Abstract
Mercury levels in sediment and predatory fish were measured for 53 localities in Suriname. The average mercury level in bottom sediment surpassed the Canadian standard for sediment in most localities, except the coastal plains. Of the predatory fish, 41 % had a mercury level above the European Union standard for human consumption of 0.5 μg g−1. Highest mercury levels were found in fish from the Brokopondo Reservoir and from the Upper Coppename River. High levels of mercury in fish in pristine areas are explained by atmospheric transportation of mercury with the northeastern trade winds followed by wet deposition. Contrary to gold mining areas, where mercury is bound to drifting sediments, in “pristine” areas the mercury is freely available for bio-accumulation and uptake. Impacts on piscivorous reptiles, birds, and mammals are unknown, but likely to be negative.
Keywords: Mercury pollution, Small-scale gold mining, Aquatic ecosystems, Pristine environment, Suriname
Introduction
Gold mining on a small- to medium-scale started in Suriname at the end of the eighteenth century (De Vletter and Hakstege 1998). This gold rush was at its height in 1907 when 1200 kg gold was produced annually, but by 1910 the annual production had already declined to 200 kg (Bosma et al. 1973). The discovery of the Serra Pelada gold mine in 1980 triggered a new gold rush that spread over Brazil and neighboring countries (Veiga 1997). The revival of small-scale gold mining in Suriname was further enhanced by an increase of the gold price, deterioration of the local economy, immigration of Brazilian miners, and the activities of foreign prospecting companies. In 2000, the Geological and Mining Survey of Suriname (GMD) estimated the number of small-scale gold miners at 25 000–35 000 (Mol et al. 2001). Veiga (1997) estimated the production in Suriname at 10 000–20 000 kg gold per year. The area where on geological grounds most gold is to be expected in Suriname, is the Greenstone Belt, a zone from northern Central to Eastern Suriname to South-eastern Suriname (dark green colored area in Fig. 1a), and this is also the area where most gold mining activities are occurring.
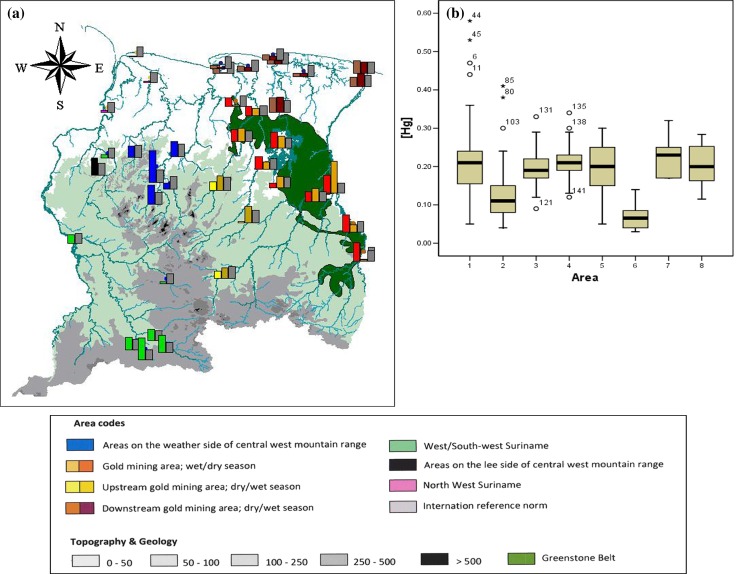
Fig. 1.
a Average mercury levels found in sediments in different river systems in Suriname; Gray bars represent the Canadian Interim Sediment Quality Guideline for Protection of Aquatic Life of 0.17 μg L−1. b Boxplot showing the distribution of mercury levels in sediment, measured in different areas. Codes for areas: 1 GMA, 2 DGMA, 3 UGMA, 4 Brokopondo Reservoir (BR), 5 WSW, 6 NW, 7 PLS, 8 PWS
During the last decennium, many small-scale operations have been scaled-up to medium-scale operations and it is therefore likely that the gold production in Suriname has increased. In artisanal gold production mercury is usually used for amalgamation of the gold, and it is estimated that for every kilogram of gold produced, 1 kg of mercury is lost to the environment (Veiga 1997). The amalgamation process usually takes place in the sluice box. In terrestrial operations the soil of creek bottom and shores is flushed into sludge by water power and this sludge is then pumped to the sluice box. Usually no tailing pond is constructed and the mud-stream from the sluice box freely enters the creek without any further treatment. In river operations a suction dredge is used to reach the gold containing bottom sediment. It is clear that apart from mercury pollution, these methods cause numerous additional environmental impacts. For the aquatic ecosystem, one of the most severe impacts is an increased sediment input into the stream causing increased turbidity (Mol and Ouboter 2004).
Of the mercury used in gold mining operations, approximately 55 % is lost to the atmosphere and 45 % is lost to streams (Pfeiffer and Lacerda 1988). If the estimate of the gold production by Veiga (1997) is correct, at least 10 000–20 000 kg of mercury is released into the environment of Suriname annually.
Mercury is a heavy metal that is toxic to many animals, especially vertebrates, including humans. Aquatic microorganisms transform metallic mercury into the extremely toxic methylmercury (Morel et al. 1998) which is assimilated rapidly by aquatic biota and accumulates in the food chain: the bio-accumulation factor (BAF) for piscivorous fish calculated by the US EPA (1997) is 6 800 000.
The enormous amount of mercury estimated to be lost to the environment in Suriname, has caused concern for many years. This led to the implementation of different research projects, resulting in more insight in the mercury levels in the main river systems in Suriname (also see De Kom et al. 1998; Pollack et al. 1998).
This paper combines the results of the different studies and intends to give an overview of the mercury levels found in the main river systems in Suriname and to clarify these mercury levels in different parts of the country, especially explaining the high levels in pristine central and western Suriname.
Materials and Methods
Study Sites
In total, 53 sites have been surveyed all over the country between 2002 and 2010. These sites can be grouped into seven different areas (see Fig. 1a):
1. Sites in the gold mining area, GMA; 2. Sites downstream of the gold mining area, DGMA; 3. Sites upstream of the gold mining area, UGMA; 4. Sites in pristine central Suriname where on geological grounds no gold and mercury deposits are present and which are situated on the weather side of the central west located mountain range, PWS; 5. Sites in West/Southwest Suriname, WSW; 6. Sites in North-west Suriname, NW; 7. Sites in areas on the lee side of the central west mountain range, not draining the mountain range, PLS.
At each site medium- to large-size streams (width >4 m) were sampled, except for the Brokopondo Reservoir (BR). Therefore, the Brokopondo Reservoir has been taken as a separate site in the analyses when comparisons between sites were made.
Sampling, Sample Treatment, and Analysis
In the first phase of the project, samples of sediment and fish muscle tissue were taken from the localities during the high and low water seasons. In total, 228 bottom sediments samples have been collected from 53 localities and 885 freshwater fishes consisting of 55 species.
Bottom sediment was taken near the shore in pre-flushed polypropylene containers. Especially recently deposited, fine sediments were taken. In addition, six core samples were taken from floodplain areas using a soil core sampler. Core samples were preferably taken to a depth of 50 cm, but in some cases the sampler could not go deeper than 25 cm because of roots. Core samples were separated in the laboratory, analyzing soil samples from 10 cm apart.
Fish was bought from local fishermen or caught overnight with gill nets. Specimens were identified with keys presented by Le Bail et al. (2000), Keith et al. (2000), and Planquette et al. (1996). After taking the wet mass and standard length of a fish, a small piece of muscle tissue was taken below the dorsal fin, weighted, and packed in ziplock bags. All samples were stored on ice till in the laboratory. In the laboratory sediment samples were dried, crushed, and sieved (50 μm), and fish samples were frozen and stored at −40 °C till analysis (usually within a few weeks, at most 3 months).
Mercury concentrations were measured with a Bacharach Mercury Analyzer using cold-vapor atomic absorption (Clesceri et al. 1998). Sediment and fish samples were first destructed by boiling with acids (adapted from EPA methods 245.1, 245.5, and 245.6, US EPA 1991). To check the accuracy of the analysis, standard solutions of mercury and reference fish material (DORM-3, National Research Council Canada) were analyzed together with a few batches of samples.
In addition to the mercury sampling, in the field water quality (temperature, pH, conductivity, dissolved oxygen, turbidity, secchi depth, and chloride) was measured to know the general condition of the stream and the form most mercury would be in under the given environmental conditions.
Data Analysis
Mercury levels were first tested between dry and wet season. Because the mercury levels significantly differed between seasons for at least one area (t test, t = −2.84, df = 4, p = 0.02), it was decided to treat dry and wet season levels separately during subsequent analysis. Mercury levels resulting from the analysis were compared with global background standards and other international standards (Canada, European Union). To detect patterns in the mercury levels found, the sites which have been grouped in areas, were statistically compared with each other using SPSS software (version 16.0). Results were mapped using Arcview 3.3 software.
Results
Sediments
Figure 1 shows the mercury levels of bottom sediments within the different areas.
Most sediment samples have a mercury level well above global background levels of 0.01–0.05 μg g−1 (Anderson 1979). Only five samples (2 %) were within global background levels; four of these were from northwestern Suriname. Sediments in the gold mining area are usually near or above the Canadian Interim Sediment Quality Guideline for Protection of Aquatic Life (Canadian Council of Ministers of the Environment 1999) of 0.17 μg g−1 soil. The estuary of the Marowijne River (eastern border river) shows relatively high levels at its mouth (max. 0.41 μg g−1). Surprisingly, mercury levels of sediments in UGMA sites (Fig. 1a) are all near or above the Canadian standard during the rainy season. Moreover in pristine areas of central, western, and southern Suriname, levels often exceed this standard (average 0.20 μg g−1, max. 0.28 μg g−1). This is especially the case in the PWS area (Fig. 1a). Levels in the most southwestern localities are high as well (Fig. 1a). Mercury levels in bottom sediments are low in NW (Fig. 1).
When looking at the average level per region (Table 1), all regions, except DGMA and NW, surpass the Canadian standard for sediments. Mercury levels from these two areas are also significantly lower than mercury levels from other locations (ANOVA, F = 10.539, df = 7, p = 0.00), see Fig. 1b.
Table 1.
Number of localities sampled, number of samples and average mercury level of water, bottom sediment, Piranha (Serrasalmus rhombeus) and Aimara (Hoplias aimara) for various areas in Suriname; also US EPA Standard for Drinking Water, US EPA Standard for freshwater chronic exposure (US EPA 1994), US EPA Wildlife Criterion for surface water, Canadian standard for sediment (Canadian Council of Ministers of the Environment, 1999), and European standard for human consumption of piscivorous fish (EC 2002)
| Area | Number of localities | Water | Bottom sediment | Serrasalmus rhombeus | Hoplias aimara | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Average Hg (μg L−1) | n | Average Hg (μg g−1) | n | Average size (cm) | Average Hg (μg g−1) | n | Average size (cm) | Average Hg (μg g−1) | ||
| Gold mining (GMA) | 9 | 40 | 0.07 ± 0.09 | 69 | 0.22 ± 0.09 | 30 | 25.6 | 0.42 ± 0.20 | 58 | 52.8 | 0.46 ± 0.23 |
| Brokopondo Reservoir (BR) | 6 | 12 | 0.38 ± 0.37 | 17 | 0.21 ± 0.06 | 36 | 29.2 | 1.38 ± 0.59 | 16 | 49.7 | 0.43 ± 0.29 |
| Downstream gold mining (DGMA) | 9 | 35 | 0.06 ± 0.06 | 50 | 0.13 ± 0.08 | 9 | 24.2 | 0.23 ± 0.13 | |||
| Upstream gold mining (UGMA) | 4 | 12 | 0.11 ± 0.09 | 18 | 0.20 ± 0.05 | 30 | 15.4 | 0.25 ± 0.21 | 35 | 47.5 | 0.43 ± 0.24 |
| Upper Coppename Basin (PWS) | 5 | 6 | 0.10 ± 0.07 | 8 | 0.20 ± 0.06 | 25 | 33.8 | 0.86 ± 0.42 | 13 | 53.3 | 0.66 ± 0.29 |
| Western Suriname (WSW) | 11 | 6 | 0.10 ± 0.15 | 6 | 0.14 ± 0.05 | 20 | 30.2 | 0.75 ± 0.44 | 24 | 50.7 | 0.65 ± 0.17 |
| Northwestern Suriname (NW) | 6 | 6 | 0.38 ± 0.50 | 12 | 0.07 ± 0.03 | ||||||
| Predicted low level (PLS) | 3 | 22 | 0.04 ± 0.02 | 42 | 0.21 ± 0.05 | 30 | 31.4 | 0.51 ± 0.19 | 4 | 70.0 | 0.74 ± 0.25 |
| International standards | 0.2 | ||||||||||
| 0.012 | 0.17 | 0.50 | 0.50 | ||||||||
| 0.00091 | |||||||||||
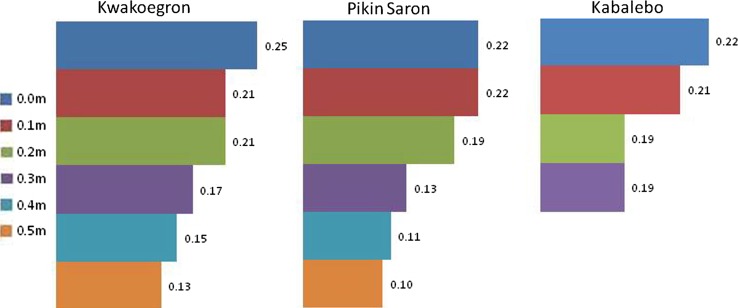
Six core samples were analyzed, four from floodplains fed by rivers running through gold mining areas and two from pristine western Suriname. The results from all core samples indicate that the mercury in these rivers is anthropogenic, the concentrations rising with the deposition of new sediment layers. Figure 2 shows the core sample mercury levels for the deeper cores.
Fig. 2.
Results of mercury analyses of core sediment samples taken from sites in two river systems; Pikin Saron (DGMA), Kwakoegron (GMA) located at the Saramacca River, downstream of gold mining areas, and Kabalebo (PLS) located at the Kabalebo River, pristine western Suriname. D0 depth 0 cm; D10 depth 10 cm; D20 depth 20 cm; D30 depth 30 cm; D40 depth 40 cm; D50 depth 50 cm
Fish
Of all collected fish 79 % belonged to piscivorous species. The most common species was the Red-eyed Piranha (Serrasalmus rhombeus) comprising 34 % of all specimens, the second commonest species was the Aimara (Hoplias aimara) with 18 % of all specimens. Both species are not known to undertake extensive river migrations (Mol et al. 2001). Of the piscivores 41 % had a mercury level above the European Union standard for human consumption of 0.5 μg g−1 (EC 2002) and 15 % above the US EPA standard for human consumption of 1 μg g−1 (US EPA 1994).
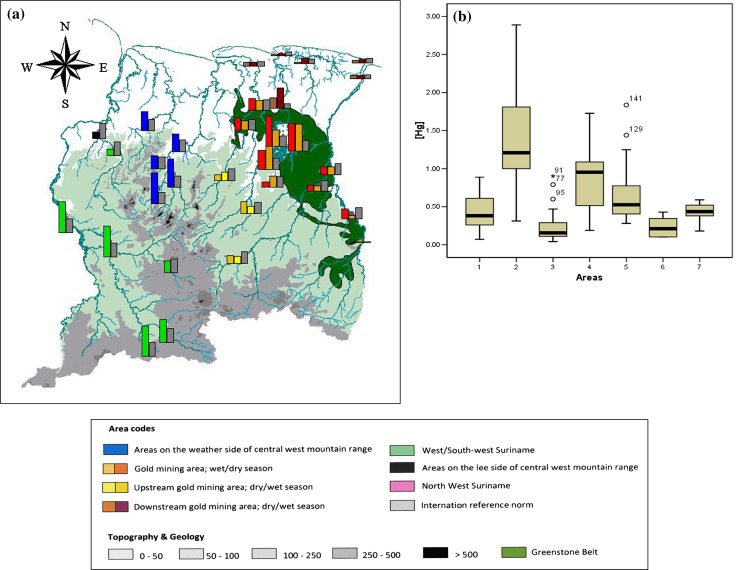
Results for the average mercury levels in muscle tissue of piscivorous fish per locality are presented in Fig. 3. In the gold mining areas levels are often above the European Union standard. Extreme high levels were measured in Brokopondo Reservoir where in some cases the levels in piranhas were six to seven times the norm for human consumption (on average two to three times). Downstream of the gold mining area, levels were usually much lower. Upstream of gold mining area, mercury values in piscivorous fish were generally below the norm, but in central and western Suriname levels were far above the norm. This concerned the same drainages that were already noted for their high levels in sediment.
Fig. 3.
a Average mercury levels found in piscivorous fishes in different river systems in Suriname; Gray bars represent the European Union standard for human consumption of 0.5 μg g−1. b Boxplot showing the distribution of mercury levels in piscivorous fishes, measured in different areas. Codes for areas: 1 GMA, 2 Brokopondo Reservoir (BR), 3 UGMA, 4 PWS, 5 WSW, 6 DGMA, 7 PLS
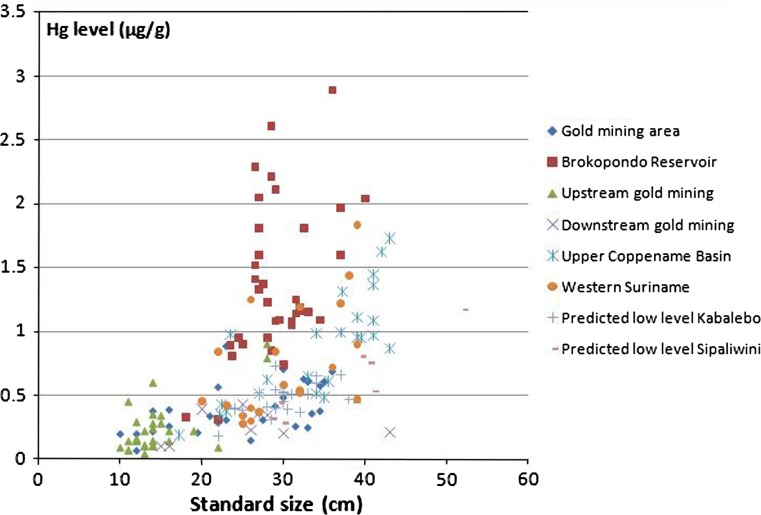
Fish may accumulate mercury during all of their lives. Consequently young (small) fish have generally lower levels than older (larger) fish. In the spatial presentation of Fig. 3, fish size is not accounted for and may influence the average levels at certain localities. To check our results we plotted piranha standard length against mercury levels (Fig. 4) and tested mercury from different areas for significant differences. Significant difference was found in the mercury levels between areas (ANOVA, F = 34.197, df = 6, p = 0.00). Piranhas from the BR have significantly higher mercury levels than piranhas from other areas, as shown in Fig. 4. Piranhas from PWS and WSW also have significant higher values than piranha from GMA. Piranha specimens in PWS are the largest encountered (Table 1) and it could therefore be argued that it is the large size (old age) of these specimens raising the mercury level. However, when excluding the largest specimens of the PWS and the smallest specimens of the GMA, to arrive at the same average size of these weighted population samples, the mercury level of the PWS is still 0.24 μg g−1 higher and the difference is significant (t test; p = 0.01). The presence of high fish-mercury levels in pristine areas is not unique for the piranha, but was also found for another common predatory fish, the aimara (Hoplias aimara) (see average Hg levels in Table 1).
Fig. 4.
Hg levels measured in Piranha (Serrasalmus rhombeus) against fish size for different areas
Creeks on the lee side of the central western mountainous areas, not draining these mountains (PLS) were predicted to have low levels of mercury (Figs. 1a, 3a). However, mercury levels in sediments were as high as for the PWS and the GMA. In S. rhombeus mercury levels are significantly lower than for the PWS (t test; p = 0.0005) and WSW (t test; p = 0.03). Sufficient data from predicted low level areas are not available for H. aimara.
Since the research took place over an extensive period of 7 years, it could be argued that mercury levels might have increased between the first surveys and later surveys. Therefore, comparisons were made for two sites that were sampled in both 2004/2005 and 2008/2009. Differences are not significant for both the sites in the PLS area (t test; p = 0.22) and the Brokopondo Reservoir (t test; p = 0.41).
Discussion
Mercury Levels in Gold Mining Areas
Mol et al. (2001) presented data for mercury concentrations in fish. The values reported for gold mining areas and Brokopondo Reservoir are in general agreement with what is presented here. The high mercury levels in Brokopondo Reservoir can probably be explained by a combination of factors: 1. Natural accumulation of heavy metals in a lake environment (e.g. Watras et al.1995 (from Bodaly et al.1993), Harris and Bodaly 1998); 2. High methylation rates within the bottom anoxic water layer of the lake caused by sulfur-reducing bacteria activity (Boudou et al.2006); 3. The low levels of mercury in the trees of the drowned forest were released in the lake; 4. The addition of mercury by gold mining. 5. A short, fine sediment-based food chain (sediment-Hemiodus-Serrasalmus) (Mol et al. 2007).
Mol et al. (2007) reported stunting in the piranha population of the Brokopondo Reservoir. This means that small piranhas may be relatively old. Applying standard length transformation on such data may cause misinterpretation of the data. Therefore, the data for S. rhombeus of the Brokopondo Reservoir in Fig. 4, should be looked at with care.
Mol et al. (2001) found some marine fish species to have accumulated high levels of mercury too. This study shows that sediment from the estuary of the Marowijne River had elevated levels of mercury, showing that mercury pollution is transported downstream the river and is therefore likely to impact the level in marine organisms as well.
Mercury pollution related to small- to medium-scale gold mining is a problem over large areas of the Amazon Basin and adjacent countries (Lacerda et al. 1991). Richard et al. (2000) present data for French Guiana, where gold mining was present over most of the country, showing values that are comparable to the values from (non-lake) gold mining areas in Suriname. Mol et al. (2001) already compared mercury data from fish from Suriname with data from Brazil. From Brazil high mercury levels in both gold mining areas (e.g., Pfeiffer et al. 1989; Lacerda et al. 1994; Malm et al. 1997) and non-gold mining areas (Lacerda et al. 1991; Silva-Forsberg et al. 1999) are reported.
Mercury Levels in Pristine Areas in Suriname
High mercury values in soils in undisturbed areas can only originate from specific geological formations with high Hg contents, from atmospheric input or from disturbance upstream. Considering the high values in the PWS area and at the most western locations, disturbance upstream is absent. We tested the impact of a geological formation in this area, the Fallawatra Formation (De Vletter et al.1988) on mercury levels. This formation mainly consists of charnockitic granulites, while most of the Surinam interior consists of granitoid rocks (De Vletter 1984). The Fallawatra Formation largely coincides with the PWS area and the mountain range west of it. The most upstream localities we measured in the PWS area (Figs. 1a, 3a) are upstream of this formation and show high levels in both sediments and fish, indicating that it is not this geological formation that is responsible for the high mercury concentrations. Roulet et al. (1998) stated that “… the natural burden of the soils is much more important than potential new inputs of anthropogenic Hg from gold mining or biomass burning, representing more than 97 % of the Hg accumulated in soils. Consequently, the deposition and incorporation of anthropogenic Hg is negligible and soils could be considered as a major reservoir of natural Hg”. It could therefore be argued that mercury levels measured in Suriname are mainly caused by Hg concentration in the natural soil. However, core sediment samples from floodplains show increasing mercury levels towards the surface, indicating that mercury levels in the rivers of Suriname have mainly an anthropogenic origin. These results are in agreement with results for the Madeira River in Brazil and the Mazaruni River in Guyana (Miller et al. 2003; Bastos et al.2006).
The atmospheric transportation of mercury from the gold mining areas in the east to southwest Suriname with northeastern trade winds seems evident. According to Pfeiffer and Lacerda (1988), 55 % of the mercury used in gold mining operations is lost to the atmosphere, among others through amalgam burning. However, no correlation with the distance from the mining operation could be found (unpublished data). Upon closer examination of the data, a pattern became clear. The amount of mercury depositing from the atmosphere can be related to the amount of precipitation an area receives and the drainage it is in. Much of the precipitation is being released on mountain ranges due to the air being pushed upward here. An area like the PWS area, almost completely surrounded by mountains above 500 m, will receive a large amount of the runoff of precipitation [e.g., Tafelberg, one of the tepu’s in this area, is reported as locality with the highest amount of precipitation in Suriname (Boedhram 1988)] and will therefore be polluted by relatively high amounts of mercury. On the basis of this hypothesis it is possible to predict what areas will receive high amounts of mercury and what areas receive less. The northwestern corner of Suriname will receive low amounts of mercury since the northeastern trade winds passing the gold mining areas will never arrive here. This prediction seems to hold for sediments (Figs. 1, 3; Table 1) (insufficient numbers of predatory fish were available from this area). Also streams on the leeside of the mountains, and not draining these mountains should be much lower in mercury. We tested this prediction sampling various left-shore tributaries of the Kabalebo River in the PLS area (Fig. 4), and indeed the prediction was proven to be true for piscivorous fish.
Relation Water Quality and Mercury Levels in Piscivorous Fish
The water quality of the Upper Coppename Basin (PWS area) is relatively well-known since it was studied during an aquatic RAP expedition of Conservation International (Ouboter and Landburg 2006) and in most parameters does not differ from other interior rivers (Haripersad-Makhanlal and Ouboter 1993). The most striking difference with streams in the gold mining area is in TSS/turbidity. The transparency of the water of the Coppename Basin differs between streams, but streams were never very turbid and secchi disk measurements sometimes surpassed 2 m. In the gold mining areas the water is very turbid from the sediment load during most of the year (e.g., Mol and Ouboter 2004). This difference may explain the higher mercury levels of fishes in pristine rivers in central and western Suriname. In gold mining areas mercury is polluting the streams directly, but much of the mercury is bound to fine sediment particles (Morel et al.1998) released by the mining operations. This mercury is not available for bio-accumulation in the biota. In pristine rivers, however, mercury enters the streams through atmospheric transportation and deposition and only a little amount is bound to sediments, most is available for methylation and bio-accumulation. Top predators in the aquatic ecosystems of pristine rivers, where deposition of mercury from the atmosphere takes place, may reach higher mercury levels this way.
In summary, the high mercury levels in pristine central and western Suriname can probably be explained by: 1. Atmospheric transportation of mercury from the gold mining areas to the southwest by the northeastern trade winds; 2. Wet deposition of atmospheric mercury, with the highest amounts polluting streams draining mountain ranges with high precipitation; 3. Mercury in pristine streams is freely available for methylation and bio-accumulation. In comparison, mercury in mining areas is to a large extent bound to fine sediment particles and therefore not freely available for bio-accumulation.
Conservation Considerations
Gold mining not only impacts areas where the miners work, but also down-wind pristine areas such as nature reserves. The mountainous areas and basins for which high mercury levels were found, coincide almost completely with the Central Suriname Nature Reserve, a 1.6 million ha wilderness area in an almost pristine stage (UNESCO 2011). Apart from some limited eco-tourism and hunting and fishing by a few small, local communities living near the borders of the reserve, no human activities occur here. However, the rivers seem to receive a high load of mercury from the atmosphere. The impact of mercury on fish needs further investigations. Laboratory experiments with fish from temperate climates show adverse impacts on behavior, gonadal development, production of sex hormones and reproduction, at methylmercury levels occurring in Hg sensitive environments (Scheuhammer et al. 2007). There is some evidence that tropical fish have a much faster uptake of mercury and a higher bio-accumulation factor (Oliveira Ribeiro et al. 2000). Tanan et al. (2006) reported effects on the retina of the tropical Hoplias malabaricus at methylmercury levels that may be encountered in the field. Possible effects from mercury on the fish population of the PWS area are not visible at present; the CI AquaRAP ichthyologists described the fishes as being “in excellent condition; the predators such as S. rhombeus and H. aimara were abundant and of very large size; the colors of the ornamental fishes were brilliant; there were almost no parasites or infections found on the fishes” (Mol et al. 2006). Of course, fish loaded with mercury is likely to present a health risk to the local communities living downstream, since fish is their main source of protein.
Mercury, and especially methylmercury, is extremely poisonous for mammals and birds. Laboratory experiments have shown behavioral, neurochemical, hormonal, and reproductive changes in birds and mammals at environmental realistic concentrations (Scheuhammer et al. 2007). In the USA an impact of high mercury concentrations on the population is not yet proven in most species. Exceptions are the Common Loon (Gavia immer) (Scheuhammer et al. 2007) and the Florida Panther (Puma concolor) (US EPA 1997). In the PWS area a negative impact is first to be expected on the otter populations. The Hoplias aimara is favorite food of the Giant Otter (Pteronura brasiliensis) and large scales of this fish are often seen at otter sites. The Giant Otter and the Neotropical Otter (Lutra longicaudis) have good populations in the PWS area (N. Duplaix, pers. comm.; Ouboter, pers. observ.), and should be monitored to see if a decline of their populations is occurring. Other (semi)aquatic top predators in the PWS area are the Spectacled Caiman (Caiman crocodiles), Smooth-fronted Caiman (Paleosuchus trigonatus), and Anaconda (Eunectes murinus) (Ouboter 1996; pers. observ.). Bio-accumulation of mercury was reported in the American Alligator (Alligator mississippiensis) (Duvall and Barron 2000; Khan and Tansel 2000), and is likely to occur in the caiman species and anaconda as well. Health effects of high mercury levels in reptiles are not well studied and need further investigations. Notwithstanding this limitation in our present knowledge, a decline of any of these top predators mentioned could occur with rising mercury levels and is likely to drive the whole aquatic ecosystem off balance. To protect the Central Suriname Nature Reserve as a pristine wilderness area, it is critical to stop the inflow of mercury from the atmosphere. Probably the only way this can be achieved is to develop and promote viable and environmental friendly alternatives to the use of mercury in the small- and medium-scale gold mining.
Acknowledgments
The various projects were funded by WWF-Guianas (Water Quality Monitoring in the Commewijne Watershed Suriname, Mercury Pollution in the Greenstone Belt, Mercury Poisoning in the Brownsweg Village) and the Schure-Beijerinck-Popping Fund (The Impact of Atmospheric Transported Mercury on the Mercury Levels in Water and Biota in the Rivers of Suriname). Several water, sediment, and fish samples were taken during RAP expeditions funded by Conservation International. Work in the field would have been impossible without the assistance of Usha Satnarain, Rawien Jairam, Joyce Metjo, and Indra Asraf-Nanden. Indra and Joyce were also responsible for mercury analysis in the laboratory.
Biographies
Paul E. Ouboter
is Director of the National Zoological Collection/Center for Environmental Research of the Anton de Kom University of Suriname. His research interests include animal biodiversity and disturbance ecology.
Gwendolyn A. Landburg
Gwendolyn A. Landburg is PhD candidate at the National Zoological Collection of Suriname/Center for Environmental Research of the Anton de Kom University of Suriname. Her research interests include disturbance ecology using amphibians as indicator species and aquatic ecology.
Jan H. M. Quik
is Head of the Chemistry Department of the Central Laboratory of the Bureau of Public Health (Ministry of Health). His research interests include environmental toxicology and monitoring of heavy metals and POPs.
Jan H. A. Mol
is Professor in Aquatic Ecology at the Anton de Kom University of Suriname. His research interests include aquatic ecology and neotropical freshwater fishes.
Frank L. van der Lugt
is Lecturer at the Faculty of Technical Science of the Anton de Kom University of Suriname. His research interests include aquatic ecology.
Contributor Information
Paul E. Ouboter, Phone: +597-494756, FAX: +597-494756, Email: p.ouboter@uvs.edu
Gwendolyn A. Landburg, Email: g.landburg@uvs.edu
Jan H. M. Quik, Email: JanQuik@bog.sr
Jan H. A. Mol, Email: fisheco@celos.sr.org
References
- Anderson, A. 1979. Mercury in soils. In The biochemistry of tertiary volcanic rocks in parts of the Virginia City Quadrangle, ed. J.O. Nriagu, 70–112. Amsterdam: Elsevier.
- Bastos, W.R., J.P.O. Gomes, R.C. Oliveira, R. Almeida, E.L. Nascimento, J.V.E. Bernardi, L.D. de Lacerda, E.G. da Silveira, et al. 2006. Mercury in the environment and the riverside population in the Madeira River Basin, Amazon, Brazil. Science of the Total Environment 368: 344–351. [DOI] [PubMed]
- Bodaly RA, Rudo JWM, Fudge RJP, Kelly CA. Mercury concentrations in fish related to size of remote Canadian Shield Lakes. Canadian Journal of Fisheries and Aquatic Sciences. 1993;50:980–987. doi: 10.1139/f93-113. [DOI] [Google Scholar]
- Boedhram, N. 1988. Climate/Rainfall. In Suriname Planatlas. Washington, DC: National Planning Office of Suriname, Paramaribo/Organization of American States.
- Bosma W, Ho Len Fat AG, Welter CC. Minerals and mining in Suriname. Contributions to the Geology of Suriname. 1973;3:71–101. [Google Scholar]
- Boudou A, Maury-Brachet R, Durrieu G, Coquery M, Dauta C. Gold mining activities and mercury contamination of freshwater systems in French Guiana—Risks towards human populations. Hydroécologie Appliquée. 2006;15:1–18. doi: 10.1051/hydro:2006007. [DOI] [Google Scholar]
- Canadian Council of Ministers of the Environment. 1999. Canadian sediment quality guidelines for the protection of aquatic life: Mercury. In Canadian environmental quality guidelines. Winnipeg: Canadian Council of Ministers of the Environment.
- Clesceri SL, Greenberg AE, Eaton AD, editors. Standard methods for the examination of water and wastewater. 20. Washington: APHA/AWWA/WEF; 1998. [Google Scholar]
- Kom JFM, Voet GB, Wolff FA. Mercury exposure of Maroon workers in the small scale goldmining in Suriname. Environmental Research. 1998;77:91–97. doi: 10.1006/enrs.1998.3835. [DOI] [PubMed] [Google Scholar]
- De Vletter, D.R. 1984. Contributions to the Geology of Suriname 8, Geological Mining Division, Mededelingen 27: 11–30.
- Vletter DR, Hakstege AL. The search for gold in Suriname. In: Wong ThE, Vletter DR, Krook L, Zonneveld JIS, Loon AJ., editors. The history of earth sciences in Suriname. Amsterdam: Netherlands Institute of Applied Geoscience TNO/Royal Netherlands Academy of Arts and Sciences; 1998. pp. 311–349. [Google Scholar]
- De Vletter, D.R., S.B. Kroonenberg, A.L. Hakstege, and R.L. Verwey. 1988. Geology and Minerals. In Suriname Planatlas. Washington, DC: National Planning Office of Suriname, Paramaribo/Organization of American States.
- Duvall AE, Barron MG. A screening level probabilistic risk assessment of mercury in Florida Everglades food webs. Ecotoxicology and Environmental Safety. 2000;47:298–305. doi: 10.1006/eesa.2000.1949. [DOI] [PubMed] [Google Scholar]
- EC. 2002. EC Regulation (221/2002) amending Commission Regulation (EC) no. 466/2001 of 8 March 2001 setting maximum levels for certain contaminants in foodstuffs. EC, Brussels.
- Haripersad-Makhanlal A, Ouboter PE. Limnology: Physics–chemical parameters and phytoplankton composition. In: Ouboter PE, editor. Freshwater ecosystems of Suriname. Dordrecht: Kluwer Academic Publishers; 1993. pp. 53–75. [Google Scholar]
- Harris, R.C., and R.A. Bodaly. 1998. Growth and dietary effects on fish mercury dynamics in two Ontario lakes. Biogeochemistry, Vol. 40 (2/3), Fourth International Conference: Mercury as Global Pollutant, March 1998, 175–187.
- Keith, P., P.Y. Le Bail, and P. Planquette. 2000. Guide to the freshwater fishes of French Guiana. Vol. 2, part I. Batrachoidiformes, Mugiliformes, Beloniformes, Cyprinodontiformes, Synbranchiformes, Perciformes, Pleuronectiformes, Tetraodontiformes. Paris: Museum National d’Histoire Naturelle (in French).
- Khan B, Tansel B. Mercury bioconcentration factors in American alligators (Alligator mississippiensis) in the Florida Everglades. Ecotoxicology and Environmental Safety. 2000;47:54–58. doi: 10.1006/eesa.2000.1923. [DOI] [PubMed] [Google Scholar]
- Lacerda LD, Bidone ED, Guimaraes AF, Pfeiffer WC. Mercury concentrations in fish from the Itacaiunas-Parauapebas River System. Carajas Region, Amazon. Anais da Academia Brasileira de Ciências. 1994;66:373–379. [PubMed] [Google Scholar]
- Lacerda LD, Salomons W, Pfeiffer WC, Bastos WR. Mercury distribution in sediment profiles of remote high Pantanal lakes, Central Brazil. Biogeochemistry. 1991;14:91–97. [Google Scholar]
- Le Bail, P.Y., P. Keith, and P. Planquette. 2000. Guide to the freshwater fishes of French Guiana. Vol. 1, part II. Siluriformes. Paris: Museum National d’Histoire Naturelle (in French).
- Malm O, Guimaraes JRD, Castro MB, Bastos WR, Viana JP, Branches FJP, Silveira EG, Pfeiffer WC. Follow-up of mercury levels in fish, human hair and urine in the Madeira and Tapajos basins, Amazon, Brazil. Water, Air, and Soil pollution. 1997;97:45–51. [Google Scholar]
- Miller JR, Lechler PJ, Bridge G. Mercury contamination of alluvial sediments within the Essequibo and Mazaruni River Basins, Guyana. Water, Air, and Soil pollution. 2003;148:139–166. doi: 10.1023/A:1025465800121. [DOI] [Google Scholar]
- Mol JH, Ouboter PE. Downstream effects of erosion from small-scale gold mining on the instream habitat and fish community of a small Neotropical rainforest stream. Conservation Biology. 2004;18:201–214. doi: 10.1111/j.1523-1739.2004.00080.x. [DOI] [Google Scholar]
- Mol JH, Ramlal JS, Lietar C, Verloo M. Mercury contamination in freshwater, estuarine and marine fishes in relation to small-scale gold mining in Suriname, South America. Environmental Research. 2001;86:183–197. doi: 10.1006/enrs.2001.4256. [DOI] [PubMed] [Google Scholar]
- Mol, J.H., Ph. Willink, B. Chernoff, and M. Cooperman. 2006. Fishes of the Coppename River, Central Suriname Nature Reserve, Suriname. In A rapid biological assessment of the aquatic ecosystems of the Coppename River Basin, Suriname, ed. Alonso, L.E., and H.J. Berrenstein. Executive Summary. RAP Bulletin of Biological Assessment 39: 67–79.
- Mol, J.H., B. de Merona, P.E. Ouboter, and S. Sahdew. 2007. The fish fauna of Brokopondo Reservoir, Suriname, during 40 years of impoundment. Neotropical Ichthyology 5(3): 351–368.
- Morel FMM, Kraepiel AML, Amyot M. The chemical cycle and bioaccumulation of mercury. Annual Review of Ecology and Systematics. 1998;29:543–566. doi: 10.1146/annurev.ecolsys.29.1.543. [DOI] [Google Scholar]
- Oliveira Ribeiro CA, Pelletier E, Pfeiffer WC, Rouleau C. Comparative uptake, bioaccumulation, and gill damages of inorganic mercury in tropical and nordic freshwater fish. Environmental Research. 2000;83:286–292. doi: 10.1006/enrs.2000.4056. [DOI] [PubMed] [Google Scholar]
- Ouboter PE. Ecological studies on crocodilians in Suriname. Niche segregation and competition in three predators. Amsterdam: SPB Academic Publishing; 1996. p. 139. [Google Scholar]
- Ouboter, P.E., and G. Landburg. 2006. Water quality of the Coppename River Basin, with notes on aquatic fauna distribution. In A rapid biological assessment of the aquatic ecosystems of the Coppename River Basin, Suriname, ed. Alonso, L.E., and H.J. Berrenstein. RAP Bulletin of Biological Assessment 39: 37–46.
- Pfeiffer WC, Lacerda LD. Mercury inputs into the Amazon region, Brazil. Environmental Technology Letters. 1988;9:325–330. doi: 10.1080/09593338809384573. [DOI] [Google Scholar]
- Pfeiffer WC, Lacerda LD, Malm O, Souza CMM, Silveira EG, Bastos WR. Mercury concentrations in inland waters of gold mining areas in Rondonia, Brazil. Science of the Total Environment. 1989;87:233–240. doi: 10.1016/0048-9697(89)90238-6. [DOI] [PubMed] [Google Scholar]
- Planquette, P., P. Keith, and P.Y. Le Bail. 1996. Guide to the freshwater fishes of French Guiana, vol. 1. Paris: Museum National d’Histoire Naturelle (in French).
- Pollack, H., J. de Kom, J. Quik, and L. Zuilen. 1998. Introducing retorts for abatement of mercury pollution in Suriname. Report to Organization of American States. Paramaribo: HWO Consultants.
- Richard S, Arnoux A, Cerdan Ph, Reynouard C, Horeau V. Mercury levels of soils, sediments and fish in French Guiana, South America. Water, Air, and Soil Pollution. 2000;124:221–244. doi: 10.1023/A:1005251016314. [DOI] [Google Scholar]
- Roulet, M., M. Lucotte, A. Saint-Aubin, S. Tran, I. Rheault, N. Farella, E. De Jesus Da Silva, et al. 1998. The geochemistry of mercury in central Amazonian soils developed on the Alter-do-Chão formation of the lower Tapajos River Valley, Para state, Brazil. Science of the Total Environment 223: 1–24. [DOI] [PubMed]
- Scheuhammer AM, Meyer MW, Sandheinrich MB, Murray MW. Effects of environmental methylmercury on the health of wild birds, mammals, and fish. AMBIO. 2007;36:12–18. doi: 10.1579/0044-7447(2007)36[12:EOEMOT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Silva-Forsberg, M.C., B.R. Forsberg, and V.K. Zeidemann. 1999. Mercury contamination in humans linked to river chemistry in the Amazon Basin. AMBIO 28(6): 519–521.
- Tanan CL, Ventura DF, Souza JM, Grotzner SR, Mela M, Gouvela A, Jr, Oliveira-Ribeiro CA. Effects of mercury intoxication on the response of horizontal cells of the retina of thraira fish (Hoplias malabaricus) Brazilian Journal of Medical and Biological Research. 2006;39:987–995. doi: 10.1590/S0100-879X2006000700017. [DOI] [PubMed] [Google Scholar]
- UNESCO. 2011. UNESCO World Heritage Centre. http://whc.unesco.org/en/list/1017. Retrieved 9 Sept 2011.
- Methods for determination of metals in environmental samples. Cincinnati: Environmental Monitoring Systems Laboratory Office of Research and Development US EPA; 1991. [Google Scholar]
- US EPA. 1994. Water quality standards handbook, 2nd ed. USEPA Water Resource Center. United States Environmental Protection Agency EPA-823-B-94-005. Washington, DC: EPA.
- US EPA. 1997. Mercury study report to Congress. Volume VI: An ecological assessment for anthropogenic mercury emissions in the United States. Office of Air Quality Planning & Standards and Office of Research and Development. United States Environmental Protection Agency EPA-452/R-97-008. Washington, DC: EPA.
- Veiga MM. Introducing new technologies for abatement of global mercury pollution in Latin America. Rio de Janeiro: UNIDO/University of British Columbia/Center for Mineral Technology; 1997. p. 94. [Google Scholar]
- Watras CJ, Morrison KA, Host Jodi S. Concentration of mercury species in relationship to other site-specific factors in the surface waters of northern Wisconsin Lakes. Limnology and Oceanography. 1995;40:556–565. doi: 10.4319/lo.1995.40.3.0556. [DOI] [Google Scholar]