Abstract
Purpose
NANOG and OCT4 are required for the maintenance of pluripotency in embryonic stem cells (ESCs). These proteins are also expressed in the inner cell mass (ICM) of the mouse pre-implantation embryo.
Methods
Immunohistochemistry was used to show the presence of NANOG and OCT4 protein, and in situ hybridization was used to localize NANOG mRNA in human embryos from two-cell to blastocyst stage, and in human ESCs (hESCs).
Results
Nanog and Oct4 were co-localized in human embryos from morula and blastocyst stages. NANOG mRNA was detected in a group of cells in the morula, in cells of the ICM of blastocysts, and evenly in hESCs. All non-differentiated hESCs expressed NANOG and OCT4 protein. Pluripotent cells expressing NANOG and Oct4 were eccentrically localized, probably in polarized cells in a human compacted morula, which appears to be different from expression in murine embryos.
Conclusion
In this study, we demonstrate that whole mount in situ hybridization is amenable to localization of mRNAs in human development, as in other species.
Keywords: Pluripotency, Embryo, Embryonic stem cells, NANOG, In situ hybridization
Introduction
Embryo development seems to be tightly regulated during each developmental stage, although the exact mechanisms are not completely understood. The transition from morula to blastocyst involves segregation of the first two cell lineages in the pre-implantation embryo, the inner cell mass (ICM), which forms the embryo, and the trophectoderm, which gives rise to the trophoblast lineage. Two transcription factors, OCT4 and NANOG, are the two most studied factors concerning formation of the ICM in mouse embryos, and it has been shown that these two intrinsic factors are required for establishment of the ICM in an undifferentiated state [3, 18, 20]. OCT4 and NANOG are expressed in human pre-implantation embryos and human embryonic stem cells (hESCs) and these transcription factors regulate stem cell pluripotency and differentiation [6, 15, 28]. The OCT4 gene is a member of the mammalian POU family of transcription factor genes. OCT4 is expressed in both ESCs and embryonic carcinoma cells [22, 26, 27, 33] and is considered to be essential for pluripotency [20, 21]. Deletion of the OCT4 gene allows blastocyst formation but failure as regards pluripotency, which results in differentiation into a trophectodermal lineage [20]. OCT4 is expressed constitutively throughout the pre-implantation period [15].
NANOG is a more recently described homeodomain-bearing protein. It acts as a transcription factor and has been described in mouse pluripotent cells [3, 18]. NANOG is expressed in a restricted number of cell types, and only in cells that also express OCT4, including ESCs [4]. In the mouse embryo, NANOG is localized in the centre of the morula and in the ICM of the blastocyst [3]. NANOG-induced self-renewal and NANOG function, but not NANOG expression, are dependent on continued OCT4 expression. OCT4 is required for NANOG-induced self-renewal [3, 17, 18]. Genetic deletion of NANOG in mouse ESCs resulted in the production of primitive endoderm-like cells, and NANOG mutant embryos were able to produce parietal endoderm. Since embryos lacking Oct4 are unable to develop so far this resulted in the assumption that NANOG function is critical during a later developmental stage than is the case for OCT4 [3, 18], and NANOG has been shown to be expressed from the 4-cell stage onwards [15]. Following implantation, when the ICM is developing into epiblast and primitive endoderm, NANOG is expressed in the epiblast cells [8].
The ability to visualize the expression of a gene in both time and space in human pre-embryos would be an essential tool in developmental biology. Whole-mount in situ hybridization has the advantage of showing the cellular location of specific mRNAs. However, to our knowledge, the method has not been used for studies on mRNA expression in human pre-embryos. Therefore, the aim of the present study was to optimise and use a novel whole-mount in situ hybridization method to determine the mRNA expression of NANOG in human embryos.
Materials and methods
Ovarian stimulation and in vitro fertilization
Down-regulation for ovarian hyperstimulation was achieved by using a long protocol gonadotrophin-releasing hormone agonist (GnRHa), nafarelin (Synarela; Syntex Nordica AB, Södertälje, Sweden) administered intra-nasally, starting on either cycle day 1 or 21. Following down-regulation, ovarian stimulation was induced using recombinant FSH (rFSH; Gonal-F, Serono Laboratories, Aubonne, Switzerland, or Puregon, NY Organon, Oss, the Netherlands). The starting dose was dependent on the subject’s age and/or previous response to ovarian stimulation. Ovarian response was monitored by serum estradiol assays and vaginal ultrasound scans. GnRHa and rFSH were administered until the leading follicle had a diameter of at least 18 mm. Maturation of the oocyte was triggered by one s.c. injection of 10,000 IU of human chorionic gonadotrophin (hCG; Profasi, Serono laboratories, Aubonne, Switzerland). Thirty-seven hours after hCG administration, oocytes were retrieved by trans-vaginal needle aspiration under ultrasonographic guidance.
Conventional IVF was performed in 20-μl droplets of medium (IVF medium, Vitrolife AB, Gothenburg, Sweden) containing about 15,000 spermatozoa, under oil (Ovoil, Vitrolife AB, Gothenburg, Sweden).
For intracytoplasmic sperm injection (ICSI), oocytes were stripped of cumulus cells by mechanical pipetting after brief exposure to hyaluronidase (HYAS; Vitrolife AB, Gothenburg, Sweden). ICSI was then performed using a Nikon-Narishige micromanipulation system. Fertilization was evaluated 18–20 h after insemination. Following fertilization, IVF and ICSI embryos were cultured in 10-μl droplets of medium under oil (G.1.2; Vitrolife AB, Gothenburg, Sweden).
Embryo transfer was carried out either on day 2 or day 3. Excess embryos, surplus to treatment, were frozen at the 2- to 8-cell stage using a three-stage propanediol cryopreservation kit (Freeze kit 1; Vitrolife AB, Gothenburg, Sweden) according to the manufacturer’s instructions. Embryos used for immunohistochemistry, n = 31, and in situ hybridization, n = 89, were cyopreserved and thawed after their 5-year storage limit in liquid nitrogen had passed. Frozen embryos were thawed using a thawing kit (Sydney IVF thawing kit, CooK IVF, Brisbane, Australia), and cultured to blastocysts in 10-μl droplets under oil in either BlastAssist System (Medicult, Jyllinge, Denmark) or blastocyst (Sidney IVF Blastocyst medium, CooK IVF, Brisbane, Australia) sequential media.
Human embryonic stem cell derivation and culture
The embryos used for embryonic stem cell culture had been donated on day 2 after fertilization after all embryos of good quality (minimum score of 2.0 out of 3.5 according to [19]) had been transferred or frozen. From an original score of 3.5, reductions of 0.5 at a time were made on the basis of the following features: more than 20 % cellular fragmentation, unequal size of blastomeres, multinuclear blastomeres, or the embryo did not fill the zona.
The blastocysts used for the present embryonic stem cell lines had been cultured in medium designed for blastocyst culture (MediCult, Ronnehavn, Denmark). Derivation of the present lines was carried out as described previously [13, 14]. Separation of the ICM from the trophectoderm cells was carried out by first removing the zona pellucida, using 0.5 % pronase (Sigma-Aldrich, Stockholm, Sweden). The trophectoderm was removed by immunosurgery as described earlier, using rabbit antihuman whole serum (Sigma) and guinea pig complement serum (Sigma-Aldrich, Stockholm, Sweden) [30]. The isolated ICMs were then placed on a feeder cell layer.
Human foreskin fibroblasts (CRL-2429; ATCC, Manassas, VA) were used as feeder cells. The feeder cells were mitotically inactivated using irradiation (35 Gy) and plated onto 2.84-cm2 dishes to form a confluent monolayer to be used as substrate cells the following day. 150,000 fibroblasts were plated for derivation of a new line, and for the passages to follow, 350,000 fibroblasts were plated. Iscove’s medium (Gibco, Invitrogen, Stockholm, Sweden) supplemented (10 %) with FCS (Gibco, Invitrogen, Stockholm, Sweden) was used as culture medium.
The culture medium used for derivation and culture of hESCs consisted of Dulbecco’s modified Eagle’s medium (Gibco, Invitrogen, Stockholm, Sweden) supplemented (20 %) with SR medium (Knockout SR, Gibco, Invitrogen, Stockholm, Sweden), 2 mM L-glutamine (Gibco, Invitrogen, Stockholm, Sweden), 1 % penicillin-streptomycin (Gibco Invitrogen Corporation), 1 % nonessential amino acids (Gibco, Invitrogen, Stockholm, Sweden), 0.5 mM 2-mercaptoethanol, 1 % insulin-selenium-transferrin (Sigma-Aldrich, Stockholm, Sweden) and bFGF (8 ng/ml; R&H Systems, Oxon, U.K.).
After an initial growth period of 12 days, the cell aggregates were removed mechanically from the original plate and transferred to fresh feeder cells. Mechanical passage was performed by cutting the colony (approximately 2,000 μm in diameter) into eight pieces using a scalpel, under a stereomicroscope. Mechanical splitting was then carried out at 5- to 8-day intervals (mean, 7 days). Non-differentiated cells, as judged by morphology, were chosen for each further passage. The doubling time of the hESCs was approximately 24 h. In vivo pluripotency was tested in embryonic bodies and teratomas. The lines used in this study were HS181, HS235 and HS237. For cryopreservation of the cells, vitrification in pulled open straws, using ethylene glycol, dimethylsulphoxide (20 % each) and 1 M sucrose as cryoprotectants.
In situ hybridization of NANOG in human preimplantation embryos
A non-radioactive whole mount in situ hybridization method was used to detect the presence of NANOG mRNA in human preimplantation embryos. The experiment was performed on batches of ten or more embryos. The embryos were placed in culture inserts, which were thereafter transferred into wells that contained the different solutions. In the first step the embryos were washed twice in phosphate-buffered saline (PBS), and then fixed overnight at 4 °C in 4 % paraformaldehyde. After fixation the embryos were washed twice in PBS with 0.1 % Tween-20 (Sigma-Aldrich, Stockholm, Sweden) (PBT). Dehydration was performed through increasing concentrations of methanol in PBS (25 %, 50 %, 75 %) and finally twice in 100 % methanol. Rehydration was carried out through decreasing concentrations of methanol/PBS (75 %, 50 %, 25 %). The embryos were then rinsed three times with PBT followed by three washes in a detergent mix of 150 mM NaCl (Sigma-Aldrich, Stockholm, Sweden), 1 % Nonidet-P-40 (Sigma-Aldrich, Stockholm, Sweden), 0.5 % sodium deoxycholate (Sigma-Aldrich, Stockholm, Sweden), 0.1 % SDS (Sigma-Aldrich, Stockholm, Sweden), 1 mM EDTA (Sigma-Aldrich, Stockholm, Sweden) and 50 mM Tris (Sigma-Aldrich, Stockholm, Sweden), pH 8.0, at room temperature. Post-fixation was carried out in 4 % paraformaldehyde (Sigma-Aldrich, Stockholm, Sweden), 0.2 % EM-grade glutaraldehyde (Sigma-Aldrich, Stockholm, Sweden) in PBT at room temperature. Thereafter, the embryos were washed in PBT followed by a wash in a 1:1 mix of hybridization buffer (HB; 50 % ultra-pure formamide (Sigma-Aldrich, Stockholm, Sweden), 5 × SSC pH 4.5, heparin at 50 μg/ml, 0.1 % Tween 20) and PBT at room temperature. Prehybridization was then performed for 3 h at 70 °C in HB containing tRNA (100 μg/ml) and sheared denatured herring sperm DNA (Sigma-Aldrich, Stockholm, Sweden) at 100 μg/ml, followed by hybridization overnight at 70 °C in HB containing tRNA (100 μg/ml) and denatured digoxigenin-labelled riboprobe (100 μg/ml). Human NANOG ORF was subcloned from IMAGE clone 664153 using ATC TCG AGG CCG CCA CCA TGA GTG TGG ATC CAG CTT GTC C and ATG CGG CCG CTC ACA CGT CTT CAG GTT GCA TGT, subcloned into pCR2.1 and verified to be free of mutations. After linearization with Xho I, the probe was labelled with digoxigenin by transcription with SP6. After hybridization the embryos were washed once in a solution containing 2 × SSC, pH 4.5, 50 % formamide and 0.1 % Tween 20 at 70 °C, then twice at room temperature and finally three times at 65 °C. After cooling to room temperature and three washes in TBST (NaCl 8 g/l, KCl 0.2 g/l, 0.25 M Tris, pH 7.5, Tween-20 (1 %)), the embryos were incubated in blocking solution (10 % heat-inactivated sheep serum) for 1 h and thereafter incubated with anti-digoxigenin Fab alkaline phosphate conjugate (Roche, Stockholm, Sweden) in TBST with 1 % heat-inactivated sheep serum. This step was followed by antibody conjugate incubation overnight at 4 °C. The antibody conjugate was removed in a series of washes, first with TBST at room temperature and then with APB. The embryos were stained using Vectashield (Vectorlab Inc., Burlingame, USA) and the embryos were thereafter rinsed in PBS containing 1 M EDTA (Sigma-Aldrich, Stockholm, Sweden). Morula- and blastocyst-stage embryos incubated without the probe served as negative controls. A total of 67 embryos were used in this study.
In situ hybridization of NANOG in human embryonic stem cells
Embryonic stem cells were fixed on dishes together with feeder cells and processed in the same way as embryos. Mouse ESCs and human skin fibroblasts served as negative control cells.
Immunostaining of NANOG and OCT4 in human embryonic stem cells
Human embryonic stem cells were fixed with 4 % paraformaldehyde in PBS for 20 min at room temperature and thereafter washed with PBS and blocked with 5 % goat serum in PBS. Permeabilisation was carried out using blocking buffer consisting of 0.02 % TritonX-100 in PBS. Primary antibodies—monoclonal mouse antibodies for OCT4 (sc-5279, Santa Cruz Biotechnologies, Santa Cruz, USA), diluted 1:80, and monoclonal goat antibody for NANOG (MAB1997, R&D systems, Minneapolis, MN USA), diluted 1:200—were added in 5 % blocking buffer overnight at 4 °C and washed three times with PBS to remove any unbound primary antibodies. Secondary antibodies—FITC-conjugated goat anti-mouse IgG and FITC-conjugated bovine anti-goat IgG (both from Chemicon)—were diluted 1:200 in 5 % blocking buffer and applied to the cells for 60 min at RT in the dark. For controls, the primary antibodies were excluded from the staining protocol. Stained cells were viewed with a Zeiss Axiovert 200 M inverted microscope equipped with fluorescence optics and appropriate filters and images were acquired using Openlab 3.1.3 software. Human skin fibroblasts served as negative control cells.
Immunostaining of NANOG and OCT4 in human preimplantation embryos
Human preimplantation embryos were rapidly transferred from culture and briefly washed in PBS containing PVP (3 mg/ml) and then fixed in 2.5 % paraformaldehyde in PBS for 15 min at room temperature. Following fixation, the embryos were permeabilised in PBS/PVP buffer containing 0.25 % Triton X 100 for 30 min. The embryos were then placed in blocking buffer containing 0.1 % BSA and 0.01 % Tween 20 in PBS for 15 min. Primary antibodies—monoclonal mouse antibody for OCT4 (Santa Cruz Biotechnologies, Santa Cruz, USA), diluted 1:100, and monoclonal goat antibody for NANOG (R&D systems, Minneapolis, MN USA), diluted 1:100—were added in blocking buffer and incubated overnight at 4 °C. The embryos were then washed 3 times for 15 min each in blocking buffer to remove any unbound primary antibodies. The secondary antibodies—Alexa 568 conjugated rabbit anti-goat (1:250) and Alexa 488 conjugated rabbit anti-mouse (1:100) antibodies (Invitrogen, Stockholm, Sweden), respectively—were diluted in blocking buffer and applied to the embryos for 60 min at RT in the dark. For negative controls, the primary antibodies were excluded from the staining protocol. After incubation the embryos were briefly washed through a series of 25, 50, 75 and 100 % citifluor (with DAPI) and then mounted on slides in antifade medium under coverslips. The coverslips were then sealed with nail varnish. Stained embryos were viewed with an inverted microscope equipped with fluorescence optics and appropriate filters.
Data from gene array studies
Data from gene array studies performed at Karolinska Institutet [34] were used for comparison with data from the present study.
Ethical considerations
The pre-implantation embryos used in this study were donated with informed consent by couples undergoing in vitro fertilization treatment. Only pre-implantation embryos that could not be used in infertility treatment were used in the study. The human embryonic stem cells were derived from the inner cell masses of blastocysts that could not be used for infertility treatment. Approval was obtained from the Research Ethics Committee of Örebro University Hospital for expression studies in human pre-implantation embryos, and from the Ethics Board of Karolinska Institutet for derivation of hES cell lines, and studies regarding their properties. Both partners of the couples involved signed an informed consent form after receiving oral and written information [1]. No reimbursement was given to the couples.
Results
Presence of NANOG and OCT4 proteins in human pre-implantation embryos
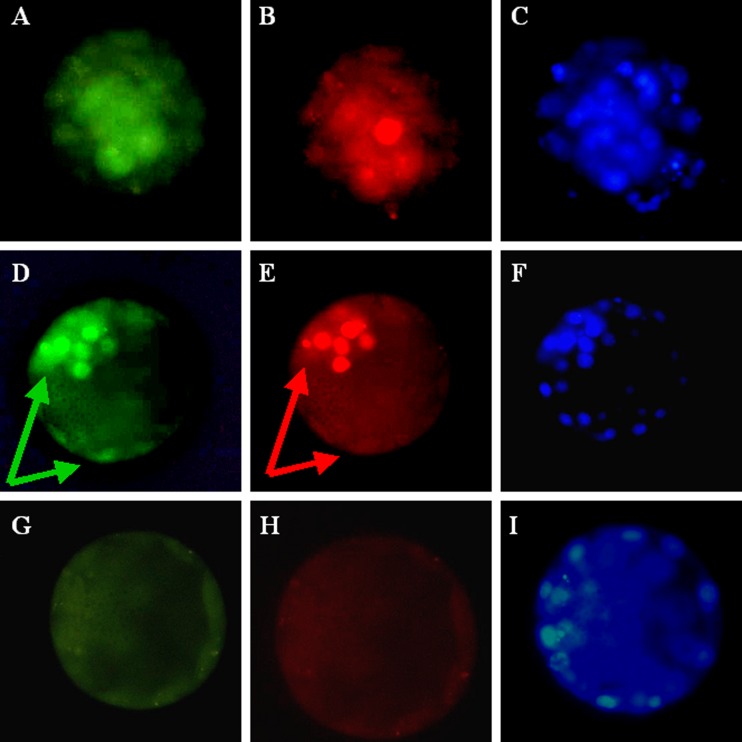
Using immunohistochemistry, we observed immunostaining of NANOG and OCT4 in human pre-implantation embryos (n = 31). This immunostaining was seen in an eccentrically located group of cells in the morula and in part of the ICM of blastocyst-stage embryos (Fig. 1a, b, c and d). Double staining showed that OCT4 and NANOG were present in the same cells of the ICM (Fig. 1d and e). There was also some staining of OCT4 in trophoblast cells, but no such staining as regards NANOG (Fig. 1d and e).
Fig. 1.
Representative pictures show double staining of OCT4 and NANOG in the human morula and blastocyst. a Immunostaining of OCT4 in the morula. b Immunostaining of NANOG in the same morula. c DAPI staining in the morula. d Staining of OCT4 in the blastocyst. Immunostaining is seen both in the inner cell mass and the trophoblast; arrows. e Staining of NANOG in the same blastocyst. Staining is seen only in the inner cell mass; arrow. f DAPI staining in the same blastocyst. g Exclusion of OCT4 antibody. h Exclusion of NANOG antibody. i DAPI staining of the embryo without primary antibodies present
Expression of NANOG mRNA in human pre-implantation embryos
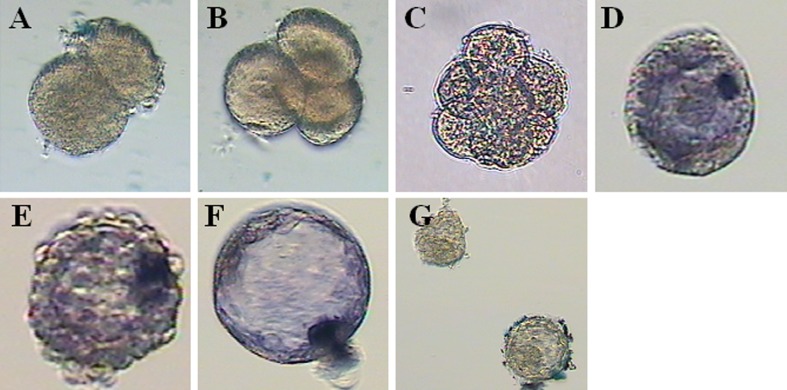
Expression of NANOG mRNA in human pre-implantation embryos was determined by using whole-mount in situ hybridization (n = 89). NANOG mRNA was expressed in some cells of the compacted morula, with eccentric localization, and in the ICM of blastocyst-stage embryos (Fig. 2d–f). No expression was detected in 2-cell, 4-cell or 8-cell stages (Fig. 2a–c). In the blastocyst, NANOG mRNA was confined to the ICM and absent in the trophectoderm (Fig. 2f).
Fig. 2.
In situ hybridization of NANOG in the human pre-implantation embryo. a 2-cell embryo, day 1. b 4-cell embryo, day 2. c 8-cell embryo, day 3. d morula, day 4. e late day 4 embryo. f blastocyst, day 5. g mouse ESCs hybridized with human NANOG primer
NANOG and OCT4 in human embryonic stem cells
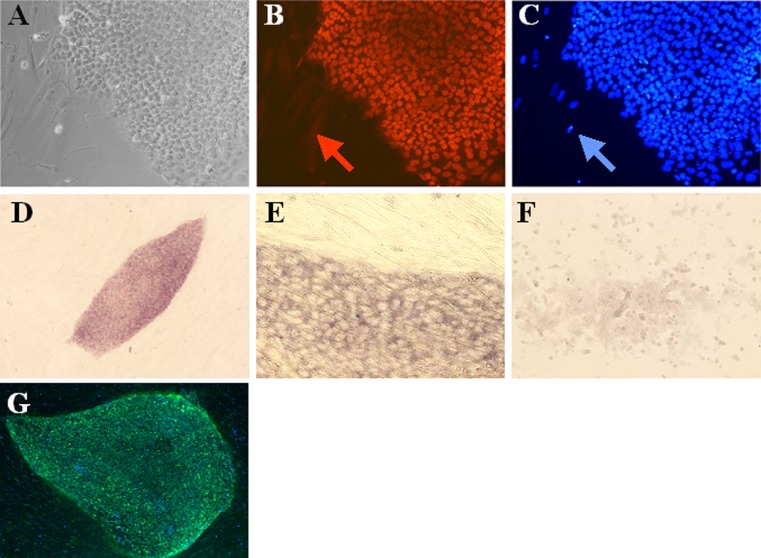
Immunostaining showed the presence of NANOG and OCT4 proteins in all embryonic stem cells, although the staining was apparently weaker in the centre of the colony (Fig. 3b and g). Nuclear Hoechst DNA staining also showed the presence of feeder cells, which were negative for NANOG and OCT4 (Fig. 3c and g). NANOG mRNA was evenly expressed in all cells in the stem cell colonies. In situ hybridization showed NANOG mRNA in a colony of HS235 hESCs on human skin fibroblast feeder cells (Fig. 3d and e). Mouse stem cells did not show any staining after in situ hybridization with human NANOG probe (Fig. 1f).
Fig. 3.
Immunostaining of OCT4 and NANOG in human embryonic stem cells, and in situ hybridization of NANOG in embryonic stem cells. a Light microscopy of human embryonic stem cells. b NANOG immunostaining in embryonic stem cells. The feeder cells do not show staining for NANOG (red arrow). c Nuclear staining of embryonic stem cells and fibroblast cells. DAPI staining is seen in feeder cells (blue arrow). d & e In situ hybridization showing NANOG mRNA in embryonic stem cells. NANOG f Mouse embryonic stem cells hybridized with human NANOG primer. g OCT4 immunostaining of stem cell line HS426 (green). Blue colour shows nuclear DAPI staining
Gene array data
Data from gene array studies showed that NANOG mRNA is present in pre-implantation embryos from day 2 and is highly expressed in hESCs (Table 1). OCT4 was weakly expressed in pre-implantation embryos and highly expressed in stem cells (Table 1). The two genes were absent in fibroblasts (Table 1).
Table 1.
Data from microarray analysis of human embryos and embryonic stem cells and fibroblasts. For Nanog, the average signal intensity from one probe set and for Oct4 the average number of three probe sets is shown
| Gene | Signal intensity | ||||
|---|---|---|---|---|---|
| Day 2 embryos | Day 3 embryos | Day 5 embryos | Stem cells | Fibroblasts | |
| Nanog | 98.6 | 194.6 | 196.8 | 391.8 | 1.3 |
| Oct4 | 32.2 | 67.1 | 46.8 | 310.9 | 5.3 |
Discussion
We localized the expression of NANOG and Oct4 in human pre-implantation embryos and in three human embryonic stem cell lines derived in our laboratory. The time of appearance (early morula), resembled that in mouse embryos [3, 12, 24, 25]. We found that the expression of NANOG in human compacted morula was not evenly distributed, but was strictly localized in the cells which apparently form the inner cell mass 1 day later. The expression of NANOG in human embryos seemed to be polarized earlier than in mouse embryos (see Fig. 2d and e and [3]).
The present study revealed NANOG and OCT4 protein and NANOG mRNA in the morula and blastocyst stages of human embryos. In mouse embryos, NANOG and OCT4 have also been seen in the morula to the hatched blastocyst stage [3, 12, 24, 25].
In the present study, some OCT4 immunostaining was also seen in the trophoblast cells of the blastocyst, although to a lesser extent than in the ICM. This has also been seen in bovine blastocysts [16, 32] and is in line with the results of a previous study showing the expression of mRNA for OCT4 in human trophoblast cells [9].
Additionally, we showed the expression of NANOG in human embryos during morula and blastocyst stages by using a whole-mount in situ hybridization technique. This method gave us the unique opportunity to visualize the expression of NANOG in both time and space in single human embryos. The protocol proved to be reliable and sensitive for visualization of NANOG gene expression in human embryos. This novel technique can be used to determine the precise expression pattern of a gene of interest at various stages of development. Furthermore, it can be used to elucidate the genes and pathways involved in cellular processes such as differentiation, proliferation and apoptosis.
Differently from mouse embryos, the expression of NANOG was not centred at the middle of the morula. This confirms that the early human embryo is polarized at early stages, and our findings show clear polarization during the morula stage. The concept of polarization of the early embryo has been discussed in connection with the expression of hCG in early embryos [10, 11]. The distribution of mitochondria has been reported to be polarized in human oocytes and early embryos [31].
Embryonic stem cells can be derived from and reintroduced to the ICM, but this does not necessarily mean that these cells are exactly equivalent. The ICM exists only transiently and does not operate as a stem cell compartment in vivo. Stem cells might arise through selection and adaptation to the culture environment [2, 29]. Two transcription factors, Oct4 and NANOG, appear to define the potency of human and mouse ESCs. NANOG plays a fundamental role in maintaining pluripotential properties of embryonic stem cells. In a recent study [28], it was shown that ICM cells in NANOG-null embryos failed to develop into viable epiblast. NANOG Octamer and SOX elements, which control NANOG transcription, are able to recruit OCT4 and SOX2, respectively.
This results in upregulation of NANOG gene expression [33]. In another study it was shown that OCT4, NANOG and SOX2 were co-expressed in porcine epiblasts [5]. Human embryonic stem cells were stained positive both for NANOG and OCT4 [12]. Furthermore, Göke J et al. [7], observed that the combinatorial binding of OCT4, NANOG and SOX2 is critical for transcription in mouse and human ES. In the present study, we showed immunostaining for both NANOG and OCT4 using both immunohistochemistry and in situ hybridisation.
Whole-mount in situ hybridization has, as far as we know, not previously been used for detection of genes in human embryos. This technique permits the direct observation of the protein synthesis capacity of particular genes. Previously, the most commonly used method has been paraffin embedding and sectioning of embryos [23]. Whole-mount in situ hybridization provides a sensitive and powerful method for detection of expression patterns of genes within an individual preimplantation embryo.
Gene array data showed the presence of NANOG mRNA from D2 stage. This analysis was performed using an additional amplifying step before array analysis, showing that this method is more sensitive in detection of a gene compared with in situ hybridization. Recently it was shown that NANOG and OCT4 cDNA are present in 4- and 8-cell human embryos, expression being restricted to the ICM [6].
We showed that the expression profile of NANOG in humans resembles that in mice, suggesting that NANOG plays a physiological role in preimplantation development of the human embryo and it also provides a useful molecular marker of pluripotency. There is clear polarization of NANOG-expressing cells in human compacted morula to those cells which are probably defined to form the ICM.
Acknowledgments
The authors thank Outi Hovatta and Jennifer Nichols for stem cells, NANOG probe and valuable comments. The study was supported by grants from the Swedish Research Council (2005–7293), the Swedish Research Council, The Swedish Society of Medicine, Karolinska Institutet, Uppsala University, Goljes Foundation and Åke Wibergs Foundation
Footnotes
Capsule
NANOG and OCT4 in human embryo and ES cells.
Contributor Information
Fredwell Hambiliki, Email: Fredham@hotmail.com.
Susanne Ström, Email: Susanne.strom@ki.se.
Pu Zhang, Email: Pu.Zhang@akademiska.se.
Anneli Stavreus-Evers, Phone: +46-18-6112831, FAX: +46-15-559775, Email: Anneli.Stavreus-Evers@kbh.uu.se.
References
- 1.Bjuresten K, Hovatta O. Donation of embryos for stem cell research—how many couples consent? Hum Reprod. 2003;18:1353–1355. doi: 10.1093/humrep/deg265. [DOI] [PubMed] [Google Scholar]
- 2.Buehr M, Smith A. Genesis of embryonic stem cells. Philos Trans R Soc Lond B Biol Sci. 2003;358:1397–4402. doi: 10.1098/rstb.2003.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of NANOG, a pluripotency sustaining factor in Embronic Stem cells. Cell. 2003;113:643–655. doi: 10.1016/S0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 4.Chambers I, Smith A. Self renewal of teratocarcinoma and embryonic stem cells. Oncogene. 2004;23:7150–7160. doi: 10.1038/sj.onc.1207930. [DOI] [PubMed] [Google Scholar]
- 5.Puy L, Lopes SM, Haagsman HP, Roelen BA. Analysis of co-expression of OCT4, NANOG and SOX2 in pluripotent cells of the porcine embryo, in vivo and in vitro. Therienology. 2011;75:513–526. doi: 10.1016/j.theriogenology.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Galán A, Montaner D, Póo E, Valbuena D, Ruiz V, Aguillar C, Dopazo J, Simon C. Functional genomics of 5- to 8-cell stage human embryos to blastomere single-cell cDNA analysis. PLoS One. 2010;5:e13615. doi: 10.1371/journal.pone.0013615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Göke J, Jung M, Behrens S, Chavez L, O’Keeffe S, Timmermann B, Lehrach H, Adjaye J, Vingron M. Combinatorial binding in human and mouse embryonic stem cells identifies conserved enhancers active in early embryonic development. PLoS Comput Biol. 2011;7:e1002304. doi: 10.1371/journal.pcbi.1002304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hart AH, Hartley L, Ibrahim M, Robb L. Identification, cloning and expression analysis of the pluripotency promoting NANOG genes in mouse and human. Dev Dyn. 2004;230:187–198. doi: 10.1002/dvdy.20034. [DOI] [PubMed] [Google Scholar]
- 9.Hansis C, Grifo JA, Krey LC. Oct-4 expression in inner cell mass and trophectoderm of human blastocysts. Mol Hum Reprod. 2000;6:999–1004. doi: 10.1093/molehr/6.11.999. [DOI] [PubMed] [Google Scholar]
- 10.Hansis C, Grifo JA, Tang Y, Krey LC. Assessment of beta-hCG and beta LH mRNA and ploidy in individual human blastomeres. RBM Online. 2002;5:156–161. doi: 10.1016/s1472-6483(10)61618-5. [DOI] [PubMed] [Google Scholar]
- 11.Hansis C, Edwards RG. Cell differentiation in the preimplantation human embryo. RBM Online. 2002;6:215–220. doi: 10.1016/s1472-6483(10)61712-9. [DOI] [PubMed] [Google Scholar]
- 12.Hatano SY, Tada M, Kimura H, Yamaguchi S, Kono T, Nakano T, Suemori H, Nakatsuji N, Tada T. Pluripotential competence of cells associated with NANOG activity. Mech Dev. 2005;122:67–79. doi: 10.1016/j.mod.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Hovatta O, Mikkola M, Gertow K, Stromberg AM, Inzunza J, Hreinsson J, Rozell B, Blennow E, Andang M, Ahrlund-Richter L. A culture system using human foreskin fibroblasts as feeder cells allows production of human embryonic stem cells. Hum Reprod. 2003;18:1404–1409. doi: 10.1093/humrep/deg290. [DOI] [PubMed] [Google Scholar]
- 14.Inzunza J, Gertow K, Stromberg MA, Matilainen E, Blennow E, Skottman H, Wolbank S, Ahrlund-Richter L, Hovatta O. Derivation of human embryonic stem cell lines in serum replacement medium using postnatal human fibroblasts as feeder cells. Stem Cells. 2005;23:544–549. doi: 10.1634/stemcells.2004-0201. [DOI] [PubMed] [Google Scholar]
- 15.Kimber SJ, Snedden SF, Bloor DJ, El-Bareg AM, Hawkhead JA, Metcalfe AD, Houghton FD, Leese HJ, Rutherford A, Lieberman BA, Brison DR. Expression of genes involved in early cell fate decisions in human embryos and their regulation by growth factors. Reproduction. 2008;135:635–647. doi: 10.1530/REP-07-0359. [DOI] [PubMed] [Google Scholar]
- 16.Kirchhof N, Carnwath JW, Lemme E, Anastassiadis K, Scholer H, Niemann H. Expression pattern of Oct-4 in preimplantation embryos of different species. Biol Reprod. 2000;63:1698–1705. doi: 10.1095/biolreprod63.6.1698. [DOI] [PubMed] [Google Scholar]
- 17.Kuroda T, Tada M, Kubota H, Kimura H, Hatano SY, Suemori H, Nakatsuji N, Tada T. Octamer and Sox elements are required for transcriptional cis regulation of NANOG gene expression. Mol Cell Biol. 2005;25:2475–2485. doi: 10.1128/MCB.25.6.2475-2485.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein NANOG is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–6642. doi: 10.1016/S0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 19.Mohr L, Trounson A. Cryopreservation of human embryos. Ann N Y Acad Sci. 1985;442:536–543. doi: 10.1111/j.1749-6632.1985.tb37562.x. [DOI] [PubMed] [Google Scholar]
- 20.Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor OCT4. Cell. 1998;95:379–391. doi: 10.1016/S0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 21.Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 22.Okamoto K, Okazawa H, Okuda A, Sakai M, Muramatsu M, Hamada H. A novel octamer binding transcription factor is differentially expressed in mouse embryonic cells. Cell. 1990;60:461–72. doi: 10.1016/0092-8674(90)90597-8. [DOI] [PubMed] [Google Scholar]
- 23.Osterlund C, Wramsby H, Pousette A. Temporal expression of platelet-derived growth factor (PDGF)-A and its receptor in human preimplantation embryos. Mol Hum Reprod. 1996;2:507–12. doi: 10.1093/molehr/2.7.507. [DOI] [PubMed] [Google Scholar]
- 24.Palmieri SL, Peter W, Hess H, Scholer HR. Oct-4 transcription factor is differentially expressed in the mouse embryo during establishment of the first two extraembryonic cell lineages involved in implantation. Dev Biol. 1994;166:259–267. doi: 10.1006/dbio.1994.1312. [DOI] [PubMed] [Google Scholar]
- 25.Reubinoff BE, Pera MF, Vajta G, Trounson AO. Effective cryopreservation of human embryonic stem cells by the open pulled straw vitrification method. Hum Reprod. 2001;16:2187–2194. doi: 10.1093/humrep/16.10.2187. [DOI] [PubMed] [Google Scholar]
- 26.Rosner MH, Vigano MA, Ozato K, Timmons PM, Poirier F, Rigby PW, Staudt LM. A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature. 1990;345:686–692. doi: 10.1038/345686a0. [DOI] [PubMed] [Google Scholar]
- 27.Scholer HR, Dressler GR, Balling R, Rohdewohld H, Gruss P. Oct-4: a germline-specific transcription factor mapping to the mouse t-complex. EMBO J. 1990;9:2185–2195. doi: 10.1002/j.1460-2075.1990.tb07388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silva J, Nichols JM, Theunissen TW, Guo G, Oosten AL, Barrandon O, Wray J, Yamanaka S, Chambers I, Smith A. NANOG is the gateway to the pluripotent ground state. Cell. 2009;138:722–737. doi: 10.1016/j.cell.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith AG. Embryo-derived stem cells: of mice and men. Ann Rev Cell Dev Biol. 2001;17:435–462. doi: 10.1146/annurev.cellbio.17.1.435. [DOI] [PubMed] [Google Scholar]
- 30.Solter D, Knowles BB. Immunosurgery of mouse blastocyst. Proc Natl Acad Sci USA. 1975;72:5099–5102. doi: 10.1073/pnas.72.12.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blerkom J, Davis P, Mathwig V, Alexander S. Domains of high polarized and low polarized mitochondria may occur in mouse and human oocytes and early embryos. Hum Reprod. 2002;17:393–406. doi: 10.1093/humrep/17.2.393. [DOI] [PubMed] [Google Scholar]
- 32.Eijk MJ, Rooijen MA, Modina S, Scesi L, Folkers G, Tol HT, Bevers MM, Fisher SR, Lewin HA, Rakacolli D, et al. Molecular cloning, genetic mapping, and developmental expression of bovine POU5F1. Biol Reprod. 1999;60:1093–1103. doi: 10.1095/biolreprod60.5.1093. [DOI] [PubMed] [Google Scholar]
- 33.Yeom YI, Fuhrmann G, Ovitt CE, Brehm A, Ohbo K, Gross M, Hubner K, Scholer HR. Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development. 1996;122:881–894. doi: 10.1242/dev.122.3.881. [DOI] [PubMed] [Google Scholar]
- 34.Zhang P, Zucchelli M, Bruce S, Hambiliki F, Stavreus-Evers A, Levkov L, Skottman H, Kerkelä E, Kere J, Hovatta O. Transcriptome profiling of human pre-implantation development. PLoS One. 2009;4(11):e7844. doi: 10.1371/journal.pone.0007844. [DOI] [PMC free article] [PubMed] [Google Scholar]