Abstract
Purposes
Clinical application of human embryonic stem cells will be possible, when cell lines are created under xeno-free and defined conditions. We aimed to establish methodologies for parthenogenetic activation, culture to blastocyst and mechanical isolation of the inner cell mass (ICM) using bovine oocytes, as a model for derivation and proliferation of human embryonic stem cells under defined xeno-free culture conditions.
Methods
Cumulus-oocyte-complexes were in vitro matured and activated using Ca2+Ionophore and 6-DMAP or in vitro fertilized (IVF). Parthenotes and biparental embryos were cultured to blastocysts, when their ICM was mechanically isolated and placed onto a substrate of fibronectin in StemPro® medium. After attachment, primary colonies were left to proliferate and stained for pluripotency markers, alkaline phosphatase and Oct-4.
Results
Parthenogenesis and fertilization presented significantly different success rates (91 and 79 %, respectively) and blastocyst formation (40 and 43 %, respectively). ICMs from parthenogenetic and IVF embryos formed primary and expanded colonies at similar rates (39 % and 33 %, respectively). Six out of eight parthenogenetic colonies tested positive for alkaline phosphatase. Three colonies were analyzed for Oct-4 and they all tested positive for this pluripotency marker.
Conclusion
Our data show that Ca2+ Ionophore, and 6-DMAP are efficient in creating large numbers of blastocysts to be employed as a model for human oocyte activation and embryo development. After mechanical isolation, parthenogetic derived ICMs showed a good rate of derivation in fibronectin and Stem-Pro forming primary and expanded colonies of putative embryonic stem cells. This methodology may be a good strategy for parthenogenetic activation of discarded human oocytes and derivation in defined conditions for future therapeutic interventions.
Keywords: Parthenogenesis, Embryonic stem cells, Defined conditions
Introduction
Since the first derivation of a human embryonic stem cell (hESC) line by Thomson and colleagues in 1998 [11], several others have been established around the world. However, the vast majority of the existing cell lines will not be suitable for clinical use, because they were created in the presence of animal components (also called xeno-components), which may introduce undesired pathogens into the cells, thus creating a serious future problem for human health. On the other hand xeno-free hESC lines have already been created. These cell lines were generated in presence of human foreskin fibroblast feeders and cultured in defined medium supplemented with human serum [4]. In addition, two independend groups claim to have generated xeno-free clinical grade hESC lines [6, 10], also using feeder layers for derivation and thereafter culturing them in feeder-free systems.
The limited access to human embryos and the ethical issues involved in manipulating them, greatly affects the experimental tests required to explore new substrates or matrices for the creation of stem cell lines with clinical grade for therapeutic use. Parthenogenetic activation is an acceptable and useful means for derivation of new human embryonic stem cell lines [7] for research and clinical applications. The bovine ovary and oocytes are considered the best model for humans, because of their very similar physiology. Thus, we decided to use this species to obtain large numbers of parthenogenetic and biparental in vitro fertilized (IVF) embryos to test the effectiveness of parthenogenetic activation and derivation for the creation of embryonic stem cell lines in defined fibronectin susbtrate and medium for cell culture.
Fibronectin is a high molecular weight glycoprotein present in the extracellular matrix that binds to integrins on the cell surface. It is secreted primarly by fibroblasts and it plays a major role in cell adhesion, migration, differentiation, wound healing and embryonic development. Fibronectin exists as a protein dimer consisting of two monomers linked by di-sulfite bonds. It has already been used as a substrate for feeder-free human embryonic stem cell lines maintenance [1, 9], but not for derivation.
This study represents the first stage of a project, which involves the manufacturing of new defined susbtrates, based on the fibronectin composition, which shall be used for derivation and maintenance of bovine and human embryonic stem cell lines under defined conditions.
Material and methods
Cumulus-oocyte-complexes (COCs) were aspirated from ovarian follicles (3–6 mm diameter) and placed in groups of 20 for in vitro maturation (IVM) in 70 μl medium drops of maturation medium (Medium 199®, Vitrogen; Cravinhos, SP, Brazil), at 38.5 °C, in 5 % CO2 in air for 22 h. COCs with expanded cumulus cells were randomly selected for parthenogenetic activation or IVF.
In vitro fertilization and embryo culture
IVF and embryo culture procedures followed the standard methodology employed routinely in Vitrogen bovine IVF laboratory [5]. Briefly, groups of 15 COCs were placed in IVF medium (H199®, Vitrogen) supplemented with 6 % BSA (Sigma-Aldrich, USA). Frozen sperm was prepared on a discontinuous Percoll (Sigma-Aldrich) gradient and added to COCs at a final concentration of 2.5 × 105/ml at day-0. On day-1 the putative zygotes were transferred to cleavage medium (CR4®, Vitrogen) and cultured to the expanded or hatching blastocyst stage on day-8 or 9. Fertilization rates were calculated as the number of 2-cell embryos on day-2.
Parthenogenetic activation
Matured COCs were exposed to M-199 supplemented with hyaluronidase (80 IU/ml) for 60 s and then gently pipetted in medium H199. Denuded oocytes were left for an additional period of 2–4 h in maturation medium, before exposure to 55 μM Ionomycin (Sigma-Aldrich) in buffered H199 medium for 5 min. Oocytes were washed three times in the same medium and transferred to CR4 medium supplemented with 2 mM 6-DMAP in a humidified atmosphere of 5 % CO2 in air at 38.5 °C for 3.5 h, before culture in CR4 medium. Observations of embryonic development were performed at 48 h and 196 h post-activation. Activation rates were calculated as the number of 2-cell embryos on day-2 (48 h).
Inner cell mass isolation
Day-9 expanded or hatching blastocysts obtained by parthenogenetic activation or IVF were manipulated in their culture dish with a pair of 32 G insulin needles under stereoscope. The blastocysts that have not hatched were gently removed from the zona pellucida by cutting it and pressing the embryo out. The ICM was isolated from the surrounding trophectodermal cells by holding the embryo with one needle and carefully slicing the ICM away with the other. We were careful to remove as much trophectodermal cells as possible, without damaging the ICM.
Derivation and culture of putative bESC colonies
The isolated ICMs were placed onto a four-well dish (Nunc) covered with fibronection (1 μg/ml) from human plasma liquid (Invitrogen) and cultured in 1 ml of StemPro® medium (Invitrogen) and basic fibroblast growth factor bFGF (Gibco). Adherence to the substrate was assessed by gently shaking the dishes in the next day. The putative primary bESC colonies that continued proliferating were maintained in StemPro and mechanically cut in small pieces for passaging after 6–7 days of culture. The cell clumps were distributed into new wells containing fresh fibronectin and StemPro. Cultures were performed in a humidified atmosphere of 5 % CO2 in air at 38.5o.C. Medium was changed every 2 days, by replacing 500 μl of the original volume with fresh medium.
Alkaline phosphatase staining
Alkaline phosphatase (AP) staining was performed using a commercially available kit (Sigma) and carried out according to manufacturer’s instructions. Briefly, grown and well spread colonies were fixed in 4 % paraformaldehyde for 10 min at room temperature washed and covered with the stain solution (napthol/fast red violent solution). The stain was incubated for 15 min in the dark at room temperature, rinsed and examined for red stained stem cell colonies under bright field using an inverted microscope (Nikon Eclipse TS100).
Immunofluorescence analysis of octamer-binding transcription factor 4 (OCT-4) expression
For Oct-4 detection, anti-mouse Oct-4 IgG (1:100; Novus Biological, USA) and goat anti-mouse IgG secondary antibodies (1:500) were used. Nuclei were counterstained with 4,6-diamidino- 2-phenylindole (DAPI). Cells were analyzed using Fluorescent microscope (Nikon Eclipse TS100).
Statistical analysis
The results of each treatment, that is, IVF or parthenogenetic activation were evaluated by Chi-square test (X2). A level of 5 % significance was used.
Overall, we performed 10 replicates of the IVF and parthenogenetic activation experiments up to blastocyst culture, without derivation. Out of these 10 replicates, seven included derivation and culture of the putative primary ESC colonies.
Results
A total of 4158 COCs were retrieved and placed for IVM before IVF or parthenogenetic activation. Table 1 shows the results of activation, fertilization blastocyst formation and derivation. There were significant differences between IVF and parthenogenesis in terms of 2-cell cleavage rates and blastocyst formation (P < 0.05). However, the number of ICMs that attached to the substrate and formed a primary colony did not differ significantly between the two treatments, IVF and parthenogenetic activation. We were not able to maintain the putative bESCells for more than two passages due to laboratory workload and financial restrictions.
Table 1.
Parthenogenetic and IVF embryonic development and derivation on fibronectin and Stem Pro medium
| Treatement | No. repetitions | No. COCs | Day-2 Cleavage (%) | Day-7 Blastocyst (%) | No. of Blastocysts placed onto fibronectin | No. repetitions | No. of ICM adhered to fibronectin (%) | No. of expanded primary colonies (%) |
|---|---|---|---|---|---|---|---|---|
| Parthenogenesis | 10 | 3312 | 3011 (91)a | 1209 (40)a | 350 | 7 | 140 (40)a | 54 (39)a |
| IVF | 10 | 846 | 674 (79)b | 363 (43)b | 90 | 7 | 45 (50)a | 15 (33)a |
*Values with different superscripts within columns are significant different (p < 0.05)
One hundred and forty parthenotes and 45 IVF ICM adhered to fibronectin, and 39 % and 33 % of these presented cell outgrowth within 2 days, respectively. They further proliferated forming monolayers of cells within 6–7 days of culture. The putative bESC colonies exhibited clear borders and characteristic ESC morphology: they were small and round with low cytoplasm:nucleus ratio (Fig. 1). The cell layers underwent two passages before we stopped them from continuing to proliferate.
Fig. 1.
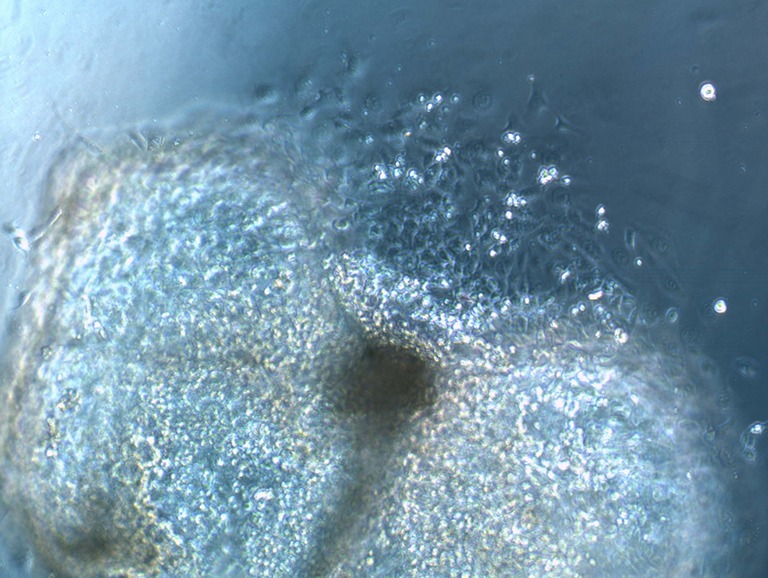
Putative bovine parthenogenetic embryonic stem cell colony
Out of the thirteen putative bESC colonies that were tested for alkaline phosphatase, eight parthenote and five IVF showed a positive reaction (Fig. 2). Also, three out of three parthenote putative bESC colonies tested positive for Oct-4.
Fig. 2.
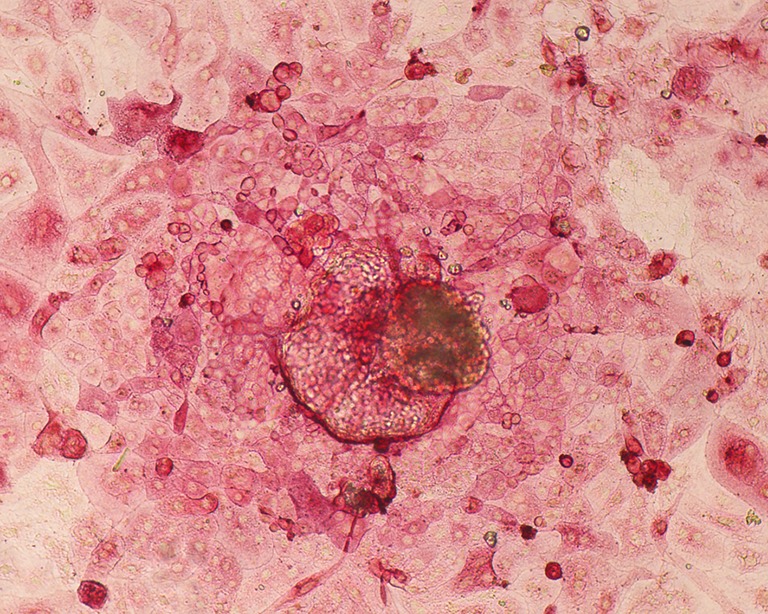
Putative bovine primary embryonic stem cell colony stained for alkaline phosphatase
Discussion
This study presents a simple and efficient methodology for the production of bovine parthenotes and isolation of ICMs for derivation and generation of putative bESC colonies using a defined substrate and medium, which may be applied as a model to work with human discarded oocytes. The newness of our work is that we successfully achieved good rates of derivation of primary bESC colonies from parthenogentic and IVF embryos in a defined commercially available medium, StemPro and a defined substrate, fibronectin. We believe that these protocols may be extrapolated for human oocytes that failed to fertilize in Assisted Reproduction cycles to generate new hESC lines under similar defined conditions.
The present activation methodology is not new [2, 12–14], but it showed a considerable higher rate of activation and development to blastocyst, when compared with previous reports using a similar protocol [15] and they agree with data reported in other studies on bovine parthenogesis [8, 12]. The successful manual isolation of the ICMs allowed us to develop an efficient protocol for derivation of bovine ESCs using defined components as fibronectin and StemPro for culture. Forty percent of the parthenogenetic ICMs and 50 % of the IVF ICMs attached to the substrate. From the adhered ICMs we obtained 39 % and 33 % of expanded colonies, respectively, suggesting that cells interacted satisfactory with fibronectin and maintained their proliferative capacity forming typical primary stem cell-like colonies. These results contrast with previously reported data, where 15 mechanically dissected bovine ICMs plated onto mouse embryonic feeder cells did not adhere to the substrate and disappeared in few days [3]. In addition, assays for pluripotency markers presented very clear results indicating that the parthenogenetic and IVF colonies created by these methodologies were primary bESCs.
One important point to make is that in our experiments, we did not observe any difference in developmental dynamics between parthenogenetic and IVF embryos, neither between the primary and the expanded colonies derived from both groups of embryos. This information reassures us to start studies with human parthenotes under the same defined conditions aiming to obtain clinical-grade parthenogetic ESC lines, once we have all the setting for this end.
Parthenogenetic activation is a useful method to create embryos for experimental and clinical work in humans, because their use overcomes the ethical concerns related to the manipulation of human biparental embryos generated for reproductive purposes. It has already been shown that parthenotes may become an important source of blastocysts to derive human embryonic stem cells (hESCs), which may benefit a considerable part of the human population as they are immunocompatible to the oocyte donors [7]. In this regard, our study adds useful information on protocols to produce high numbers of parthenogenetic blastocysts for derivation and a successful means of ICM isolation and culture under defined conditions.
We were not able to continue the culture of the putative bESC due to laboratory workload and financial restrictions. Our aim is to follow up these results using the same protocols and new chemically defined matrices to produce well-established bovine and human embryonic cell lines. We are aware that bovine embryonic cell lines are not easy to keep in culture and possibly, it will be more challenging to maintain them proliferating under defined conditions, than human ESCs. In a recent work, Jin and colleagues [16] compared different methods of cell passaging, feeder cell layers and medium conditions for bovine embryonic stem cell-like cells maintenance. They found that a murine embryonic fibroblast feeder layer is more suitable for embryonic stem cell-like cells continued proliferation, than bovine embryonic fibroblasts. However, feeder cells represent a potential source of putative pathogens and thus a component we shall not try to employ in our work as others did [4, 6, 10].
In conclusion, we believe that the present results show that the combination of fibronectin and StemPro was effective in producing a good number of primary putative bESC colonies, which were able to proliferate and demonstrate pluripotency markers after extended culture. Despite the announcement of the creation of the first clinical-grade hESC line [6], with potential therapeutic applications, there is still the need for more research to develop protocols that are economically viable and repeatable under different laboratory settings and also safe, for clinical use.
Footnotes
Capsule
Bovine parthenotes may be used as a model for the creation of human embryonic stem cells derived and cultured under defined conditions.
References
- 1.Amit M, Shariki C, Margulets V, Itskovitz-Eldor J. Feeder layer- and serum-free culture of human embryos stem cells. Biol Reprod. 2004;70:837–845. doi: 10.1095/biolreprod.103.021147. [DOI] [PubMed] [Google Scholar]
- 2.Bhak JS, Lee SL, Ock SA, Mohana Kumar B, Choe SY, Rho GJ. Developmental rate and ploidy of embryos produced by nuclear transfer with different activation treatments in cattle. Anim Reprod Sci. 2006;92:37–49. doi: 10.1016/j.anireprosci.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 3.Cao S, Wang F, Chen Z, Liu Z, Mei C, Wu H, Huang J, Li C, Zhou L, Lui L. Isolation and culture of primary bovine embryonic stem cell colonies by a novel method. J Exp Zool. 2009;331A:368–376. doi: 10.1002/jez.535. [DOI] [PubMed] [Google Scholar]
- 4.Ellerström C, Strehl R, Moya K, Anderson K, Berch C, Lundin K, Hyllner J, Semb H. Derivation of a xeno-free human embryonic stem cell line. Stem Cells. 2006;24:2170–2176. doi: 10.1634/stemcells.2006-0130. [DOI] [PubMed] [Google Scholar]
- 5.Ferreira RM, Ayres H, Chiaratti MR, Ferraz ML, Araújo AB, Rodrigues CA, Watanabe YF, Vireque AA, Joaquim DC, Smith LC, Meirelles FV, Baruselli PS. The low fertility of repeat-breeder cows during summer heat stress is related to a low oocyte competence to develop into blastocysts. J Dairy Sci. 2011;94:2383–2392. doi: 10.3168/jds.2010-3904. [DOI] [PubMed] [Google Scholar]
- 6.Ilic D, Stephenson E, Wood V, Jacquet L, Stevenson D, Petrova A, Kadeva N, Codognotto S, Patel H, Semple M, Corwell G, Ogilvie C, Braude P. Derivation and feeder-free propagation of human embryonic stem cells under xeno-free conditions. Cytotherapy. 2012;14:122–128. doi: 10.3109/14653249.2011.623692. [DOI] [PubMed] [Google Scholar]
- 7.Mai Q, Yui Y, Li T, Wang L, Chen M-J, Huang S-Z, Zhou C, Zhou Q. Derivation of human embryonic stem cell lines from parthenogenetic blastocysts. Cell Res. 2007;17:1008–1019. doi: 10.1038/cr.2007.102. [DOI] [PubMed] [Google Scholar]
- 8.Méo SC, Yamazaki W, Ferreira CR, Perecin F, Saraiva NZ, Leal CL, Garcia JM. Parthenogenetic activation of bovine oocytes using single and combined strontium, ionomycin and 6-dimethylaminopurine treatments. Zygote. 2007;15:295–306. doi: 10.1017/S0967199407004285. [DOI] [PubMed] [Google Scholar]
- 9.Noaksson K, Zoric N, Zeng X, Rao MS, Hyllner J, Semb H, Kubista M, Sartipy P. Monitoring differentiation of human embryonic stem cells using real-time PCR. Stem Cells. 2005;23:1460–1467. doi: 10.1634/stemcells.2005-0093. [DOI] [PubMed] [Google Scholar]
- 10.Tannenbaum SE, Turetsky TT, Singer O, Aizenman F, Kirshberg S, Ilouz N, Gil Y, Berman-Zaken Y, Perlman TS, Geva N, Levy O, Arbell D, Simon A, Ben-Meir A, Shufaro Y, Laufer N, Reubinoff BE. Derivation of xeno-free and GMP-grade human embryonic stem cells – platforms for future clinical applications. PLoS One. 2012;7:e35325. doi: 10.1371/journal.pone.0035325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 12.Velde A, Liu L, Bols PE, Ysebaert MT, Yang X. Cell allocation and chromosomal complement of parthenogenetic and IVF bovine embryos. Mol Reprod Dev. 1999;54:57–62. doi: 10.1002/(SICI)1098-2795(199909)54:1<57::AID-MRD8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Wang ZG, Wang W, Yu SD, Xu ZR. Effects of different activation protocols on preimplantation development, apoptosis and ploidy of bovine parthenogenetic embryos. Anim Reprod Sci. 2008;105:292–301. doi: 10.1016/j.anireprosci.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Winger QA, Fuente R, King A, Armstrong DT, Watson AJ. Bovine parthenogenesis is characterized by abnormal chromosomal complements: implications for maternal and paternal co-dependence during early bovine development. Dev Genet. 1997;21:160–166. doi: 10.1002/(SICI)1520-6408(1997)21:2<160::AID-DVG5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Paffoni A, Brevini TA, Somigliana E, Restelli L, Gandolfi F, Ragni G. In-vitro development of human oocytes after parthenogenetic activation or intracytoplasmic sperm injection. Fertil Steril. 2007;87:77–82. doi: 10.1016/j.fertnstert.2006.05.063. [DOI] [PubMed] [Google Scholar]
- 16.Jin M, Wu A, Dorzhin S, Yue Q, Ma Y, Liu D. Culture conditions for bovine embryonic stem cell-like cells isolated from blastocysts after external fertilization. Cytotechnology. 2012;64:379–89. doi: 10.1007/s10616-011-9408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


