Abstract
Purpose
To investigate whether embryo shape is a useful morphologic predictor of developmental competence in IVF cycles.
Methods
Two hundred eighteen day 3 single embryo transfer (SET) cycles and 225 day 3 double embryo transfer (DET) cycles in which only 8-cell non-fragmented embryos with symmetric blastomeres were transferred and in which the developmental fate of each embryo was known were analyzed for IVF outcomes with respect to embryo shape. Embryo shape was quantitatively calculated after digitizing embryo images using MATLAB, where a score of 1.0 represented a perfectly circular embryo.
Results
The SET data did not reveal a significant impact of embryo shape on embryo developmental fate. The DET data revealed a trend toward the best outcomes in cycles where both embryos exhibited “roundness” scores in the highest tertiles (T3) for embryo shape. However only one subgroup (T2/T1—one embryo in the middle shape tertile (T2) and one in the lowest shape tertile (T1)) was associated with significantly lower odds of live-birth as compared to the referent group (T3/T3). When SET and DET data were combined, embryo shape was not found to be a predictor of IVF outcome.
Conclusions
Based on this retrospective analysis, the weak association of day 3 embryo shape with implantation potential suggests that this morphological characteristic is unlikely to be a useful additional marker for embryo selection after cell number, fragmentation, and blastomere symmetry. Further studies are planned to assess applicability of these conclusions to embryos of varying stages and grades.
Keywords: IVF, Embryo morphology, Day 3 embryo shape, Implantation
Introduction
Determination of embryo developmental competence has been a significant focus of assisted reproduction since the inception of IVF. Embryo morphology has long been a means of selecting the most promising embryos for transfer [1–4]. As the field has evolved, increasing effort has been applied to developing more advanced techniques, such as metabolomic profiling of spent culture media and preimplantation genetic screening (PGS), to determine which embryo or embryos have the highest chance for implantation [5, 6]. However, recent prospective trials have not shown improved selection of developmentally competent embryos using metabolomic analyses of spent culture media [7, 8], and it remains unclear whether more invasive techniques such as PGS should be widely implemented. Thus, embryo morphologic assessment remains the standard method for embryo selection in clinical IVF.
Embryo cell number, cytoplasmic fragmentation, and blastomere symmetry are the primary morphologic parameters evaluated for day 3 embryos. The question remains, however, whether there are additional phenotypic embryo characteristics that could augment the grading rubric routinely employed when evaluating cleavage stage embryos. We have noticed that embryo shape is not uniformly spherical; while some embryos are perfectly round, others appear significantly ovoid or oblong in shape. To our knowledge, no previous study has described the significance of this variation. In the following analysis, we set out to quantitatively evaluate shape as a fourth embryo morphologic parameter on day 3, and subsequently tested the hypothesis that the most spherical embryos would exhibit the highest implantation potential.
Materials and methods
This study was IRB approved for retrospective analysis of clinical IVF records by Partners’ Healthcare Institutional Review Board.
Cycle inclusion criteria
Cycles selected for inclusion were any IVF cycle from 2006 to 2010 in which either single embryo transfer or double embryo transfer on day 3 occurred and in which the developmental fate of all transferred embryos was known (i.e. double embryo transfer resulted in either no implantation or confirmed dizygotic twin gestation). Only cycles characterized by eight cell embryos with the lowest fragmentation and perfectly symmetric blastomeres were included.
Clinical protocols
Controlled ovarian hyperstimulation using either GnRH agonist down-regulation, co-flare, or GnRH antagonists to suppress ovulation were employed as per our routine protocols [9]. When >4 follicles were observed, with two lead follicles measuring >18 mm in diameter, 10,000 IU of intramuscular hCG (Profasi: Serono, Geneva, Switzerland or Novarel: Ferring Pharmaceuticals Inc., Suffern, NY) was administered. Oocyte retrieval was scheduled for 36 h post hCG administration. Luteal progesterone supplementation was started 24 h after retrieval, with either daily intramuscular progesterone (50 mg), daily 8 % progesterone vaginal gel (Crinone; Wyeth-Ayerst, Madison, NJ), or vaginal progesterone suppositories three times daily (200 mg). A Wallace catheter (Marlow/Cooper Surgical, Shelton, CT) was routinely used to perform embryo transfer.
Laboratory protocols
Oocytes intended for intracytoplasmic sperm injection were denuded of their surrounding cumulus cells so as to grade nuclear maturity. Metaphase II oocytes were injected 3–5 h after retrieval. In non-ICSI cycles employing conventional insemination, individual oocytes were incubated with ∼50,000 motile spermatozoa without undergoing cumulus stripping. Zygotes exhibiting two pronuclei were subsequently cultured in 25 μL drops of G1.3 or G1.5 medium (VitroLife, Denver, CO) or Global medium (IVF Online, Guelph, ON), overlaid with 8 mL of equilibrated oil in Falcon 1007 culture dishes (Becton Dickinson Labware, Franklin Lakes, NJ). Incubators were set to 37 °C with 5 % CO2 in humidified air.
Embryos were assessed on day 3 prior to transfer for cell number, fragmentation, and asymmetry. Assessments were performed by a team of 6–8 embryologists proficient in embryo grading. Fragmentation scores between 0 and 4 were assigned to each embryo, corresponding to 0, 1–9, 10–25, 26–50, or >50 % cytoplasmic fragmentation. Asymmetry scores between 1 and 3 were given to embryos based on uniformity in blastomere size and shape. A score of 1 represented perfect symmetry and a score of 3 represented severe asymmetry. Embryos exhibiting eight cells with the least fragmentation and highest symmetry were preferentially selected for transfer. Embryos were photographed immediately prior to transfer at 200x magnification.
So as to remove the potential confounding or effect heterogeneity by other morphologic characteristics previously observed to be associated with live-birth, only cycles with transfer of eight cell embryos with 0 fragmentation and a symmetry score of 1 were included in the present study. Moreover, cycles with embryo images that were blurry (thus not allowing for exact embryo measurement) were excluded from the study.
Image analyses
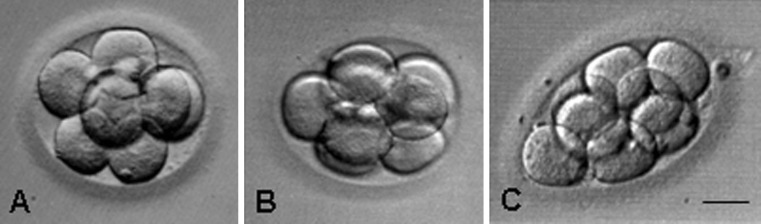
Photographic images were scanned and subsequently analyzed with Matlab (R2011b, Mathworks, Natick, MA). Various image processing functions from the Matlab Imaging Processing Toolbox were utilized to process and clean up the images. Using functions from the toolbox, each image was converted to black and white, and pixels that did not belong to the embryo image were removed. The edge of the embryo was detected by a function that traced the boundaries of the embryo as depicted by the outside perimeter of the blastomeres. Measurements of area were obtained using algorithms that calculated area based on the number of pixels in the edge-defined blastomere. Perimeter was computed using an algorithm that calculates the distance between adjoining pairs of pixels around the border of a defined region. “Roundness” was calculated according to the geometric formula 4*pi*area/perimeter2, where a value of 1.0 represented a perfect circle. Embryo roundness was divided into tertiles, with T3 representing the top 1/3rd of embryos exhibiting values closest to 1.0 (circular), and T1 the 1/3rd of least-circular embryos. Representative examples of embryos in the three tertiles are shown in Fig. 1.
Fig. 1.
Representative photographic images of embryos in the three tertiles; a = T3, b = T2 and c = T1. The bar represents 30 μm
Statistical analyses
As the relation between embryo shape and odds of live-birth was not hypothesized to be linear, embryo shape was analyzed categorically. Tertiles were preferred over quartiles due to the narrow distribution of embryo shape. For double embryo transfers, a six-category cycle-level exposure was created to define the embryo shape of both embryos: T3/T3 (both embryos transferred were in the top tertile), T3/T2, T3/T1, T2/T2, T2/T1, T1/T1 (both embryos transferred were in the bottom tertile).
Multivariable logistic regression was performed to study the association between embryo shape and live-birth. Patient age was included as a covariate in the model a priori. Calendar year, cycle attempt number, day 3 FSH, infertility diagnosis (female factor, male factor, unexplained), peak estradiol, number of oocytes retrieved, number of mature oocytes, percent of normally fertilized embryos (2PN), ICSI, and assisted hatching were tested individually as potential confounders and included in the final model if the addition of the covariate changed the odds ratio for the main exposure by greater than 10 % [10]. Diagnosis, day 3 FSH, % 2PN, and number of embryos frozen met these criteria and were included, with age, as covariates in the final model.
All statistical analyses were performed with Statistical Analysis Software (SAS®) version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Results
A total of 443 IVF cycles met criteria for study inclusion. 218 cycles were characterized by single embryo transfer of a day 3, 8-cell embryo with no fragmentation and symmetric blastomeres. 225 cycles were characterized by double embryo transfer of two 8-cell embryos with no fragmentation and symmetric blastomeres in which either both embryos implanted or neither implanted. As shown (Table 1), age was similar between the two groups, as was ovarian response to stimulation. Assisted hatching was more common in the double embryo transfer group among those with zero or 100 % livebirth fate, and this group had, on average, 1.6 fewer day 3 embryos frozen.
Table 1.
Demographic characteristics of 218 SETs and 225 DETs between 2006 and 2010
| SET (n = 218) | DET (n = 225) | |
|---|---|---|
| Age | ||
| Mean (SD) | 32.7 (3.7) | 33.6 (4.6) |
| Median (Q1–Q3) | 33.0 (30.7–34.6) | 34.2 (31.0–37.1) |
| Minimum–maximum | 20.7–43.5 | 21.8–41.0 |
| Attempt number | ||
| 1 | 170 (78.0 %) | 138 (61.3 %) |
| 2 | 35 (16.1 %) | 52 (23.1 %) |
| 3 | 13 (6.0 %) | 35 (15.6 %) |
| Day 3 FSH | ||
| Mean (SD) | 7.1 (2.6) | 7.2 (3.8) |
| Median (Q1–Q3) | 6.8 (5.7–8.0) | 6.8 (5.2–8.2) |
| Minimum–maximum | 0.8–30.0 | 2.9–45.3 |
| Diagnosis | ||
| Female factor | 110 (50.7 %) | 87 (48.1 %) |
| Male factor | 56 (25.8 %) | 46 (25.4 %) |
| Unexplained | 51 (23.5 %) | 48 (26.5 %) |
| Peak Estradiol | ||
| Mean (SD) | 2391.4 (913.7) | 2275.5 (882.5) |
| Median (Q1–Q3) | 2360 (1681–3163) | 2191 (1540–2973) |
| Minimum–maximum | 474–4178 | 511–5168 |
| Total number of eggs | ||
| Mean (SD) | 17.8 (8.0) | 17.8 (8.3) |
| Median (Q1–Q3) | 18 (12–22) | 17 (12–22) |
| Minimum–maximum | 2–43 | 3–44 |
| Number of mature eggs | ||
| Mean (SD) | 15.2 (7.0) | 15.1 (7.4) |
| Median (Q1–Q3) | 15 (10–20) | 14 (10–19) |
| Minimum–maximum | 1–38 | 2–41 |
| Fertilization rate | ||
| Mean (SD) | 76.5 (18.7) | 79.6 (13.2) |
| Median (Q1–Q3) | 80.5 (70.0–88.9) | 81.8 (71.4–87.5) |
| Minimum–maximum | 11.1–100.0 | 35.7–100.0 |
| ICSI cycle | 77 (35.3 %) | 89 (39.6 %) |
| Assisted hatching | 36 (16.5 %) | 96 (42.7 %) |
| Number of embryos frozen | ||
| Mean (SD) | 7.4 (3.7) | 5.8 (4.4) |
| Median (Q1–Q3) | 7.0 (5.0–9.0) | 5.0 (3.0–7.5) |
| Minimum–maximum | 1.0–27.0 | 1.0–28.0 |
Table 2 presents live-birth rates as a function of embryo shape for the single embryo transfer cycles. T3, representing the most-round tertile, was utilized as the referent group against which less round embryos were compared. T3 embryos had roundness scores in the range 0.89–0.96 as compared to T2 (0.80–0.88) and T1 (0.12–0.79), again where a value of 1.0 represented perfectly round embryos. The percentage of live-births were not different among the three tertiles; the odds of live-birth did not differ with respect to the referent population when controlling for age, diagnosis, day 3 FSH, % 2PN zygotes, and number of embryos frozen as confounding variables.
Table 2.
Association between embryo shape and live-birth among 218 single embryo transfer cycles between 2006 and 2010
| T3 (n = 55) | T2 (n = 58) | T1 (n = 105) | |
|---|---|---|---|
| % Live-birth | 29 (52.7 %) | 30 (51.7 %) | 54 (51.4 %) |
| OR (95 % CI)a | 1.00 (Referent) | 1.03 (0.46–2.28) | 0.89 (0.43–1.82) |
aOdds ratios (OR) and 95 % confidence intervals (CI) are from a logistic regression model adjusted for age, diagnosis (female, male, unexplained), day 3 FSH, percent 2PN, and number of embryos frozen
Table 3 presents live-birth rates for cycles where two embryos were transferred and in which the developmental fate of each embryo was known. Cycles in which both embryos were in the highest tertile for round shape (T3/T3) were the referent population against which other tertile combinations were compared. The highest live-birth rate was observed in the referent population, with an overall decline in pregnancy rates as roundness decreased. However, only the T2/T1 group showed statistically significantly lower odds of live-birth as compared to the T3/T3 group when adjusting for age, diagnosis, day 3 FSH, fertilization, and embryos frozen (OR = 0.32, 95 % CI = 0.12–0.87) .
Table 3.
Association between embryo shape and live-birth among 255 double embryo transfer cycles between 2006 and 2010
| T3/T3 (n = 42) | T3/T2 (n = 55) | T3/T1 (n = 26) | T2/T2 (n = 36) | T2/T1 (n = 42) | T1/T1 (n = 24) | |
|---|---|---|---|---|---|---|
| % Live-birth | 23 (54.8 %) | 29 (52.7 %) | 13 (50.0 %) | 16 (44.4 %) | 14 (33.3 %) | 9 (37.5 %) |
| Odds ratioa | 1.00 (Referent) | 0.63 (0.26–1.55) | 0.68 (0.23–2.05) | 0.45 (0.16–1.22) | 0.32 (0.12–0.87) | 0.39 (0.12–1.22) |
aOdds ratios (OR) and 95 % confidence intervals (CI) are from a logistic regression model adjusted for age, diagnosis (female, male, unexplained), day 3 FSH, percent 2PN, and number of embryos frozen
Among double embryo transfers, combining the bottom three combinations (T2/T2, T2/T1, T1/T1), the odds of live-birth were 62 % lower as compared to the referent population (OR = 0.38, 95 % CI = 0.17–0.86). Comparison of the two groups with at least one embryo of intermediate roundness (T2/T2, T2/T1) to the three tertile groups containing at least one T3 embryo (T3/T3, T3/T2, T3/T1) again revealed a statistically significantly lower odds of live-birth (OR = 0.50, CI 0.26–0.95). Combining all data from both the SET and DET cycles, there were no differences observed in the odds of live-birth with respect to embryo shape (data not shown).
Discussion
In the present study, we set out to assess whether embryo shape on day 3 is correlated with IVF outcomes following day 3 transfer. Using MATLAB, a software program capable of reading digital embryo images and quantifying two-dimensional shape, we evaluated the hypothesis that transfer of the most circular embryos would lead to increased live-birth rates. Embryos were given a roundness coefficient ranging from 0 to 1.0, where 1.0 represented a perfect circle. Cycles were only included in which one or two embryos of the highest quality were transferred (8-cells, no fragmentation, perfect blastomere symmetry) and in which embryo fate was known. Embryo shape was evaluated in tertiles, where the most round embryos were considered the referent group against which less-round embryos were evaluated. To our knowledge, this is the first and only study assessing the shape of embryos on day 3 as a morphologic predictor of implantation.
While cycles with double embryo transfer where both embryos scored in the lowest tertile did have statistically lower odds of live-birth, no consistent relations were observed for the single embryo transfer group, or when single embryo transfer and double embryo transfer cycles were combined. Still, however, it was interesting to note that when the tertiles in the double embryo transfer cycles were collapsed into two groups, the less-round group did exhibit lowers odds as evidenced by a 62 % reduction in the odds of live-birth.
The reasons for the discordance in results between the single and double embryo transfer groups are unknown. However, the SET group likely included patients having a better prognosis for success based on their a priori selection for an SET, and as evidenced by their lower mean age (32.7 vs. 33.6y), increased percentage of patients undergoing their first IVF attempt (78.0 % vs. 61.3 %), and more embryos frozen (7.4 vs. 5.8). It remains to be determined whether this overall improved prognosis in our SET patients explains the apparent irrelevance of embryo shape to implantation potential in this subgroup.
Several limitations of this study should be acknowledged. The images used to quantify embryo shape were static, 2-D images. Time lapse photography of developing embryos confirm that development is a dynamic process in which expansion and contraction occurs [11]; thus a single embryo image may not be an accurate portrayal of an embryo’s true dynamic 3-D shape. While we were able to assess how circular embryos were in two dimensions, this is only a proxy for measuring the true three dimensional shape; it is possible that embryos which were not truly spherical appeared to be circular in two dimensions if the picture was taken at a specific angle. Still, however, we feel that our two dimensional images were able to give a fair approximation of the overall three dimensional shape of the embryos. A further limitation relates to our intentional inclusion of only those embryos with known developmental fate. Therefore, by design, DET cycles with 50 % live birth rate—prevalent in the general clinical population—were excluded from this investigation. It is possible that cycles transferred with T2/T1 are less likely to have a twin birth than a cycle transferred with T3/T3, and thus we may be excluding a disproportionate number of births from T2/T1. While inclusion of such cycles would not enable us to associate precisely embryo shape with resulting live birth, exclusion may temper applicability of our observations to this third patient group. Finally, it remains to be seen whether our observations pertain to embryos of lesser quality than those included in this study.
Conclusion
The present results suggest that day 3 embryo shape may be weakly associated with IVF outcome. However, based on the above findings, it is overall unlikely that embryo shape as an additional morphologic criteria for embryo evaluation would offer much benefit above that which is afforded by assessment of cell number, fragmentation, and blastomere symmetry as a tool for embryo selection. A prospective randomized trial is now planned to confirm these conclusions in which embryos will be selected for transfer with and without consideration of shape and then followed for implantation potential.
Acknowledgments
Conflict of interest
The authors can identify no potential conflicts of interest, neither financial nor any other, involved in the writing or publication of this manuscript.
Footnotes
Capsule
Embryo shape on day 3 is only weakly associated with implantation potential.
References
- 1.Racowsky C, Combelles CM, Nureddin A, Pan Y, Finn A, Miles L, et al. Day 3 and day 5 morphological predictors of embryo viability. Reprod Biomed Online. 2003;6:323–331. doi: 10.1016/S1472-6483(10)61852-4. [DOI] [PubMed] [Google Scholar]
- 2.Giorgetti C, Terriou P, Auquier P, Hans E, Spach JL, Salzmann J, et al. Embryo score to predict implantation after in-vitro fertilization: based on 957 single embryo transfers. Hum Reprod. 1995;10:2427–2431. doi: 10.1093/oxfordjournals.humrep.a136312. [DOI] [PubMed] [Google Scholar]
- 3.Scott L, Alvero R, Leondires M, Miller B. The morphology of human pronuclear embryos is positively related to blastocyst development and implantation. Hum Reprod. 2000;15:2394–2403. doi: 10.1093/humrep/15.11.2394. [DOI] [PubMed] [Google Scholar]
- 4.Ziebe S, Petersen K, Lindenberg S, Andersen AG, Gabrielsen A, Andersen AN. Embryo morphology or cleavage stage: how to select the best embryos for transfer after in-vitro fertilization. Hum Reprod. 1997;12:1545–1549. doi: 10.1093/humrep/12.7.1545. [DOI] [PubMed] [Google Scholar]
- 5.Seli E, Botros L, Sakkas D, Burns DH. Noninvasive metabolomic profiling of embryo culture media using proton nuclear magnetic resonance correlates with reproductive potential of embryos in women undergoing in vitro fertilization. Fertil Steril. 2008;90:2183–2189. doi: 10.1016/j.fertnstert.2008.07.1739. [DOI] [PubMed] [Google Scholar]
- 6.Mastenbroek S, Twisk M, Veen F, Repping S. Preimplantation genetic screening: a systematic review and meta-analysis of RCTs. Hum Reprod Update. 2011;17:454–466. doi: 10.1093/humupd/dmr003. [DOI] [PubMed] [Google Scholar]
- 7.Hardarson T, Ahlström A, Rogberg L, Botros L, Hillensjö T, Westlander G, et al. Non-invasive metabolomic profiling of Day 2 and 5 embryo culture medium: a prospective randomized trial. Hum Reprod. 2012;27(1):89–96. doi: 10.1093/humrep/der373. [DOI] [PubMed] [Google Scholar]
- 8.Vergouw CG, Kieslinger DC, Kostelijk EH, Botros LL, Schats R, Hompes PG, et al. Day 3 embryo selection by metabolomic profiling of culture medium with near-infrared spectroscopy as an adjunct to morphology: a randomized controlled trial. Hum Reprod. 2012 May 30. [Epub ahead of print] [DOI] [PubMed]
- 9.Reichman DE, Missmer SA, Berry KF, Ginsburg ES, Racowsky C. Effect of time between human chorionic gonadotropin injection and egg retrieval is age dependent. Fertil Steril. 2011;95:1990–5. [DOI] [PubMed]
- 10.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79(3):340–349. doi: 10.2105/AJPH.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirkegaard K, Agerholm IE, Ingerslev HJ. Time-lapse monitoring as a tool for clinical embryo assessment. Assist Reprod Genet. 2012;29(6):565–572. doi: 10.1007/s10815-012-9750-x. [DOI] [PubMed] [Google Scholar]