Abstract
The principal sensory (PrV) nucleus-based trigeminal lemniscus conveys whisker-specific neural patterns to the ventroposteromedial (VPM) nucleus of the thalamus and subsequently to the primary somatosensory cortex. Here we examined the perinatal development of this pathway with carbocyanine dye labeling in embryonic and early postnatal mouse brains. We developed a novel preparation in which the embryonic hindbrain and the diencephalon are flattened out, allowing a birds-eye view of the PrV lemniscus in its entirety. For postnatal brains we used another novel approach by sectioning the brain along an empirically determined oblique horizontal angle, again preserving the trigeminothalamic pathway. PrV neurons are born along the hindbrain ventricular zone and migrate radially for a short distance to coalesce into a nucleus adjacent to the ascending trigeminal tract. During migration of the spindle-shaped cell bodies, slender axonal processes grow along the opposite direction towards the floor plate. As early as embryonic day (E) 11, pioneering axons tipped with large growth cones cross the ventral midline and immediately make a right angle turn. By E13 many PrV axons form fascicles crossing the midline and follow a rostral course. PrV axons reach the midbrain by E15 and the thalamus by E17. While the target recognition and invasion occurs prenatally, organization of PrV axon terminals into whisker-specific rows and patches takes place during the first 4 postnatal (P) days. Initially diffuse and exuberant projections in the VPM at P1 coalesce into row and whisker specific terminal zones by P4.
Keywords: barrelettes, barreloids, ventroposteromedial nucleus, somatosensory
Orofacial somatosensory information is carried to the ventroposteromedial (VPM) nucleus of the thalamus via the trigeminothalamic projection cells of the principal sensory trigeminal (PrV) nucleus. In rodents, a portion of the maxillary nerve (the infraorbital nerve) carries whisker-specific information to the PrV and the spinal trigeminal nucleus where whisker-specific neural patterns, “barrelettes” form (Belford and Killackey, 1979; Ma and Woolsey, 1984, Erzurumlu and Killackey, 1982; Bates and Killackey, 1985.) Selective lesion experiments in neonatal rats showed that the PrV alone conveys whisker-specific patterning to the VPM (Killackey and Fleming, 1985). The PrV-based lemniscal pathway is a crossed projection and develops perinatally (see Leamey and Ho, 1998 for the rat and Leamey et al., 1998 for the wallaby). Little is known about the development of connectivity between the PrV and the VPM in the mouse. Given the emerging mouse mutant phenotypes with midline axonal crossing defects, we conducted a descriptive developmental study of the crossed trigeminothalamic projections in this species.
The infraorbital nerve innervates the mystacial vibrissae and the sinus hairs. Central counterparts of the infraorbital nerve axons form focalized terminal arbors in the PrV and spinal nuclei of the trigeminal nerve in the pontine brainstem and the medulla. The spatial distribution of these terminal arbor patches replicates the patterned organization of the whiskers (Erzurumlu and Jhaveri, 1992, Waite et al., 2000). In the PrV, a specific class of neurons recognizes the patterning of the presynaptic trigeminal arbors and the dendritic trees of these neurons embrace the presynaptic terminal patches. Collectively the presynaptic terminal endings and neurons with their polarized dendritic fields form individual barrelettes, each barrelette corresponding to a single whisker or sinus hair on the snout (Ma and Woolsey, 1984; Lee et al., 2005). The barrelette patterns were first identified using mitochondrial enzyme histochemistry succinic dehydrogenase (Belford and Killackey, 1979; Durham and Woolsey, 1984) as discontinuous patches of dense synaptic zones; in later years another histochemical staining method for mitochondrial enzyme cytochrome oxidase (Wong-Riley and Welt, 1980) became the choice method of demonstrating whisker-specific neural patterning in the rodent brain. The barrelette neurons are also the trigeminothalamic projection neurons; their axons form the trigeminal lemniscus and instruct barreloid formation in the VPM (Bates et al., 1982; Killackey and Fleming, 1985).
The PrV originates from the rostral hindbrain (Marín and Puelles, 1995; Oury et al. 2006). Genetic fate mapping studies in mice indicate that the PrV neurons are derived from rhombomeres r2 and r3 (Oury et al., 2006). Fluorescent reporter gene markers for the r2 and r3 rhombomeres revealed that the neurons in the ventral (barrelette) region of the PrV are mostly derived from r3 and those in the dorsal portions of the PrV from r2 (Oury et al., 2006). It has been suggested that the spatial segregation of r2 and r3 postmitotic progenies within the PrV underlies the parcellation of mandibular and maxillo-ophthalmic projection zones in the VPM (Oury et al., 2006; Erzurumlu et al., 2010).
Like the PrV, the VPM has distinct and non-overlapping zones devoted to the representation of the mandibular and maxillo-ophthalmic sensory periphery. Within this somatotopic representation PrV-based lemniscal afferent terminals and VPM neurons form clusters termed “barreloids” (Van der Loos, 1976). Clearly the somatotopy and the patterning within it are brought to the VPM by the axons of the contralateral PrV neurons. So far, only two studies, one in the rat (Leamey and Ho, 1998) and the other in the wallaby (Leamey et al., 1998), have examined the development of this pathway. In the present study we fill the gap for the mouse and introduce wholemount embryonic preparations and oblique horizontal slices from postnatal mouse brains that maintain the connections between the PrV and the thalamus intact. Aside from descriptive anatomical results presented here, these preparations are also suitable for in vitro electrophysiological recordings to study development of the synaptic connectivity between the trigeminal brainstem and the thalamus.
MATERIALS AND METHODS
C57BL/6J mice were used at embryonic day (E) 11, 13, 15 and 17 and postnatal days (P) 1–4. Timed-pregnant females were purchased from Jackson Laboratories. The plug date was considered E0.. The day of birth was considered P0. Pregnant mice were overdosed with pentobarbital (administered at > 100 mg/kg intraperitoneal). The embryos were removed by Cesarean section and placed in ice-cold phosphate buffer saline (PBS, pH 7.4). Animal handling was in accordance with NIH guidelines and a protocol approved by the UMB Animal Use and Care Committee.
Embryonic wholemount and postnatal trigeminothalamic slice preparations and carbocyanine dye labeling
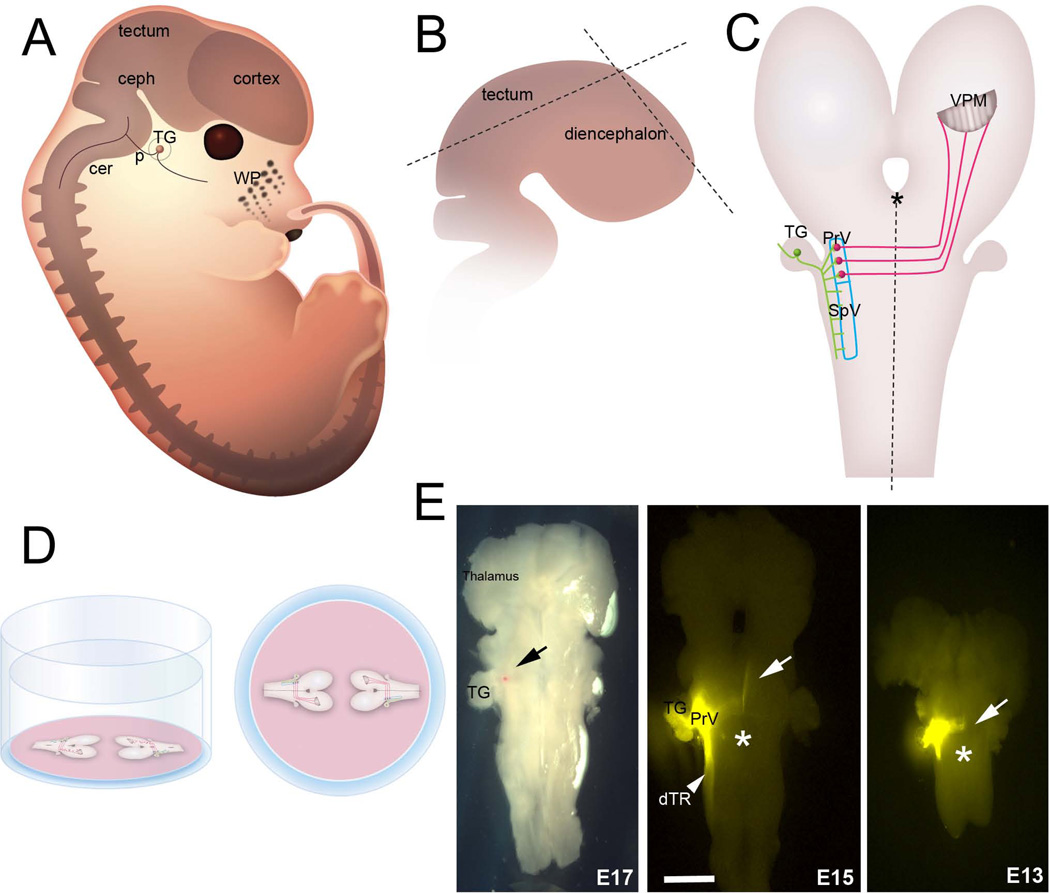
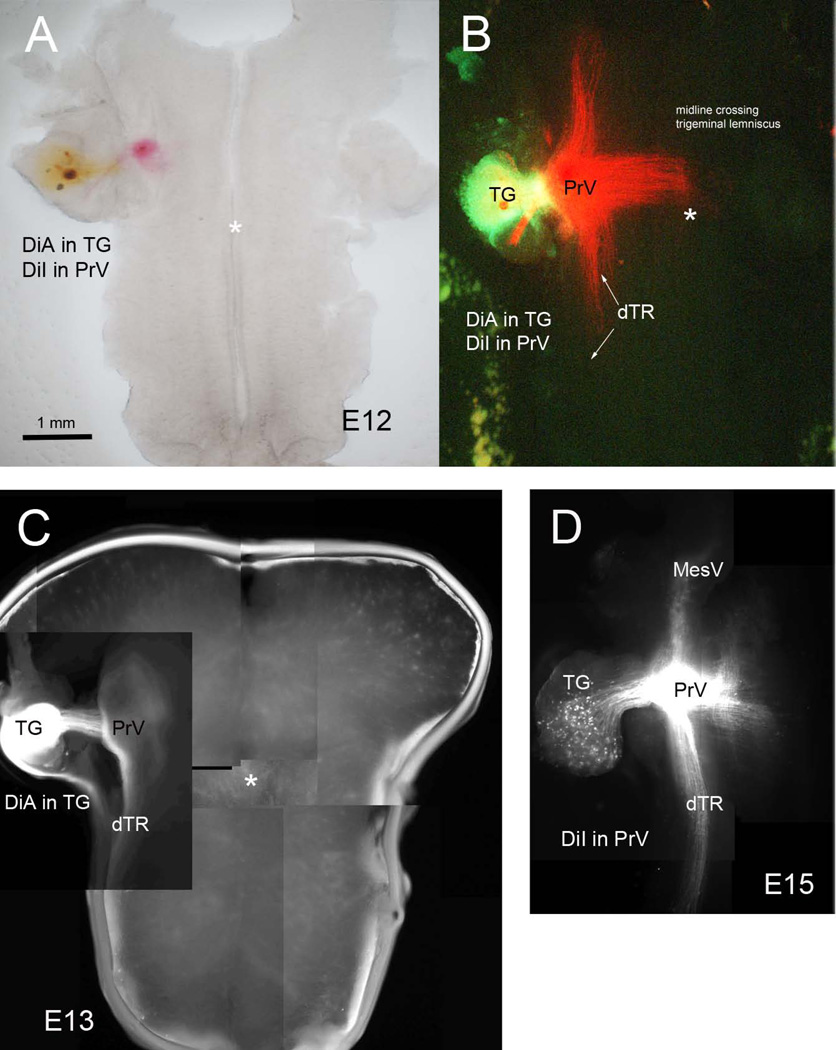
We developed two preparations that allow visualization of the PrV trigeminothalamic projections in their entirety in embryonic and early postnatal mice. From E10 to E17 the embryonic CNS, when viewed from a sagittal angle, is along a sideways S-shaped plane due to cephalic, pontine and cervical flexures (Fig. 1A). For embryonic wholemounts we first remove the entire brain, including the upper cervical spinal cord, under a stereo dissecting microscope. The brain is placed in a petri dish filled with Gey’s balanced salt solution (Invitrogen) and the telencephalic vesicles are removed. The meninges covering the diencephalon and the brainstem are carefully peeled away with fine forceps. At all embryonic ages, tectum is a prominent structure in the midbrain; using micro scissors the tectum is removed along a plane from the caudal edge to the pretectal area (see Fig. 1B). Next the dorsal and ventral midline is excised from the midbrain through the diencephalon (Fig. 1B). This leaves an intact caudal brainstem with the midbrain and diencephalon on either side free. The specimen is then transferred to a microporous Millicell membrane and held straight with the dorsal side down. The GBSS is gently suctioned out around the specimen as it flattens out with the ventral side of the entire brainstem-diencephalon facing up (Fig. 1C). Finally, the Millicell membrane is placed in 6-well culture plates, each well filled with 1ml of 4% paraformaldehyde. (Fig. 1D) The plates are sealed with parafilm and kept in the refrigerator overnight. The next day, small crystals of carbocyanine dye 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (DiI, Invitrogen) are inserted into the PrV region (Fig. 1E, Fig. 2A, B, D). In many cases the trigeminal ganglia are also dissected out attached to the brainstem at the pontine levels (Fig. 2). Presence of the ganglion is a solid landmark in aiding the dye placement. In initial localization studies we implanted the TG with a crystal of DiA and charted the location of central trigeminal axons and the position of the PrV, then we implanted DiI in the PrV (Fig. 2). DiI labeling of the PrV also led to retrograde labeling of the trigeminal ganglion cells and their axons that travel in the descending trigeminal tract (Fig. 1E, Fig. 2B, D). This labeling pattern was taken as an index of proper targeting of DiI placement in the PrV proper.
Figure 1.
Preparation of the embryonic trigeminal lemniscal pathway wholemounts. A: Schematic drawing of an E15 mouse embryo from a parasagittal view. B: For embryonic brainstem wholemounts, the cerebral cortex and tectum are removed, a cut is made along the dorsal diencephalic midline (dashed lines) and the entire specimen is splayed out (C) on a Millicell membrane as described in the Methods. D: Side view and birds-eye view of two brainstem wholemounts on a Millicell membrane in a six-well plate. Up to 4 explants can be flattened and kept on the same membrane. E: DiI labeling in flattened brainstem specimens at 3 different embryonic ages. The red dot (black arrow) in the E17 specimen is an exemplary DiI crystal. In other photomicrographs, white arrows point to the axons arriving at and crossing the midline. Asterisks indicate the midline. WP: whisker pad, ceph: cephalic flexure, p: pontine flexure, cer: cervical flexure, TG: trigeminal ganglion, PrV: principal sensory nucleus of the trigeminal nerve, SpV: spinal trigeminal nucleus; VPM: ventroposteromedial nucleus of the thalamus. Scale bar = 300 µm.
Figure 2.
Double carbocyanine dye labeling to localize the position of the PrV in embryonic wholemounts. Placement of DiA in the ganglion and DiI in the PrV (A and B). DiA in the ganglion (C) allowed initial localization of the PrV. Backlabeling of the trigeminal ganglion (TG) cells and the descending trigeminal tract (dTR) also ensured that DiI placement was in the PrV (D).
After E17 flattening of the diencephalon and the brainstem is not feasible. For postnatal ages we used an oblique horizontal plane to obtain slices containing the PrV-VPM pathway intact. In aldehyde fixed brains small crystals of DiI were inserted into the brainstem at the level of the trigeminal root entry zone. Labeled brains were stored at 37°C in dark for 2–4 weeks. After dye diffusion, the cerebral cortex was removed on one side, allowing the visualization of the dorsal thalamus. Using a fine felt tip pen, a small mark was placed over the dorsal thalamus and another one laterally at the trigeminal root entry zone of the pontine flexure (Fig. 7A). The brain was then embedded in agarose and blocked with the ventral side flat. The two marks were visualized under a stereomicroscope and a straight line between them was used as the optimal angle and the block was re-trimmed with the cutting surface parallel to the line between the thalamus and the PrV; µm-thick sections were cut using a vibratome (Leica). In a few postnatal brains, the DiI labeling from the PrV was traced using serial vibratome sections in the coronal plane.
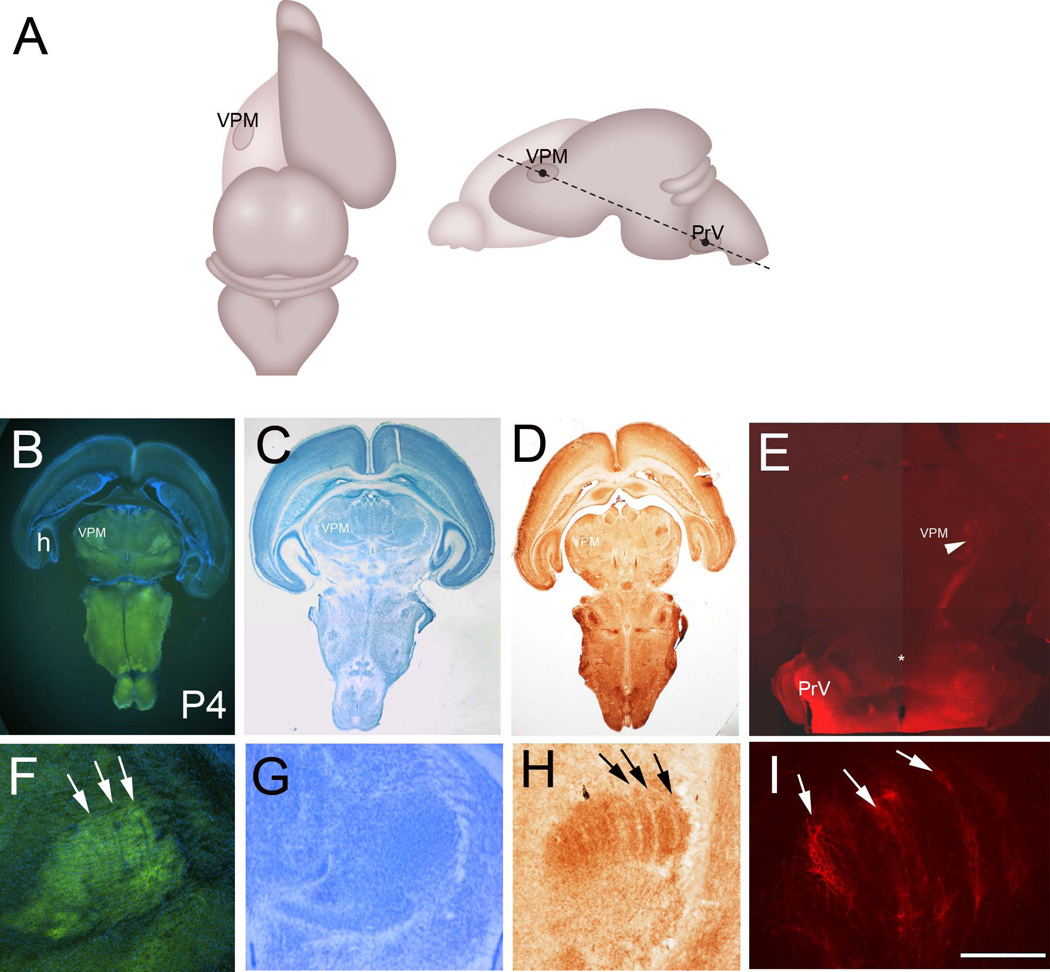
Figure 7.
A: Schematic illustration of the oblique horizontal plane of sectioning to preserve the PrV-thalamic pathway. B: VGLUT2 immunostained oblique horizontal section with DAPI counterstain from a P4 brain. C: Nissl stain. D: Cytochrome oxidase histochemistry. E: DiI labeling at P4. H: hippocampus; VPM: ventroposteromedial nucleus. F–I show higher magnification views of the VPM with VGLUT2 immunohistochemistry (F), Nissl (G), CO (H) staining and DiI (I) labeling. Barreloid rows are clearly visible with VGLUT2 immuno- and CO histochemistry (arrows). Note also alignment of axon terminals along bands (arrows in I), reflecting the barreloid organization in the VPM. Scale bar in F = 200µm, I = 200µm.
Images were collected using Nikon AZ100 and Nikon 90i microscopes and NIS Elements software. The photomicrographs were adjusted for brightness, contrast, sharpness and evenness of illumination.
Histochemistry and immunocytochemistry
Late P1 and P3 brains were sectioned in the oblique horizontal plane (as described above) at a thickness of 50 µm using a vibratome (Leica). Alternate sections were processed for cytochrome oxidase (CO) histochemistry or immunohistochemistry for vesicular glutamate transporter (VGLUT) 2.
For CO histochemistry, the sections were incubated with PBS containing 0.5mg/ml cytochrome C, 0.5 mg/ml diaminobenzidine (DAB, both from Sigma, St. Louis, MO), and 50 mg/ml sucrose for 6–7 hours at 37°C, in a shaker incubator. The reaction was stopped when the sections turned brown by several rinses with PBS.
In the rodent brain presynaptic afferent terminals and their postsynaptic partners form whisker-specific patterns. CO histochemistry is a well-established method of visualizing whisker-specific patterning in the rodent brain. Cytochrome oxidase is mitochondrial enzyme and mitochondria are abundant at the synaptic sites. However, CO histochemistry does not allow differentiation of pre- and postsynaptic elements. Stains that allow visualization of dendrites or Nissl stains are routinely used for the patterning of postsynaptic elements in this system and a couple of antibodies have become established ways of visualizing the distribution of presynaptic afferent inputs along the whisker-barrel neuraxis. Serotonin transporter (5-HTT) and vesicular glutamate transporters 1 and 2 (VGLUT1 and 2) are highly reliable markers of presynaptic afferent terminal patterns along trigeminal centers of the developing rodents and their patterned expression corresponds to patterned distribution of presynaptic afferent terminals labeled with carbocyanine dyes (Lebrand et al., 1998; Rebsam et al., 2002; Nakamura et al., 2005; Liguz-Lecznar and Skangiel-Kramska, 2007). In this study we used VGLUT2 immunohistochemistry to complement CO histochemistry for visualization of whisker-related trigeminothalamic patterning. The two markers showed similar patterning in the VPM. For VGLUT2 immunohistochemistry, the sections were incubated at 4°C with guinea pig anti-VGLUT2 (Millipore, AB2251, 1:1000) for 48 hours, followed by incubation in FITC-conjugated donkey anti-guinea pig antibody (Jackson Immuno Research, 1:500). The sections were rinsed in PBS, counterstained with fluorescent nuclear stain DAPI, mounted on subbed slides, and coverslipped. Control sections were processed the same way without the primary antibody and there was no patterned staining.
Antibody characterization
Please see Table 1 for identification of the antibody used. VGLUT2 is localized to synaptic vesicles at glutamatergic synapses. Polyclonal VGLUT2 antiserum recognized a single band of approximately 52 kDa molecular weight on Western blots of rat brain lysate (manufacturer’s data sheet). Preadsorption with the immunogen peptide abolishes immunolabeling in mouse and rat brain sections as reported by the manufacturer (see also Soiza-Reilly and Commons, 2011). VGLUT2 antibodies have been used as a marker of axon terminal patterning in the mouse thalamus and neocortex (Nakamura et al., 2005; Liguz-Lecznar and Skangiel-Kramska, 2007). We used this VGLUT2 antibody as a tissue/pattern marker and the staining localization and patterns in normal neonatal mouse thalamus and cortex were identical with previous reports on developing mouse forebrain (Nakamura et al., 2005; Liguz-Lecznar and Skangiel-Kramska, 2007). Additionally, immunostaining pattern we see in the thalamus is similar to what we see with DiI labeling of trigeminothalamic axons from the PrV or with CO histochemistry (Fig. 7).
Table 1.
Primary Antibody Used for Visualization of Trigeminothalamic Axon Patterning in the VPM
| Antigen | Immunogen | Manufacturer | Dilution |
|---|---|---|---|
| Vesicular glutamate transporter 2 (VGLUT2) | Recombinant GST-tagged C-terminal peptide of rat VGLUT2 (VQESAQDAYSYKDRDDYS)* | Millipore (Billerica, MA), guinea pig polyclonal, AB2251, Lot# NG1827430 | 1:1,000 |
RESULTS
The earliest cohort of newly born PrV neurons migrates ventrolaterally from the ventricular zone of the rhombomeres r2 and r3 (Oury et al., 2006). We saw isolated axons crossing the midline at E10, which also corresponds to the onset of neurogenesis of PrV neurons. Thus, PrV neurons extend their axons across the midline as soon as they have achieved neuronal identities around E10.
Our observations on the E11 embryos revealed that PrV neurons emit single axonal processes towards the midline floor plate that grow in the opposite direction of their migratory path. A group of pioneering axons from the presumptive PrV zone crosses the midline at E11 (Fig. 3A, B). Few of these axons, tipped with growth cones make a sharp rostral turn while others appear to have just crossed the midline or stalled after crossing (Fig 3B). Some of the turning axons follow the immediate edge of the midline while others extend about 10–40 µm before making their rostral turn (Fig. 3B)
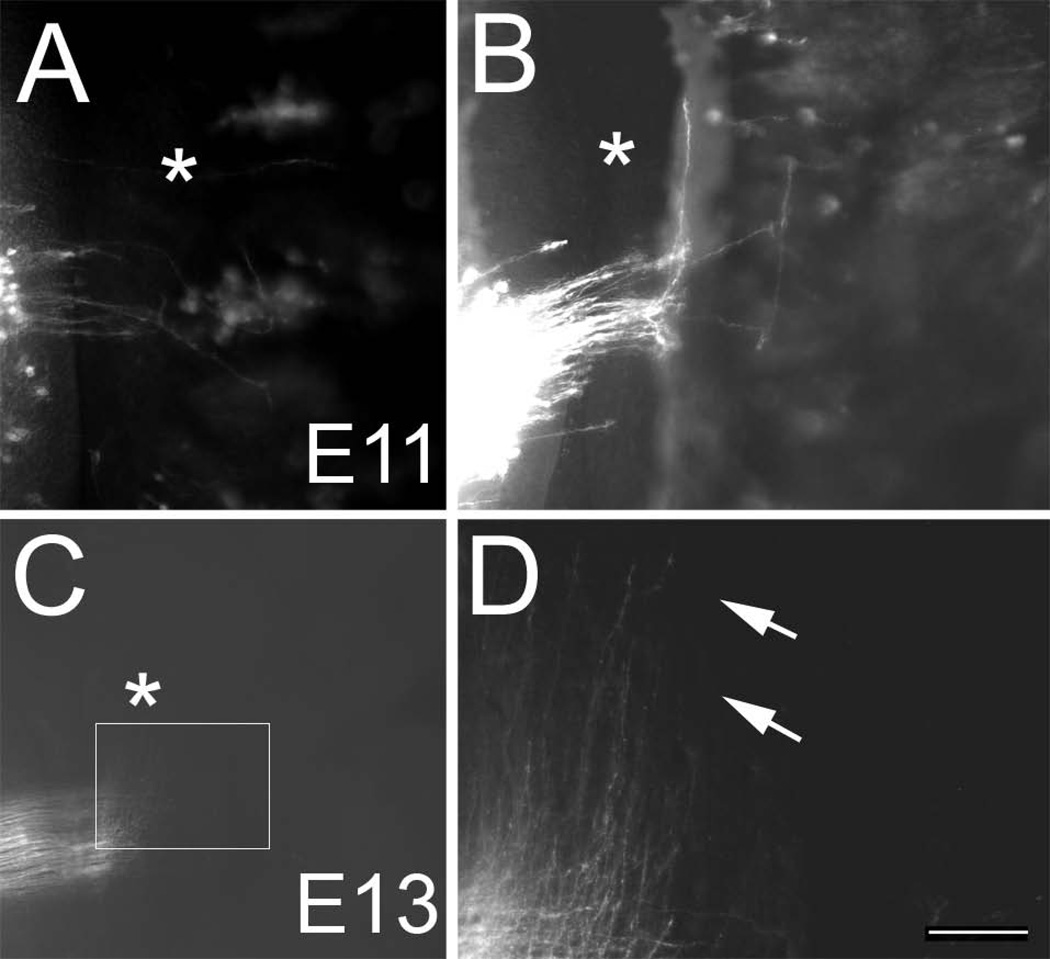
Figure 3.
Onset of midline crossing by PrV axons. A, B: Examples of pioneering PrV axons crossing the midline on E11. Note that the PrV axons do not cross the midline en masse, rather a few pioneers lead the way, stall on the other side of the midline and make an abrupt rostral turn. Asterisks indicate the midline. C: A large group of PrV axons have crossed the midline by E13, made a sharp rostral turn and waves of growth cone-tipped axons are advancing towards the midbrain. D: higher magnification view of the boxed area in C. Pioneering front of the PrV lemniscal pathway (arrows). Scale bar = 50 µm for B and 100 µm for D.
On E13 waves of axon fronts are seen advancing towards the midbrain while some are still crossing the midline (Fig. 3C, D). Two days later, a much larger bundle of midline crossing axons are labeled from the presumptive PrV. Vast majority of these axons cross the midline as parallel arrays and make a sharp rostral turn immediately after crossing the midline (Fig. 4A, B). As this bundle takes a rostral course, it begins to fan out laterally. At the front edge of the pathway several axons with growth cones are visible. The leading edge of the pathway has much fewer axons compared to caudally towards the zone of midline crossing. Light microscopic examination of the pathway indicates successive waves of PrV axons that cross the midline and turn rostrally between E11 and E13. At these early embryonic stages, the trajectory of the PrV axons just after the midline crossing in wholemount preparations gives the impression that there is a twisting of the ribbon-shaped pathway such that axons originating from more caudal points turn abruptly compared to those from rostral origination points hence inverting the rostrocaudal axis (width) of the ribbon (lemniscus) to a lateral to medial axis as it advances rostrally. At tegmental levels more thinning and spreading out of the pathway occurred and it now resembled a true ribbon, “lemniscus”.
Figure 4.
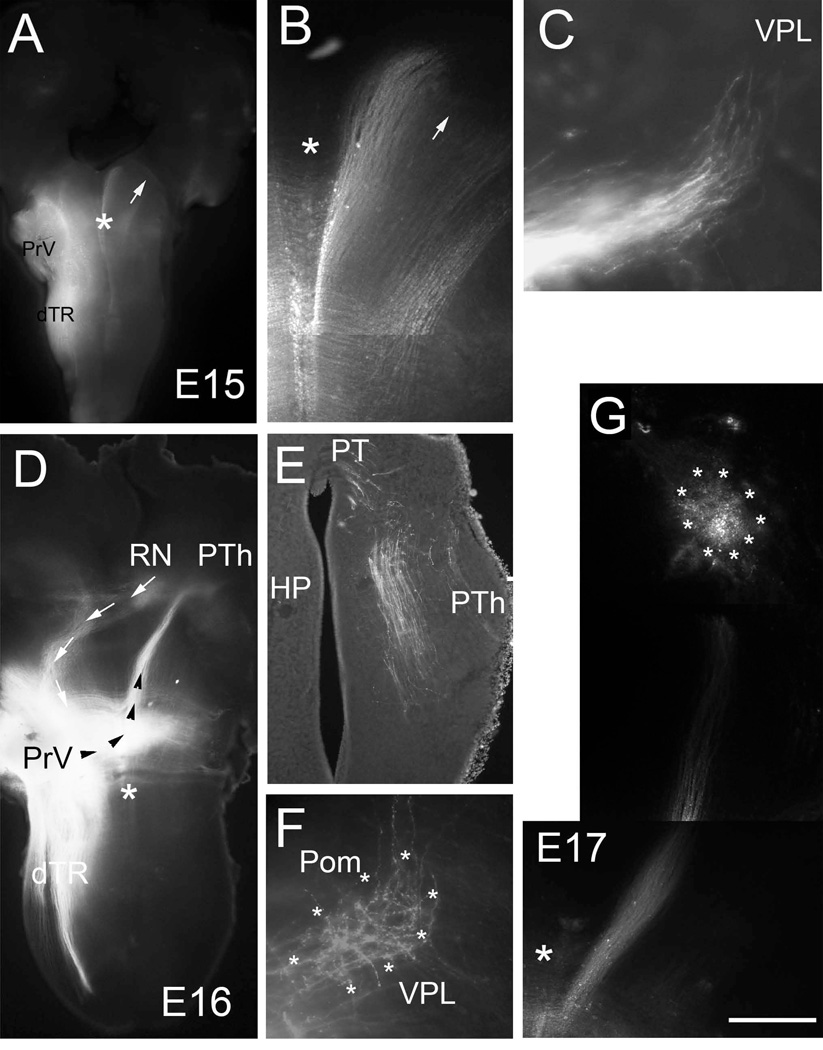
Developmental progression of the trigeminothalamic tract between E15-E17. Low (A,) and high (B) power views of the PrV lemniscal pathway on E15. At this age, trigeminal axons have reached the midbrain tegmental levels and spread out to form a ribbon. Arrows point to the same frontier edge of the lemniscal fibers. C: In a few late E15 cases, trigeminal axons are seen approaching the VPL (coronal section). D: Low power view of a brainstem-diencephalon flat mount from an E16 case. The trigeminal axons (black arrowheads) have reached the posterior thalamus (PTh) slightly rostral to the red nucleus (RN). In this case rubrotrigeminal (to trigeminal motor nucleus) axons are also labeled as a contralaterally projecting pathway (white arrows). E: A coronal section through the pretectal (PT)- posterior thalamus (PTh) area from another E16 case showing the progression of trigeminothalamic axons in this region; some axons are destined towards the tectum. E17 marks the time when PrV axons arrive at ventroposteromedial thalamic nucleus (VPM). F: Initial termination pattern in the VPM (boundaries marked by asterisks; E17-coronal section) at E17 is diffuse but mostly restricted to the VPM nucleus. G: Photomontage of low power views of the PrV lemniscal pathway in a wholemount preparation. Scale bar = 1mm for A, 500 µm for D and 200 µm for G. Large asterisks mark the midline; HP: habenulopeduncular tract; Pom: posteromedial thalamic nucleus; dTR: descending trigeminal tract.
By E15 PrV lemniscal axons have ascended to the contralateral tegmentum (Fig. 1E, Fig. 4A, B). We also followed their course in some cases by sectioning the brain in the coronal plane. Such sections from late E15 embryos showed the PrV axons approaching the developing almond shaped VPM/VPL complex from its ventral aspect in a mediolateral direction (Fig. 4C). At E16, PrV axons could be traced to the posterior thalamus just caudal to the VPM/VPL complex. In the wallaby there is a distinct waiting period of trigeminal axons in the thalamus before they invade the VPM (Leamey et al., 1998). The development of the trigeminothalamic and thalamocortical pathways in the wallaby is a protracted period, while in rodents this period is much shorter. Thus we cannot conclude with certainty whether there is a “waiting period” for trigeminothalamic axons before they invade the VPM. Even if there is, this period is probably like a “pause” rather than waiting for longer than a 24 hr. period.
Over the course of next couple of days lemniscal axons begin invading the VPM/VPL complex and form exuberant terminal branches that cover large spans in the region (Fig. 4E, F). Initial exuberance of the PrV terminals in the VPM can also be seen in oblique horizontal sections from early postnatal animals (Fig. 5B).
Figure 5.
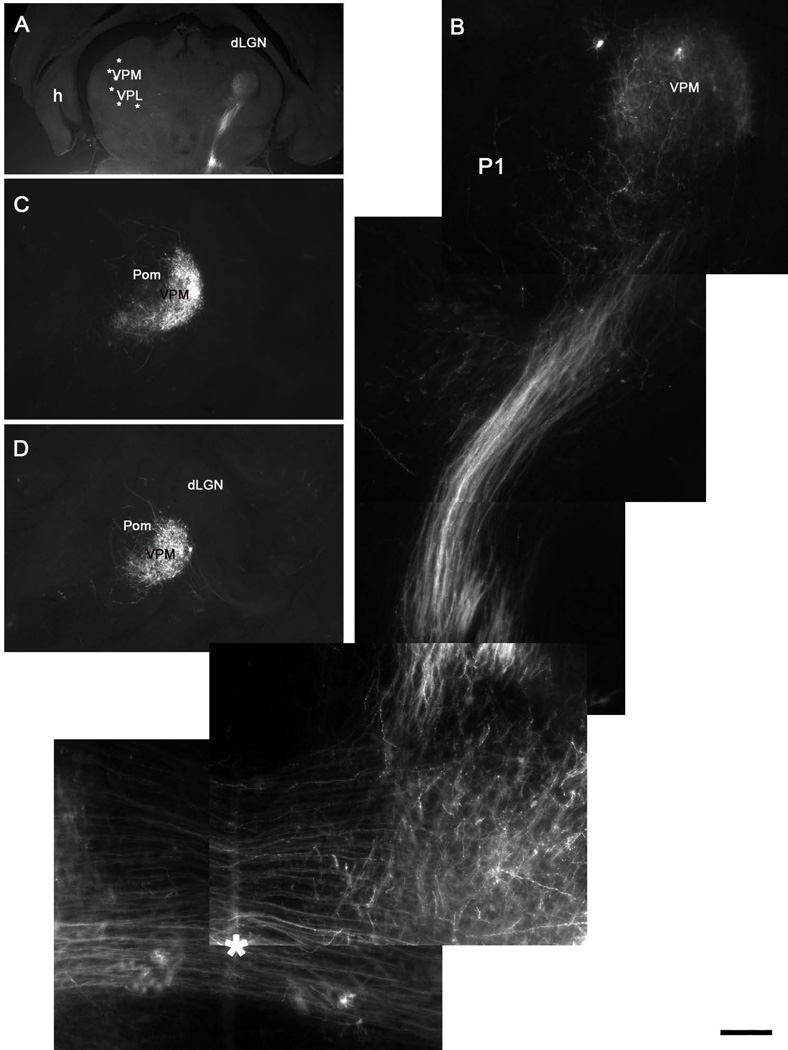
Postnatal development of the trigeminal lemniscal pathway. A, B: Oblique horizontal sections show that the trigeminal lemniscus has invaded the VPM and axons branch diffusely across the entire nucleus at P1. C, D: Consecutive coronal sections showing the confinement of PrV axons to the VPM, with only a few axons spilling into the neighboring posteromedial nucleus (Pom). Note that no lemniscal axons enter the dorsal lateral geniculate nucleus (dLGN). Asterisk in B indicates the midline crossing of the PrV axons. Scale bar = 1200µm for A, 200µm for B and 500µm for C, D.
Initially, PrV axon terminals occupy larger space compared to later ages (compare Fig. 4F, 5B, 6B, and 7I). Even at initial stages of terminal area invasion very few lemniscal axons, if any go beyond the boundaries of the VPM, indicating that axon guidance cues and boundary markers restrict terminal arborization field of the PrV axons from the onset. A few axons are seen to go into the posteromedial (Pom) nucleus, but none cross dorsolaterally into the neighboring dorsal lateral geniculate nucleus. The reverse, a transient retinogeniculate projection overspill into the VPM has been noted in the hamster (Frost, 1986). In contrast to the development of trigeminal afferent arbors in the PrV (Lee et al., 2005a) and thalamocortical terminal arbors in the barrel cortex (Agmon et al., 1993; Lee et al., 2005b), sculpting of patterned, barrelette-specific PrV arbors in the VPM develop from an initially overlapping and exuberant terminal branches.
Figure 6.
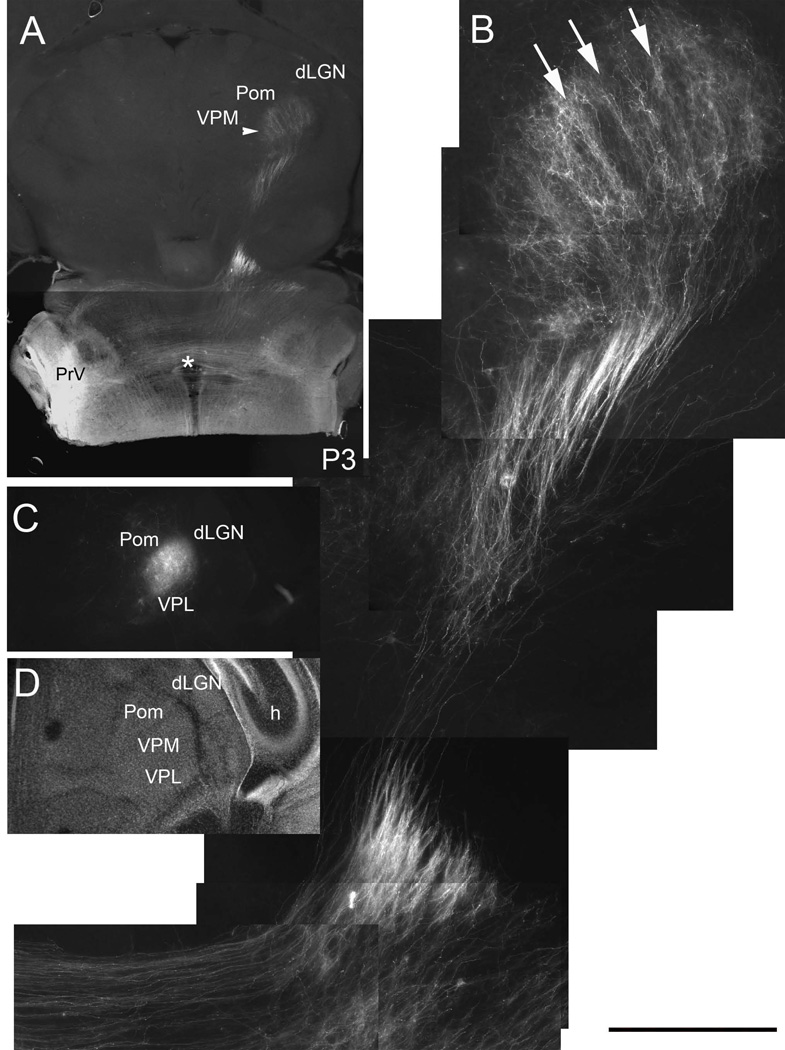
Emergence of barreloid rows on P3. Low power (A) and higher magnification (B) views of the PrV lemniscal pathway in oblique horizontal sections. Note that rows (arrows) of axon terminal bands are emerging from an initially diffuse terminal projection field in the VPM. C, D: DiI labeling and Nissl staining in coronal sections through the VPM at P3. H: hippocampus, dLGN: dorsal lateral geniculate nucleus, VPL: ventroposterolateral nucleus, Pom: posteromedial nucleus, asterisk indicates the midline crossing of the PrV axons. Scale bar = 400µm for B.
Both DiI labeling, glutamate transporter (VGLUT2) immunohistochemistry and cytochrome oxidase histochemistry reveal emergence of whisker row-related segmentation in the VPM by P3 (Figs. 6 and 7B–I). Nissl staining shows uniform distribution of VPM cells (Fig. 7G), which develop a “barreloid” pattern after P4. Thus, as in the PrV and the barrel cortex, afferent terminal patterning precedes cellular patterning into whisker-specific neuronal modules, the barreloids.
DISCUSSION
The barreloids were identified thirty years ago (Van der Loos, 1976, Belford and Killackey, 1979), however, little is known about their axonal connectivity with the barrelettes in the brainstem. A combined anterograde tracing and electrophysiological study in the rat showed that trigeminothalamic fibers reach the thalamus by E17, begin branching in the VPM by E18, and start elaborating arbors shortly before birth (Leamey and Ho, 1998). A brief progression of the lemniscal pathway was described in a study focusing on the PrV defects in drg11 knockout mice (Ding et al., 2003). Here, we used an embryonic wholemount preparation in which the entire trigeminal lemniscal pathway can be visualized with carbocyanine dye labeling from the earliest time points (E11) up to E17. We also developed an oblique horizontal slice preparation in which the PrV and VPM/VPL with lemniscal projections are preserved in neonatal mice. We found that PrV axons aim towards the midline by E11, cross the midline and make a sharp rostral turn by E13, advance through the tegmentum by E15 and arrive at VPM by E17. Whisker-specific patterning of PrV axon terminals in the VPM develops from an initially diffuse projection segregating into bands corresponding to whisker rows and then patches corresponding to single whiskers by P3.
Differentiation of the PrV-thalamic projection neurons
Little is known about the molecular mechanisms underlying the formation and specification of the PrV, a small brainstem nucleus which relays somatotopic whisker patterns to the downstream thalamic and cortical somatosensory centers. The developing PrV expresses transcription factors drg11 and lmx1b (Ding et al., 2003; Jacquin et al., 2008; Xiang et al., 2010) and genetic invalidations of these transcription factors lead to aberrant distribution of PrV neurons, abnormal trigeminal afferent projections, increased cell death, delayed trigeminothalamic projections and failure to develop whisker-specific patterns, the barrelettes.
The PrV originates from the rostral hindbrain (Marín and Puelles, 1995; Oury et al. 2006), and there is evidence indicating that progeny of rhombomeres 2 and 3 cells are segregated in the dorsal and ventral portions of the PrV (Oury et al., 2006). The rat PrV neurons are generated between E12 and E17 (Altman and Bayer, 1980; Miller and Muller, 1989; Al-Ghoul and Miller, 1993). Similarly, in the mouse, PrV progenitors first emerge from the r2-r3 ventricular zone around E10.5 and by E15.5 the nucleus is mostly formed (Ding et al., 2003). Newborn PrV neurons settle in the ventrolateral hindbrain in an ‘inside-out’ fashion with early generated neurons being located more medially than the later born neurons (Al-Ghoul and Miller, 1993). Our results show that as soon as the PrV neurons are generated they take a tangential migratory route between the floor plate and the lateral side of the pontine flexure. Immature, spindle shaped neurons emit a single axonal process that is attracted towards the floor plate while the cell body and a primitive dendritic process migrates in the opposite direction. While we have not directly observed this with time-lapse imaging, DiI labeling at E10–12 embryos captured PrV neurons in their various stages of axonal extension, midline crossing and cell body extending away from the floor plate.
Members of the semaphorin family are also expressed in the developing hindbrain and they may play a role in preferential growth and migration of dendrites and cell bodies towards the laterally positioned trigeminal tract while their axons grow in the opposite direction towards the midline. Similar bidirectional growth of apical dendrites and axonal processes under the influence of semaphorins has ben reported for various types of neurons (Polleux et al., 2000; Tran et al., 2007)
Midline crossing of the trigeminal lemniscus
The major somatosensory and motor pathways are crossed axonal tracts and this arrangement leads to representation of the somatosensory space on one side of the face and body in the contralateral thalamus and neocortex and motor control of the skeletal musculature in the contralateral cortex. Axonal crossing is a common feature of the vertebrate nervous system and for all the crossing axons the midline acts as an intermediate target by secreting attractants and repellents channeling them to the proper side of the neural space. The major descending motor pathway, the corticospinal tract (CST) axons arise from subpopulations of somatosensory and motor layer V cortical neurons and grow ipsilaterally through the internal capsule, down the cerebral peduncles, through the pons medial to the inferior olive where they abruptly cross forming the pyramidal decussation. CST crossing defects in human mutation of the Dcc gene leads to motor abnormalities such as mirror movements (Vulliemoz et al., 2005; Srour et al., 2010).
Ascending somatosensory axons remain ipsilateral until their first synaptic relay in the dorsal column nuclei. Axons from the dorsal column nuclei, along with their counterparts from the PrV cross the midline, form the medial lemniscus and project to the contralateral thalamic VPM/VPL complex. Thalamocortical axons from the VPM/VPL target the layer IV of the primary somatosensory cortex of the same side. Mutations in Robo3 gene in humans lead to horizontal gaze palsy with progressive scoliosis and hindbrain dysplasia (HGPPS) (Jen et al., 2004). In HGPPS patients the CST and the dorsal column lemniscal pathway fail to cross. In addition the abducens nucleus is hypoplastic affecting horizontal gaze. Similar midline crossing defects have been observed in robo3 knockout mice (Sabatier et al., 2004), which apparently are postnatal lethal.
Midline crossing defects and their consequences are presented in the accompanying paper (Mirza et al., 2012) showing that slit-mediated repulsion and netrin1-mediated attraction are essential in proper crossing of the PrV axons. Immediately after crossing the midline, the PrV axons make a rostrally directed hairpin turn and stay close to the midline. As development progresses, the PrV axons are displaced more laterally and they fan out towards the midbrain. We do not know which attractant/repellent cues guide this phase of the lemniscal pathway formation. In the absence of netrin-1 receptor dcc, PrV axons are repelled by the midline and they lose preference for rostrally directed growth, they defasciculate and are dispersed both rostrally and caudally (Mirza et al., 2012).
At early embryonic stages (E11–13) we noted that the frontline of the PrV lemniscal pathway is not uniform. Some axons have crossed, made a hairpin turn and advanced considerably along the pathway while many others are just crossing the midline and have not made a sharp turn. Thus a pioneering group of axons lay down the lemniscal pathway and others follow them. This is probably related to the differential time of neurogenesis (E10-E15) of PrV neurons. Real time imaging studies are needed to determine the actual progression of neurogenesis and axonal advancement across the midline and along the lemniscal pathway. If the final positioning of earliest generated PrV neurons medially and later generated neurons laterally within the rat PrV also holds for the mouse PrV, it would be interesting to find out whether the PrV-VPM axons that are destined to terminate medially in the VPM arrive first.
“Barrelettes” and “barreloids”
Central trigeminal afferents develop discrete patches of synaptic terminals, the distribution of which replicate the patterned organization of the whiskers on the snout (Erzurumlu and Jhaveri, 1992, Waite et al., 2000). Whisker-specific patterning in the brainstem emerges by E19–20 in the rat (Chiaia et al., 1992; Waite et al., 2000), and at birth in mice (Ma, 1993; Li et al., 1994). Thus, the PrV is the first neural station where a topographic and patterned map of the snout is established. The mouse (and rat) PrV contains three classes of neurons: barrelette neurons, interbarrelette neurons and GABAergic interneurons. Polarized dendritic trees and whisker-specific patterning characterize barrelette neurons. Physiologically they are distinguished by specific membrane properties and synaptic responses. Barrelette neurons display a transient K+ (IA) current and receive monosynaptic excitatory and disynaptic inhibitory inputs upon stimulation of the trigeminal tract (TrV) (Lo et al., 1999).
Whisker-specific neural patterning in the VPM, and subsequently in the primary somatosensory cortex, depends solely upon the inputs from the barrelette cells (Killackey and Fleming, 1985). The rodent VPM contains primarily barreloid cells, which project to the barrel cortex, and inhibitory inputs to the VPM come from the zona incerta and reticular nucleus (Sherman and Guillery, 1996; Deschênes et al., 2005; Trageser et al., 2006). VPM cells, like barrelette neurons, orient their dendrites and somata in relation to PrV afferent terminal patches, forming the “barreloids.” Barreloid cells in turn, convey the whisker-specific patterns to the layer IV of the primary somatosensory cortex, where spiny stellate cells form the “barrels”. The first step in matching the cortical barrel patterns to the arrangement of whiskers and sinus hairs on the snout is somatotopic projections from the PrV to the VPM and formation of barreloids instructed by the PrV barrelette cells.
Previously, we showed that in the PrV and the barrel cortex whisker-related afferent terminals start out as simple branches and develop focalized terminal arbors in an NMDAR-dependent fashion (Lee et al., 2005 a, b; Erzurumlu and Iwasato, 2006). Surprisingly, PrV lemniscal terminal arbors in VPM differ from their whisker-related primary sensory trigeminal afferent and thalamocortical counterparts. Lemniscal axons arrive in VPM at E17 and initially form diffuse arbors. From this exuberant terminal field, whisker-related row patterns and restricted terminal patches arise gradually and become distinct by few days after birth.
In the rodent, feline, and primate species eye-specific laminar segregation of retinal terminals develops from initially overlapping terminal fields in the lateral geniculate nucleus (LGN) of the thalamus (Huberman, 2007). The trigeminothalamic afferent terminal segregation and patterning appears to follow a parallel developmental scheme to that seen in the LGN. Pruning of axon terminal fields from an initially exuberant arborization was previously noted for the wallaby, a species in which the development, invasion and patterning of PrV-based lemniscal axons in the VPM occurs over a month-long period (Leamey et al., 1998). Thus, development of discrete lemniscal terminal arbors in the VPM follows a similar pruning scheme that is seen in other sensory thalamic nuclei as well as across species. The molecular substrates of patterning of the trigeminothalamic terminals remain to be elucidated.
Acknowledgements
Research supported by NIH/NINDS 5P01NS49048 and 5RO1NS037070
We thank S. Zhao, C. Bernardelli and F. Akkentli for their technical assistance.
Footnotes
Conflict of interest statement.
The authors state that there is no conflict of interest.
Role of authors
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: RSE. Acquisition of data: BGK, RSE. Analysis and interpretation of data: RSE, BGK. Drafting of the manuscript: BGK, RSE. Critical revision of the manuscript for important intellectual content: RSE. Obtained funding: RSE. Technical support: SZ, CB, FA. Study supervision: RSE.
LITERATURE CITED
- Agmon A, Yang LT, O'Dowd DK, Jones EG. Organized growth of thalamocortical axons from the deep tier of terminations into layer IV of developing mouse barrel cortex. J Neurosci. 1993;13:5365–5382. doi: 10.1523/JNEUROSCI.13-12-05365.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ghoul WM, Miller WM. Orderly migration of neurons to the principal sensory nucleus of the trigeminal nerve of the rat. J Comp Neurol. 1993;330:464–475. doi: 10.1002/cne.903300403. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the brain stem in the rat IV. Thymidine-autoradiographic study of the time of origin of neurons in the pontine region. J Comp Neurol. 1980;194:905–929. doi: 10.1002/cne.901940411. [DOI] [PubMed] [Google Scholar]
- Bates CA, Killackey HP. The organization of the neonatal rat's brainstem trigeminal complex and its role in the formation of central trigeminal patterns. J Comp Neurol. 1985;240:265–287. doi: 10.1002/cne.902400305. [DOI] [PubMed] [Google Scholar]
- Belford GR, Killackey HP. The development of vibrissae representation in subcortical trigeminal centers of the neonatal rat. J Comp Neurol. 1979;188:63–74. doi: 10.1002/cne.901880106. [DOI] [PubMed] [Google Scholar]
- Chiaia NL, Bennett-Clarke CA, Eck M, White FA, Crissman RS, Rhoades RW. Evidence for prenatal competition among the central arbors of trigeminal primary afferent neurons. J Neurosci. 1992;12:62–76. doi: 10.1523/JNEUROSCI.12-01-00062.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschênes M, Timofeeva E, Lavallée P, Dufresne C. The vibrissal system as a model of thalamic operations. Prog Brain Res. 2005;149:31–40. doi: 10.1016/S0079-6123(05)49003-2. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Yin J, Xu HM, Jacquin MF, Chen ZF. Formation of whisker-related principal sensory nucleus-based lemniscal pathway requires a paired homeodomain transcription factor, Drg11. J Neurosci. 2003;23:7246–7254. doi: 10.1523/JNEUROSCI.23-19-07246.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham D, Woolsey TA. Effects of neonatal whisker lesions on mouse central trigeminal pathways. J Comp Neurol. 1984;223:424–447. doi: 10.1002/cne.902230308. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Jhaveri S. Trigeminal ganglion cell processes are spatially ordered prior to the differentiation of the vibrissa pad. J Neurosci. 1992;12:3946–3955. doi: 10.1523/JNEUROSCI.12-10-03946.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS, Killackey HP. Critical and sensitive periods in neurobiology. Curr Top Dev Biol. 1982;17:207–240. doi: 10.1016/s0070-2153(08)60522-0. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Iwasato T. Erzurumlu R, Guido W, Molnár Z. Development and plasticity in sensory thalamus and cortex. New York: Springer; 2006. Patterning of the somatosensory maps with NMDA receptors; pp. 158–182. [Google Scholar]
- Erzurumlu RS, Murakami Y, Rijli FM. Mapping the face in the somatosensory brainastem. Nat Rev Neurosci. 2010;11:252–263. doi: 10.1038/nrn2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost DO. Development of anomalous retinal projections to nonvisual thalamic nuclei in Syrian hamsters: a quantitative study. J Comp Neurol. 1986;252:95–105. doi: 10.1002/cne.902520106. [DOI] [PubMed] [Google Scholar]
- Huberman AD. Mehanisms of eye-specific visual circuit development. Curr Opin Neurobiol. 2007;17:73–80. doi: 10.1016/j.conb.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Jacquin MF, Arends JJ, Xiang C, Shapiro LA, Ribak CE, Chen ZF. In DRG11 knock-out mice, trigeminal cell death is extensive and does not account for failed brainstem patterning. J Neurosci. 2008;28:3577–3585. doi: 10.1523/JNEUROSCI.4203-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen JC, Chan WM, Bosley TM, Wan J, Carr JR, Rüb U, Shattuck D, Salamon G, Kudo LC, Ou J, Lin DD, Salih MA, Kansu T, Al Dhalaan H, Al Zayed Z, MacDonald DB, Stigsby B, Plaitakis A, Dretakis EK, Gottlob I, Pieh C, Traboulsi EI, Wang Q, Wang L, Andrews C, Yamada K, Demer JL, Karim S, Alger JR, Geschwind DH, Deller T, Sicotte NL, Nelson SF, Baloh RW, Engle EC. Mutations in human ROBO gene disrupt hindbrain axon pathway crossing and morphogenesis. Science. 2004;304:1509–1513. doi: 10.1126/science.1096437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killackey HP, Fleming K. The role of the principal sensory nucleus in central trigeminal pattern formation. Brain Res. 1985;354:141–145. doi: 10.1016/0165-3806(85)90077-x. [DOI] [PubMed] [Google Scholar]
- Leamey CA, Ho SM. Afferent arrival and onset of functional activity in the trigeminothalamic pathway of the rat. Dev Brain Res. 1998;105:195–207. doi: 10.1016/s0165-3806(97)00170-3. [DOI] [PubMed] [Google Scholar]
- Leamey CA, Ho SM, Marotte LR. Morphological development of afferent segregation and onset of synaptic transmission in the trigeminothalamic pathway of the wallaby (Macropus eugenii) J Comp Neurol. 1998;399:47–60. doi: 10.1002/(sici)1096-9861(19980914)399:1<47::aid-cne4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Lee LJ, Iwasato T, Itohara S, Erzurumlu RS. Exuberant thalamocortical axon arborization in cortex-specific NMDAR1 knockout mice. J Comp Neurol. 2005a;485:280–292. doi: 10.1002/cne.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LJ, Lo F-S, Erzurumlu RS. NMDA receptor-dependent regulation of axonal and dendritic branching. J Neurosci. 2005b;25:2304–2311. doi: 10.1523/JNEUROSCI.4902-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Erzurumlu RS, Chen C, Jhaveri S, Tonegawa S. Whisker related neuronal patterns fail to develop in the trigeminal brainstem nuclei of NMDAR1 knockout mice. Cell. 1994;76:427–437. doi: 10.1016/0092-8674(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Liguz-Lecznar M, Skangiel-Kramska J. Vesicular glutamate transporters VGLUT1 and VGLUT2 in the developing mouse barrel cortex. Int J Dev Neurosci. 2007;25:107–114. doi: 10.1016/j.ijdevneu.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Lo FS, Guido W, Erzurumlu RS. Electrophysiological properties and synaptic responses of cells in the trigeminal principal sensory nucleus of postnatal rats. J Neurophysiol. 1999;82:2765–2775. doi: 10.1152/jn.1999.82.5.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma PM. Barrelettes: architectonic vibrissal representations in the brainstem trigeminal complex of the mouse: II. normal postnatal development. J Comp Neurol. 1993;327:376–397. doi: 10.1002/cne.903270306. [DOI] [PubMed] [Google Scholar]
- Ma PM, Woolsey TA. Cytoarchitectonic correlates of the vibrissae in the medullary trigeminal complex of the mouse. Brain Res. 1984;306:374–379. doi: 10.1016/0006-8993(84)90390-1. [DOI] [PubMed] [Google Scholar]
- Marín F, Puelles L. Morphological fate of rhombomeres in quail/chick chimeras: a segmental analysis of hindbrain nuclei. Eur J Neurosci. 1995;7:1714–1738. doi: 10.1111/j.1460-9568.1995.tb00693.x. [DOI] [PubMed] [Google Scholar]
- Miller MW, Muller SJ. Structure and histogenesis of the principal sensory nucleus of the trigeminal nerve: effects of prenatal exposure to ethanol. J Comp Neurol. 1989;282:570–580. doi: 10.1002/cne.902820408. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Hioki H, Fujiyama F, Kaneko T. Postnatal changes of vesicular glutamate transporter (VGluT)1 and VGluT2 immunoreactivities and their colocalization in the mouse forebrain. J Comp Neurol. 2005;492:263–288. doi: 10.1002/cne.20705. [DOI] [PubMed] [Google Scholar]
- Oury F, Murakami Y, Renaud JS, Pasqualetti M, Charnay P, Ren SY, Rijli FM. Hoxa2- and rhombomere-dependent development of the mouse facial somatosensory map. Science. 2006;313:1408–1413. doi: 10.1126/science.1130042. [DOI] [PubMed] [Google Scholar]
- Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for apical dendrites. Nature. 2000;404:567–573. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- Sabatier C, Plump AS, Le Ma, Brose K, Tamada A, Murakami F, Lee EY, Tessier-Lavigne M. The divergent Robo family protein rig-1/Robo3 is a negative regulator of slit responsiveness required for midline crossing by commissural axons. Cell. 2004;117:157–169. doi: 10.1016/s0092-8674(04)00303-4. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Functional organization of thalamocortical relays. J Neurophysiol. 1996;76:1367–1395. doi: 10.1152/jn.1996.76.3.1367. [DOI] [PubMed] [Google Scholar]
- Soiza-Reilly M, Commons KG. Quantitative analysis of glutamatergic innervation of the mouse dorsal raphe nucleus using array tomography. J Comp Neurol. 2011;519:3802–38014. doi: 10.1002/cne.22734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srour M, Rivière JB, Pham JM, Dubé MP, Girard S, Morin S, Dion PA, Asselin G, Rochefort D, Hince P, Diab S, Sharafaddinzadeh N, Chouinard S, Théoret H, Charron F, Rouleau GA. Mutations in DCC cause congenital mirror movements. Science. 2010;328:592. doi: 10.1126/science.1186463. [DOI] [PubMed] [Google Scholar]
- Tran TS, Kolodkin AL, Bharadwaj R. Semaphorin regulation of cell morphology. Annu Rev Cell Dev Biol. 2007;23:263–292. doi: 10.1146/annurev.cellbio.22.010605.093554. [DOI] [PubMed] [Google Scholar]
- Trageser JC, Burke KA, Masri R, Li Y, Sellers L, Keller A. State-dependent gating of sensory inputs by zona incerta. J Neurophysiol. 2006;96:1456–1463. doi: 10.1152/jn.00423.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Loos H. Barreloids in mouse somatosensory thalamus. Neurosci Lett. 1976;2:1–6. doi: 10.1016/0304-3940(76)90036-7. [DOI] [PubMed] [Google Scholar]
- Vulliemoz S, Raineteau O, Jabaudon D. Reaching beyond the midline: why are human brains cross wired? Lancet Neurol. 2005;4:87–99. doi: 10.1016/S1474-4422(05)00990-7. [DOI] [PubMed] [Google Scholar]
- Waite PM, Ho SM, Henderson TA. Afferent ingrowth and onset of activity in the rat trigeminal nucleus. Eur J Neurosci. 2000;12:2781–2792. doi: 10.1046/j.1460-9568.2000.00161.x. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT, Welt C. Histochemical changes in cytochrome oxidase of cortical barrels after vibrissal removal in neonatal and adult mice. Proc Natl Acad Sci USA. 1980;77:2333–2337. doi: 10.1073/pnas.77.4.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang C, Zhang KH, Yin J, Arends JJ, Erzurumlu RS, Jacquin MF, Chen ZF. The transcription factor, Lmx1b, is necessary for the development of the principal trigeminal nucleus-based lemniscal pathway. Mol Cell Neurosci. 2010;44:394–403. doi: 10.1016/j.mcn.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]