Abstract
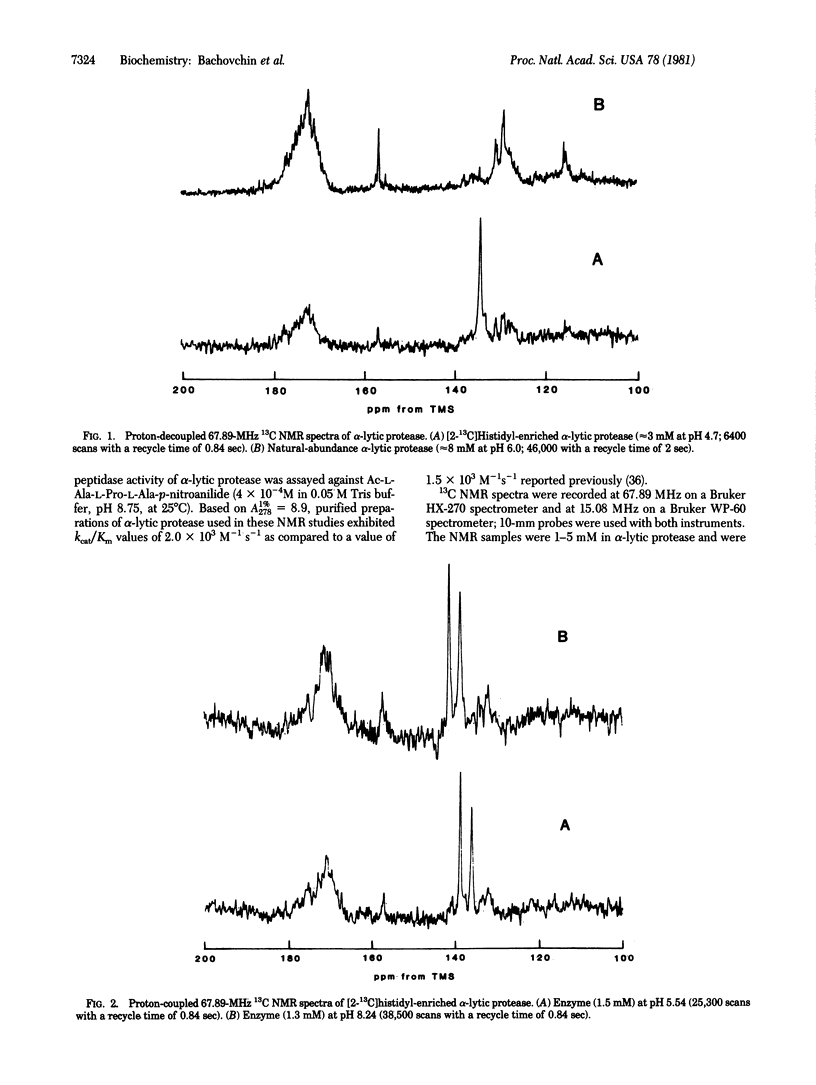
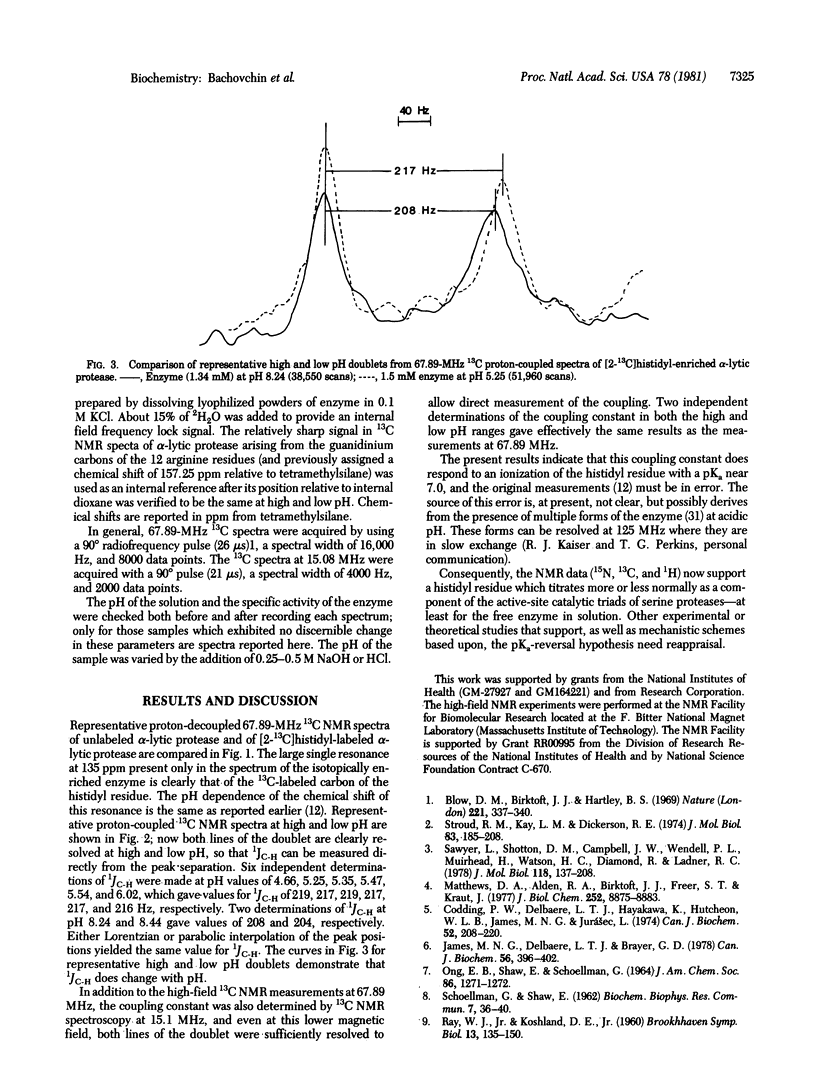
L-Histidine, 90% 13C enriched at the C2 position, was incorporated into the catalytic triad of alpha-lytic protease (EC 3.4.21.12) with the aid of histidine-requiring mutant of Lysobacter enzymogenes (ATC 29487), and the pH dependence of the coupling constant between this carbon atom and its directly bonded proton was reinvestigated. The high degree of specific 13C isotopic enrichment attainable with the auxotroph permits direct observation and measurement of this coupling constant in proton-coupled 13C NMR spectra at 67.89 MHz and at 15.1 MHz. In contrast to the earlier study, the present study indicate that this coupling constant does respond to a microscopic ionization with pKa near 7.0; moreover, the magnitude of the values of 1JC-H observed are in accord with those expected for titration of the histidyl residue. We conclude that the original measurement must be in error and that this coupling constant now also supports a histidyl residue that titrates more or less normally as a component of the catalytic triad of serine proteases.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amidon G. L. Theoretical calculations on an acetic acid, 5-methylimidazole, methanol hydrogen bond network: a model charge relay system. J Theor Biol. 1974 Jul;46(1):101–109. doi: 10.1016/0022-5193(74)90141-6. [DOI] [PubMed] [Google Scholar]
- Blow D. M., Birktoft J. J., Hartley B. S. Role of a buried acid group in the mechanism of action of chymotrypsin. Nature. 1969 Jan 25;221(5178):337–340. doi: 10.1038/221337a0. [DOI] [PubMed] [Google Scholar]
- Bruice T. C. Some pertinent aspects of mechanism as determined with small molecules. Annu Rev Biochem. 1976;45:331–373. doi: 10.1146/annurev.bi.45.070176.001555. [DOI] [PubMed] [Google Scholar]
- Codding P. W., Delbaere L. T., Hayakawa K., Hutcheon W. L., James M. N., Jurásek L. The 4.5 Angstrom resolution structure of a bacterial serine protease from Streptomyces griseus. Can J Biochem. 1974 Mar;52(3):208–220. doi: 10.1139/o74-034. [DOI] [PubMed] [Google Scholar]
- Egan W., Shindo H., Cohen J. S. Carbon-13 nuclear magnetic resonance studies of proteins. Annu Rev Biophys Bioeng. 1977;6:383–417. doi: 10.1146/annurev.bb.06.060177.002123. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Forgac M. D., Richards J. H. Mechanism of action of serine proteases: tetrahedral intermediate and concerted proton transfer. Biochemistry. 1976 Dec 14;15(25):5581–5588. doi: 10.1021/bi00670a024. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Smallcombe S. H., Whitaker D. R., Richards J. H. Carbon nuclear magnetic resonance studies of the histidine residue in alpha-lytic protease. Implications for the catalytic mechanism of serine proteases. Biochemistry. 1973 Nov 6;12(23):4732–4743. doi: 10.1021/bi00747a028. [DOI] [PubMed] [Google Scholar]
- James M. N., Delbaere L. T., Brayer G. D. Amino acid sequence alignment of bacterial and mammalian pancreatic serine proteases based on topological equivalences. Can J Biochem. 1978 Jun;56(6):396–402. doi: 10.1139/o78-062. [DOI] [PubMed] [Google Scholar]
- Kitayama H. P., Fukutome H. A molecular orbital theoretical study on the acid-base property and reactivity of the charge relay system in alpha chymotrypsin. J Theor Biol. 1976 Jul 21;60(01):1–18. doi: 10.1016/0022-5193(76)90152-1. [DOI] [PubMed] [Google Scholar]
- Koeppe R. E., 2nd, Stroud R. M. Mechanism of hydrolysis by serine proteases: direct determination of the pKa's of aspartyl-102 and aspartyl-194 in bovine trypsin using difference infrared spectroscopy. Biochemistry. 1976 Aug 10;15(16):3450–3458. doi: 10.1021/bi00661a009. [DOI] [PubMed] [Google Scholar]
- Komiyama M., Bender M. L., Utaka M., Takeda A. Model for "charge-relay": acceleration by carboxylate anion in intramolecular general base-catalyzed ester hydrolysis by the imidazolyl group. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2634–2638. doi: 10.1073/pnas.74.7.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama M., Roesel T. R., Bender M. L. Intramolecular general base-catalyzed ester hydrolyses by the imidazolyl group. Proc Natl Acad Sci U S A. 1977 Jan;74(1):23–25. doi: 10.1073/pnas.74.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markley J. L., Ibañez I. B. Zymogen activation in serine proteinases. Proton magnetic resonance pH titration studies of the two histidines of bovine chymotrypsinogen A and chymotrypsin Aalpha. Biochemistry. 1978 Oct 31;17(22):4627–4640. doi: 10.1021/bi00615a008. [DOI] [PubMed] [Google Scholar]
- Markley J. L., Porubcan M. A. The charge-relay system of serine proteinases: proton magnetic resonance titration studies of the four histidines of porcine trypsin. J Mol Biol. 1976 Apr 15;102(3):487–509. doi: 10.1016/0022-2836(76)90330-2. [DOI] [PubMed] [Google Scholar]
- Matthews D. A., Alden R. A., Birktoft J. J., Freer T., Kraut J. Re-examination of the charge relay system in subtilisin comparison with other serine proteases. J Biol Chem. 1977 Dec 25;252(24):8875–8883. [PubMed] [Google Scholar]
- RAY W. J., Jr, KOSHLAND D. E., Jr Comparative structural studies of phosphoglucomutase and chymotrypsin. Brookhaven Symp Biol. 1960 Nov;13:135–150. [PubMed] [Google Scholar]
- Robillard G., Shulman R. G. High resolution nuclear magnetic resonance studies of the active site of chymotrypsin. I. The hydrogen bonded protons of the "charge relay" system. J Mol Biol. 1974 Jul 5;86(3):519–540. doi: 10.1016/0022-2836(74)90178-8. [DOI] [PubMed] [Google Scholar]
- Robillard G., Shulman R. G. High resolution nuclear magnetic resonance studies of the active site of chymotrypsin. II. Polarization of histidine 57 by substrate analogues and competitive inhibitors. J Mol Biol. 1974 Jul 5;86(3):541–558. doi: 10.1016/0022-2836(74)90179-x. [DOI] [PubMed] [Google Scholar]
- Robillard G., Shulman R. G. High resolution nuclear magnetic resonance study of the histidine--aspartate hydrogen bond in chymotrypsin and chymotrypsinogen. J Mol Biol. 1972 Nov 14;71(2):507–511. doi: 10.1016/0022-2836(72)90366-x. [DOI] [PubMed] [Google Scholar]
- Rogers G. A., Bruice T. C. Synthesis and evaluation of a model for the so-called "charge-relay" system of the serine esterases. J Am Chem Soc. 1974 Apr 17;96(8):2473–2481. doi: 10.1021/ja00815a028. [DOI] [PubMed] [Google Scholar]
- SCHOELLMANN G., SHAW E. A new method for labelling the active center of chymotrypsin. Biochem Biophys Res Commun. 1962 Feb 20;7:36–40. doi: 10.1016/0006-291x(62)90140-7. [DOI] [PubMed] [Google Scholar]
- Sawyer L., Shotton D. M., Campbell J. W., Wendell P. L., Muirhead H., Watson H. C. The atomic structure of crystalline porcine pancreatic elastase at 2.5 A resolution: comparisons with the structure of alpha-chymotrypsin. J Mol Biol. 1978 Jan 15;118(2):137–208. doi: 10.1016/0022-2836(78)90412-6. [DOI] [PubMed] [Google Scholar]
- Scheiner S., Kleier D. A., Lipscomb W. N. Molecular orbital studies of enzyme activity: I: Charge relay system and tetrahedral intermediate in acylation of serine proteinases. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2606–2610. doi: 10.1073/pnas.72.7.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiner S., Lipscomb W. N. Molecular orbital studies of enzyme activity: catalytic mechanism of serine proteinases. Proc Natl Acad Sci U S A. 1976 Feb;73(2):432–436. doi: 10.1073/pnas.73.2.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud R. M., Kay L. M., Dickerson R. E. The structure of bovine trypsin: electron density maps of the inhibited enzyme at 5 Angstrom and at 2-7 Angstron resolution. J Mol Biol. 1974 Feb 25;83(2):185–208. doi: 10.1016/0022-2836(74)90387-8. [DOI] [PubMed] [Google Scholar]
- WEIL L., JAMES S., BUCHERT A. R. Photo-oxidation of crystalline chymotrypsin in the presence of methylene blue. Arch Biochem Biophys. 1953 Oct;46(2):266–278. doi: 10.1016/0003-9861(53)90200-8. [DOI] [PubMed] [Google Scholar]



