent ER retention signals in a lower eukaryote. J Cell Sci. 1993;105(Pt 4):1101–1113. doi: 10.1242/jcs.105.4.1101. [DOI] [PubMed] [Google Scholar]
- 48.de Ibarra AA, Howard JG, Snary D. Monoclonal antibodies to Leishmania tropica major: specificities and antigen location. Parasitology. 1982;85(Pt 3):523–531. doi: 10.1017/s0031182000056304. [DOI] [PubMed] [Google Scholar]
- 49.Zhang K, Barron T, Turco SJ, Beverley SM. The LPG1 gene family of Leishmania major. Mol. Biochem. Parasitol. 2004;136:11–23. doi: 10.1016/j.molbiopara.2004.02.012. PMC Journal – In Process. PMID: 15138063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Titus RG, et al. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985;7(5):545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 51.Hattori M, Arai H, Inoue K. Purification and characterization of bovine brain platelet-activating factor acetylhydrolase. J Biol Chem. 1993;268(25):18748–18753. [PubMed] [Google Scholar]
- 52.Farber SA, et al. Characterization of Ca2+-dependent phospholipase A2 activity during zebrafish embryogenesis. J Biol Chem. 1999;274(27):19338–19346. doi: 10.1074/jbc.274.27.19338. [DOI] [PubMed] [Google Scholar]
- 53.Rice SQ, et al. Expression, purification and characterization of a human serine-dependent phospholipase A2 with high specificity for oxidized phospholipids and platelet activating factor. Biochem J. 1998;330(Pt 3):1309–1315. doi: 10.1042/bj3301309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sacks DL, Perkins PV. Identification of an infective stage of Leishmania promastigotes. Science. 1984;223(4643):1417–1419. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- 55.McConville MJ, et al. Structure of the lipophosphoglycan from Leishmania major. J Biol Chem. 1990;265(32):19611–19623. [PubMed] [Google Scholar]
- 56.Turco SJ, et al. Structure of the phosphosaccharide-inositol core of the Leishmania donovani lipophosphoglycan. J Biol Chem. 1989;264(12):6711–6715. [PubMed] [Google Scholar]
- 57.Button LL, McMaster WR. Molecular cloning of the major surface antigen of leishmania. J Exp Med. 1988;167(2):724–729. doi: 10.1084/jem.167.2.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schneider P, et al. Structure of the glycosyl-phosphatidylinositol membrane anchor of the Leishmania major promastigote surface protease. J Biol Chem. 1990;265(28):16955–16964. [PubMed] [Google Scholar]
- 59.Stafforini DM, et al. Mammalian platelet-activating factor acetylhydrolases. Biochim Biophys Acta. 1996;1301(3):161–173. doi: 10.1016/0005-2760(96)00040-9. [DOI] [PubMed] [Google Scholar]
- 60.Hattori K, et al. Purification and characterization of platelet-activating factor acetylhydrolase II from bovine liver cytosol. J Biol Chem. 1995;270(38):22308–22313. doi: 10.1074/jbc.270.38.22308. [DOI] [PubMed] [Google Scholar]
- 61.Marathe GK, et al. Inflammatory platelet-activating factor-like phospholipids in oxidized low density lipoproteins are fragmented alkyl phosphatidylcholines. J Biol Chem. 1999;274(40):28395–28404. doi: 10.1074/jbc.274.40.28395. [DOI] [PubMed] [Google Scholar]
- 62.Androulakis N, et al. Molecular and mechanistic characterization of platelet-activating factor-like bioactivity produced upon LDL oxidation. J Lipid Res. 2005;46(9):1923–1932. doi: 10.1194/jlr.M500074-JLR200. [DOI] [PubMed] [Google Scholar]
- 63.McConville MJ, et al. Secretory pathway of trypanosomatid parasites. Microbiol Mol Biol Rev. 2002;66(1):122–154. doi: 10.1128/MMBR.66.1.122-154.2002. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dutra PM, et al. Stimulation of Leishmania tropica protein kinase CK2 activities by platelet-activating factor (PAF) Acta Trop. 2009;111(3):247–254. doi: 10.1016/j.actatropica.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 65.Rosa MS, et al. Platelet-activating factor (PAF) modulates peritoneal mouse macrophage infection by Leishmania amazonensis. Curr Microbiol. 2001;43(1):33–37. doi: 10.1007/s002840010256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
0.4 unit of purified PLA2 (from bovine pancreas) was incubated with NBD-PC for 1 hour and the release of NBD-labeled fatty acid (FA) was detected by TLC as described in Materials and Methods (2.7). NBD-FA (FA standard) and NBD-PC (PC standard) were included in the TLC to illustrate the migration of FA and PC. The arrow indicates the direction of TLC. O: origin. F: solvent front.
Whole cell lysates from stationary phase day 1 (A) and day 3 (B) promastigotes were separated on SDS-PAGE (5 × 105 cells per lane) and probed with antibodies against LPG, GP63, or α-tubulin (to control loading). The numbers indicate relative signal intensity acquired using a FluorChem E imager.
WT, pla2−, and pla2−/+PLA2 promastigotes were labeled with monoclonal antibodies against LPG (A) or GP63 (B), followed by incubation with FITC-labeled goat anti-mouse IgG secondary antibody. Scale bars = 10 µm.
Whole cell lysates (1 × 106 cells per lane) and culture supernatant (10 µl per lane) from log phase promastigotes were subjected to western blot analysis using an anti-GFP antibody. The PLA2-GFP fusion protein was detected in the whole cell lysate of pla2−/+PLA2-GFP at the predicted molecular weight (~69 KDa) but not in the culture supernatant.
Log phase promastigotes of pla2−/+PLA2-GFP were stained with Mitotracker 580 and visualized by microscopy as described in Materials and Methods (2.7). (A) DIC image; (B) DNA staining with Hoechst 33342; (C) PLA2-GFP epifluorescence; (D) Staining result with Mitotracker; (E) Merge of C and D.