Abstract
Purpose
Our objective was to identify a marker for oocyte aneuploidy in follicular fluid (FF) in women with an increased risk of oocyte aneuploidy after controlled ovarian hyperstimulation.
Materials and methods
Three groups of oocytes were constituted for polar body screening by FISH (chromosomes 13, 16, 18, 21 and 22): Group 1, advanced maternal age (n = 156); Group 2, implantation failure (i.e. no pregnancy after the transfer of more than 10 embryos; n = 101) and Group 3, implantation failure and advanced maternal age (n = 56). FSH and other proteins were assayed in the corresponding FF samples.
Results
Of the 313 oocytes assessed, 35.78 % were abnormal. We found a significant difference between the follicular FSH levels in normal oocytes and abnormal oocytes (4.85 ± 1.75 IU/L vs. 5.41 ± 2.47 IU/L, respectively; p = 0.021). We found that the greater the number of chromosomal abnormalities per oocyte (between 0 and 3), the higher the follicular FSH level.
Conclusion
High FF FSH levels were associated with oocyte aneuploidy in women having undergone controlled ovarian hyperstimulation.
Keywords: Oocyte, Polar body, FISH, Aneuploidy, FSH
Introduction
In humans, 50 % of fertilized embryos do not implant [31] and most of these implantation failures are related to aneuploidy. Moreover, these abnormalities are predominantly oocyte-related, since between 20 % and 30 % of human oocytes have defects of this type [48]. When screened with respect to 10 chromosomes, only 36 % of embryos were found to be normal after ovarian hyperstimulation for IVF [3]. However, it is not clear why our species has such a high chromosomal risk.
Meiosis I errors can occur via one of two mechanisms: (a) reduced cohesion between sister chromatids, leading to premature separation of sister chromatids (PSSC) and an abnormality affecting a single chromatid (many authors consider that this is the major event in oocytes from older women [21, 44, 45, 66, 69]), and (b) a malsegregation of a whole chromosome (comprising 2 chromatids at this stage) due to a change in the spindle structure, i.e. non-disjunction (ND).
Over the last 15 years or so, it has been unambiguously demonstrated that oocyte chromosomal abnormalities are strongly related to maternal age [5, 15, 22, 25, 44–46]. Many reports on the FISH-based preconception screening (PCS) of 5 chromosomes have revealed a first polar body (PB) abnormality rate of between 22 % and 42 % in women over the age of 36 [65], 1998, [30, 41, 52, 66, 67, 69]. Besides age, factors like hyperstimulation treatment may also influence the results. The effect of superovulation and the relative importance of meiosis I and II in the generation of aneuploidy are still subject to debate in the literature. Although most errors in natural human reproduction were thought to occur during meiosis I (though with some dependence on the chromosome involved) [23, 24], recent comparative genomic hybridization (CGH)/microarray data on first and second polar bodies after IVF prompted different conclusions [15, 33]. Pre-IVF ovarian stimulation could perturb meiosis in aging oocytes and also modify the aetiology of meiotic errors [15]. Baart’s randomized, prospective study of an embryo screening programme [4] showed that the embryonic aneuploidy rate was significantly higher in women undergoing a conventional, high-dose FSH hyperstimulation protocol than in women undergoing “mild” (i.e. low-dose) hyperstimulation. An impact of ovarian stimulation on aneuploidy has also been suggested by many authors [4, 12, 32, 40], although other groups have reported contradictory results [10, 36, 68]. In a study of human in vitro matured oocytes, the error rate for the first meiotic division increased significantly [75] with increasing FSH levels in the culture medium. Lastly, the results of an European collaborative study on first and second PB screening in aged women [21] were compatible with the hypothesis in which aneuploidies are more frequent in aged women undergoing IVF than in natural conception of same age women. The study also suggested that the type of aneuploidy differs in these two situations since in this series, over half of aneuploidies resulted from errors in the second meiotic division. More generally in humans, many lines of evidence argue in favour of interactions between the hormonal environment (especially FSH levels), the quality of oocyte maturation, the quality of meiosis and the risk of aneuploidy. Kan [28] found a positive correlation between perifollicular vascularisation during ovarian stimulation, normal chromosome content in the oocyte and the embryo implantation rate. Perifollicular hypoxia may have a harmful impact on the configuration of the meiotic spindle and on embryo quality [62, 63]. Smoking is thought to dampen the response to ovarian stimulation and increase the oocyte aneuploidy rate [76]. Recent work has underlined the importance of factors in the ovarian environment (such as the level of dehydroepiandrosterone) [19] and the oocyte’s micro-environment (i.e. the cells in the cumulus-oocyte complex) on oocyte quality [1, 2, 17].
Results obtained in mice also suggest that subtle changes in the regulation of folliculogenesis and oocyte maturation are capable of inducing aneuploidy [49, 50]. have shown that FSH modifies the spindle configuration. Hence, exposure to high doses of FSH during in vitro maturation could accelerate nuclear maturation and increase the aneuploidy rate in metaphase II. An elevated oocyte aneuploidy rate has been observed in an LH gene knock-out mouse [26]. Furthermore, [61] showed that repeated FSH stimulation can alter meiotic spindle quality in the mouse. Lastly, aging-associated aneuploidy in mice can be prevented by caloric restriction [51].
Hence, the objective of the present study was to identify follicular fluid (FF) markers that are predictive of the risk of aneuploidy, in order to gain insight into the mechanism involved in various groups of women with an increased aneuploidy risk.
In addition to older women, a number of other subpopulations present an elevated oocyte aneuploidy rate. This includes women with implantation failure (i.e. the lack of a pregnancy after the transfer of at least 10 embryos) [53, 70]. However, non-invasive, early screening of these abnormalities is not yet possible.
We evaluated the predictive potential of several factors involved in oogenesis and ovulation: FSH, LH, P4, E2, testosterone and anti-Müllerian hormone (AMH). Plasma AMH may be a marker of ovarian function and the likelihood of pregnancy in Assisted Reproductive Technology (ART) programmes [11, 27, 34, 47] and thus also appears to be a good candidate marker of oocyte quality.
Most of the previous studies of FF proteins involved pooled samples, which prevented an individual analysis of each follicle obtained after ovarian stimulation and the associated outcome for each oocyte. Our study is novel in that we tracked individual oocytes and corresponding FF samples.
Materials and methods
The study population
We recruited all the women with normal karyotype participating in our intracytoplasmic sperm injection (ICSI) programme with a PCS protocol for age or implantation failure at the Poissy Saint-Germain-en-Laye ART Centre. First PB diagnosis is performed before fertilization in patients who have a normal karyotype but present known risk factors for oocyte aneuploidy and not in a control group without any aneuploidy risk factor due to the restrictions of french bioethics law. In France, these couples with normal karyotypes though at risk for oocyte aneuploidy can not undergo pre-implantation genetic diagnosis (PGD) because the latter is not authorized when the parents are free of genetic abnormalities (even in cases of advanced maternal age and repeated implantation failure). Seventy-eight oocytes retrievals for FISH PCS test were performed on the first PB, with the individual collection of FF samples. Three groups were constituted, as a function of the indication:
(advanced maternal age): 28 patients, 40 ICSI with PCS attempts (156 oocytes with a PB diagnosis) in patients over the age of 36 (mean ± SD age: 38 years and 11 months ± 1 year and 11 months) but below the threshold for implantation failure indication (i.e. fewer than 10 embryo transferred prior to the attempt in question). 32/40 attempts (80 %) were ICSI for severe male infertility.
(implantation failure): 17 patients, 20 ICSI with PCS attempts (101 oocytes with a PB diagnosis) in patients under the age of 36 (mean ± SD age: 32 years and 1 month ± 2 years and 7 months) and with implantation failure (i.e. more than 10 embryos transferred (mean: 14.5 ± 3.6) and no pregnancy prior to the attempt in question). 12/20 attempts (60 %) were ICSI for severe male infertility.
(implantation failure + advanced maternal age): 15 patients, 18 ICSI with PCS attempts (56 oocytes with a PB diagnosis) in women over the age of 36 (mean ± SD age: 38 years and 3 months ± 1 year and 7 months) with implantation failure (13 ± 3 embryos transferred and no pregnancy prior to the concerned attempt). 13/18 attempts (72 %) were ICSI for severe male infertility.
Methods
Ovarian stimulation and FF collection
Follicular development was stimulated with recombinant FSH (Gonal F® from Merck serono orPuregon® from MSD) and was monitored by E2 mesurements and transvaginal ultrasound from day 5 after stimulation. Ovulation was induced with 250 μg of recombinant hCG (ovitrelle® from Merck serono). Oocyte puncture and FF sampling were performed on individual follicles. The FF’s volume and aspect (haemorrhagic, haematic, orange or yellow) were noted. Ten samples (3 %) were excluded because the blood contamination level was greater than 3 % (corresponding to a haemorrhagic aspect). The non-excluded FF samples (with a haematic, orange or yellow aspect) were divided into 250 μl aliquots and frozen at −80 °C for subsequent analysis.
Assay for FF markers
Assays for some putative FF markers (FSH and LH) were performed on an ARCHITECT system in Two-step, using an immunoassay microparticulate by chemoluminescence (CMIA) (Abbott, Rungis, France).
Oestradiol, progesterone and testosterone were performed on an ARCHITECT system in one step, using an immunoassay microparticulate by chemoluminescence (CMIA) (Abbott, Rungis, France).
Anti-Müllerian hormone was assayed quantitatively with the AMH/MIS ELISA kit (Immunotech, Marseilles, France).
Total protein was assayed on a COBAS INTEGRA 800 system, using a Colorimetric test (Roche, USA).
FISH PCS test
Polar body biopsy was carried out as previously described [69] using the Zilos TK laser (Hamilton Thorne Biosciences, Boston USA). Immediately after oocyte retrieval and corona radiata removal, 3 or 4 laser impacts (180 mW, 0.5 ms pulse) were made in the zona pellucida. The PB was then extracted (from 340 oocytes, in all) with a biopsy micropipette (Humagen, Charlottesville, VA, USA) and placed in 0.5 μl of water on a siliconized slide. The drop was air-dried and two drops of Carnoy solution were added.
The slide was transferred into a series of solutions: methanol at room temperature for 5 min, 2× SSC at 37 °C for 10 min, 1 % paraformaldehyde at room temperature for 10 min, PBS at room temperature for 5 min, 0.1 N HCl with 30 μl/40 ml of 10 % active pepsin solution for 10 min, PBS at room temperature for 5 min and, lastly, 70 %, 85 % and then 100 % ethanol solutions for 2 min each.
A 3 μl drop of probe solution was placed on each PB. Codenaturation and hybridization were automatically performed in a Hybrite system (Vysis) (at 73 °C for 4 min and at 37 °C for 4 h).
The slide was then washed in a 0.7× SSC/0.3 % NP40 solution for 1 min 45 s and then in a 2× SSC/0.1 % NP40 solution for 15 s. This was followed by counterstaining with an antifade solution and analysis with a five-filter fluorescence microscope (Olympus BX60) and the PathVysion imaging system.
Polar bodies were analysed using the MultiVysionTM Polar Body Kit probe panel hybridization mixture (Abbott), which includes LSI® 13 (13q14, labelled with SpectrumRedTM), CEP® 16 (satellite II D16Z3, labelled with SpectrumAquaTM), CEP® 18 (alpha satellite D18Z1, labelled with SpectrumBlueTM), LSI® 21 (21q22.13–21q2.2, labelled with SpectrumGreenTM) and LSI® 22 (22q11.2, labelled with SpectrumGoldTM). Chromosomes 13, 16, 18, 21 and 22 were chosen because those aneuploïdies are the most viable autosome aneuploïdies in the human species.
Each first PB chromosome normally consists of two chromatids. Locus-specific probes always give a doublet signal corresponding to each of the two chromatids; hence, each chromosome was represented by either a doublet of a distinct colour (sometimes very close) or by two separate signals. Centromeric probes usually give strong signals for one chromosome or a doublet with very close signals. The absence of spot (0 spot; no chromosome present in polar body) or the presence of 4 signals (2 chromosomes present in polar body) was interpreted as ND and 1 or 3 spots were interpreted as PSSC. Two separate signals were considered to correspond to balanced separation of sister chromatids (rather than an abnormality) and were therefore not included in the PSSC count.
313/340 oocytes could be successfully diagnosed and we had 27 technical failures
The ICSI procedure
We decided in the 3 groups to inject all oocytes with normal FISH results or lacking a FISH analysis. Indeed some oocytes were immature in the morning at the time of the biopsy but expelled their polar body a few hours later and could be injected in the afternoon. The reason to do so was to inject as many oocytes as possible to preserve all the possibilities of pregnancies since the efficiency of the procedure in terms of pregnancy rates has not yet been demonstrated. The opening in the zona pellucida was maintained in a 12 or 6 o’clock position, in order to keep the spindle away from the microinjection site. Oocytes were then microinjected using a 7 μm outer diameter ICSI micropipette (Humagen).
Statistical analysis
Statistical analyses (chi-squared, Fisher, ANOVA and t tests) in Tables 1, 2, 3 and 4, and ANOVA and t test in Figs. 1, 2 and 3, were performed using Statview software. The threshold for statistical significance was set to p < 0.05.
Table 1.
Patients characteristics in the 3 study subgroups and stimulation data
| Group 1 : Advanced maternalage | Group 2 : Implantation faillure | Group 3 : Implantation faillure + advanced maternal age | p value | |
|---|---|---|---|---|
| Number of patients with severe male infertility (%) | 25/28 (89 %) | 12/17 (70.5 %) | 11/15 (73 %) | NS |
| Number of attempts with severe male infertility (%) | 32/40 (80 %) | 12/20 (60 %) | 13/18 (72 %) | NS |
| Age (mean ± SD) | 38 years and 11 months ± 1 year and 11 months | 32 years and 1 months ± 2 year and 7 months | 38 years and 3 months ± 1 year and 7 months | 1 vs 2 and 2 vs 3: p < 0.0001 |
| 1 vs 3: NS | ||||
| FSH units administered per attempt (mean ± SD) | 2237.86 ± 624.94 | 2836.83 ± 2827.50 | 2805.00 ± 1011.73 | 1 vs 2: p = 0.0004 |
| 1 vs 3:p = 0.006 | ||||
| 2 vs 3: NS | ||||
| E2 serum level 36 hours before oocytes retrieval (mean ± SD) | 2013.47 ± 688.06 | 2299.82 ± 1049.85 | 1592.85 ± 689.50 | 1 vs 2: p = 0.0004 |
| 1 vs 3 and 2 vs 3:p < 0.0001 | ||||
| Number of oocytes retrieval (mean ± SD) | 8.27 ± 3.19 | 9.25 ± 2.54 | 6.29 ± 2.27 | 1 vs 2:p = 0.0005 |
| 1 vs 3 and 2 vs 3:p < 0.0001 | ||||
| Day 3 basal FSH (mean ± SD) | 6.79 ± 0.68 | 6.43 ± 3.49 | 9.94 ± 6.00 | 1 vs 2: NS |
| 1 vs 3 and 2 vs 3:p = 0.003 | ||||
| Day 3 LH (mean ± SD) | 5.72 ± 2.86 | 4.53 ± 1.35 | 4.20 ± 1.69 | 1 vs 2 vs 3: NS |
| Day 3 E2 (mean ± SD) | 38.14 ± 15.09 | 31.33 ± 12.05 | 56.50 ± 23.33 | 1 vs 2 vs 3: NS |
| Follicular fluid volume (ml)(mean ± SD) | 3.95 ± 1.95 | 3.63 ± 1.94 | 3.99 ± 2.67 | 1 vs 2 vs 3: NS |
| FSH levels in FF (IU/L)(mean ± SD) | 5.021 ± 1.75 | 5.02 ± 2.44 | 5.17 ± 2.08 | 1 vs 2 vs 3: NS |
Table 2.
ART outcomes in the three study subgroups
| Group 1: Advanced maternal age | Group 2: Implantation failure | Group 3: Implantation failure + advanced maternal age | Total | |
|---|---|---|---|---|
| Number of ICSI attempts | 40 | 20 | 18 | 78 |
| Number of oocytes with PB diagnosis | 156 | 101 | 56 | 313 |
| Number of oocytes with an abnormal PB (%) | 55 out of 156 (35.25 %) | 32 out of 101 (31.68 %) | 25 out of 56 (44.64 %) | 112 out of 313 (35.78 %) |
| Number of oocytes injected with a normal PB (%) | 101 out of 156 (64.75 %) | 69 out of 101 (68.32 %) | 31 out of 56 (45.34 %) | 201 out of 313 (64.21 %) |
| Number of oocytes injected with no PB diagnosis | 92 | 55 | 32 | 179 |
| Number of two-pronucleates with a PB diagnosis | 70 out of 101 (69.30 %) | 47 out of 69 (68.11 %) | 28 out of 31 (90.32 %) | 145 out of 201 (72.14 %) |
| Number of embryos derived from oocytes with a PB diagnosis (%) | 68 out of 70 (97.14 %) | 42 out of 47 (89.36 %) | 23 out of 28 (82.14 %) | 133 out of 145 (91.72 %) |
| Number of embryos per transfer | 1.97 | 2.59 | 2.06 | 2.14 |
| Clinical pregnancy rate per transfer (%) | 7 out of 38 (18.42 %) | 4 out of 17 (23.52 %) | 4 out of 16 (25.00 %) | 15 out of 71 (21.12 %) |
Table 3.
Patients characteristics, and stimulation characteristics for normal and abnormal PB characteristics
| FF volume (ml : mean ± SD) | Age (mean ± SD) | Day 3 basal FSH (mean ± SD) | number of oocytes retrieval (mean ± SD) | E2 serum level 36 hours before oocytes retrievel (mean ± SD) | FSH units administered per attempts (mean ± SD) | |
|---|---|---|---|---|---|---|
| Ooytes with an abnormal PB (n = 112) | 4,21 ± 2.46 | 37.02 ± 3.58 | 7.13 ± 2.75 | 8.01 ± 2.94 | 1846.188 ± 875.56 | 2587.05 ± 1687.93 |
| Oocytes with a normal PB (n = 201) | 3.66 ± 1.84 | 36.09 ± 3.83 | 6.75 ± 2.41 | 8.19 ± 3.06 | 2026.20 ± 781.068 | 2384.20 ± 1352.69 |
| p value | *p = 0.027 | *p = 0.036 | p = 0.20 | p = 0.61 | p = 0.06 | p = 0.24 |
Table 4.
Follicular fluid levels (LH, E2, P4, AMH, Testosterone and total protein) for normal and abnormal 1st PBs in the three subgroups
| LH (FF) mean ± SD | E2×1000 (FF) mean ± SD | P4×1000 (FF) mean ± SD | AMH (FF) mean ± SD | Testostérone (FF) mean ± SD | Total protein (FF) mean ± SD | |
|---|---|---|---|---|---|---|
| oocytes with an abnormal PB (n = 112) | undetectable | 839.50 ± 523.06 | 19.34 ± 8.81 | 1.53 ± 1.81 | 5.15 ± 1.94 | 52.41 ± 11.10 |
| oocytes with a normal PB (n = 201) | undetectable | 788.61 ± 462.64 | 18.12 ± 8.53 | 1.74 ± 2.13 | 5.14 ± 1.65 | 52.58 ± 12.34 |
| p value | p = 0.37 | p = 0.23 | p = 0.38 | p = 0.95 | p = 0.90 |
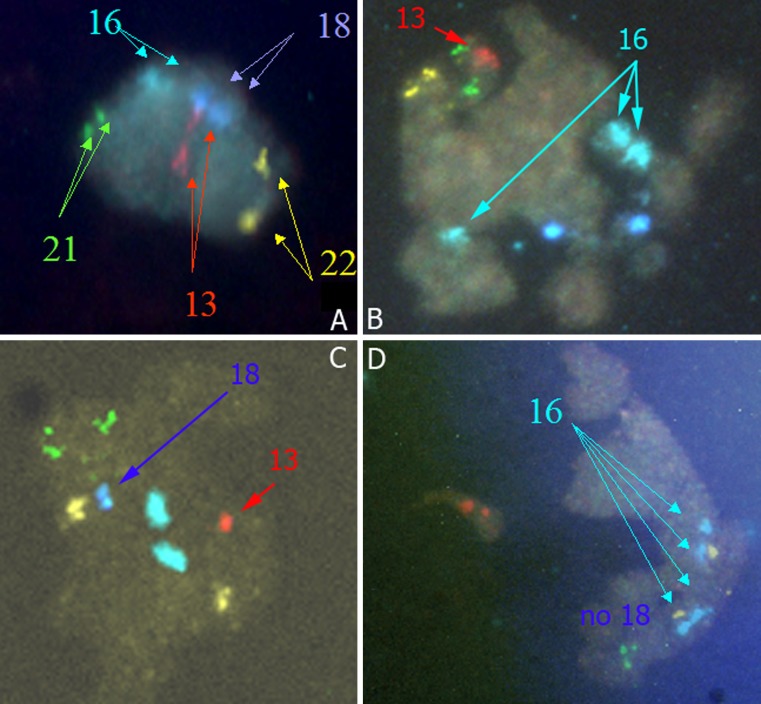
Fig. 1.
Different types of FISH results in a polar body: A- Normal polar body (PB) (2 spots for each chromosome 13, 16, 18, 21, 22). B- Premature separation of sister chromatids (PSSC) with 3 spots for chromosome 16 (1 chromosome 16 and 1 super numerary chromatid 16). C- Premature separation of sister chromatids (PSSC) of chromosomes 13 and 18 with only 1 spot (only 1 chromatid) for chromosomes 13 and 18. D- Non-disjunction (ND) of chromosome 16 with 4 spots for chromosome 16 (2 chromosomes 16)
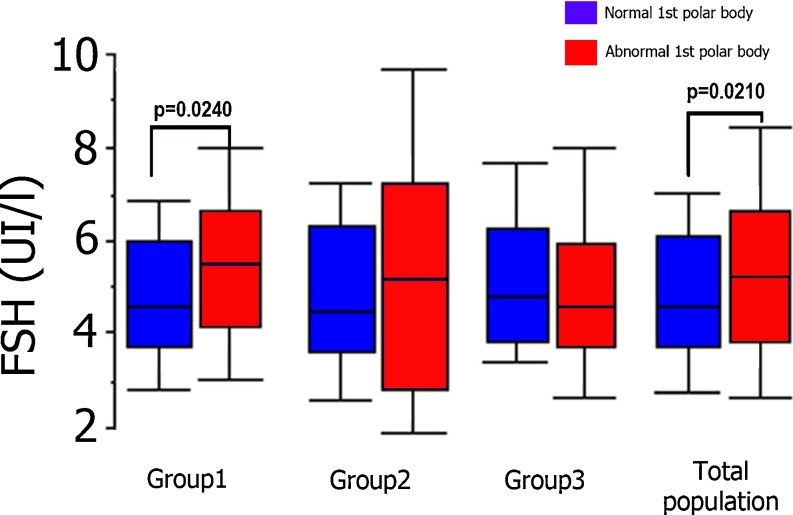
Fig. 2.
Follicular fluid FSH levels for normal and abnormal 1st PBs in the three subgroups and for the study population as a whole (Group 1: Advanced maternal age; Group 2: Implantation failure; Group 3: Implantation failure + advanced maternal age)
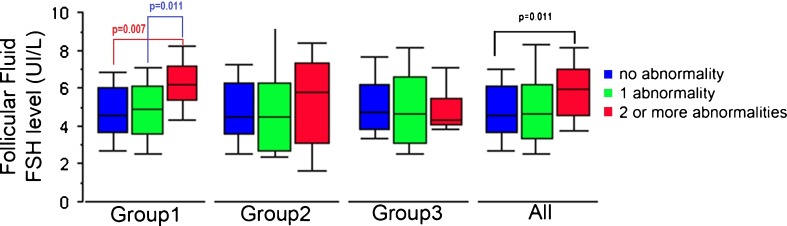
Fig. 3.
Number of chromosomal abnormalities in the PB (n = 313) and follicular fluid FSH levels in the three study subgroups and the study population as a whole (Group 1: Advanced maternal age; Group 2: Implantation failure; Group 3: Implantation failure + advanced maternal age)
Results
The FF properties (haemorrhagic, haematic, orange or yellow) were noted. In an initial step, we evaluated the degree of blood contamination in a series of FF samples as a function of their colour and the red blood cell count. Ten samples (3 %) were excluded because the level blood contamination was greater than 3 % (corresponding to a haemorrhagic aspect).
Patient’s characteristics and ovarian stimulation data
In Table (1) the patient’s characteristics and the ovarian stimulation data of the 3 groups are shown. Obviously, the ages are different, between group 1 (aged women) and 2 (implantation failure) (mean ± SD: 38 years and 11 months ±1 year and 11 months vs 32 years and 1 months ±2 year and 7 months; p < 0.0001) and between group 2 and 3 (mean ± SD: 32 years and 1 months ±2 year and 7 months vs 38 years and 3 months ±1 year and 7 months; p < 0.0001) but not different between group 1 and 3. The basal day 3 FSH in group 3 is higher than in group 1 and 2 (p = 0.003). The quantity of FSH administrated was lower in group 1 than in group 2 and 3 (p = 0.016). E2 levels 36 h before oocytes retrieval were also lower in group 1 and 3(implantation failure + advanced maternal age) than in group 2 but FSH levels in follicular fluid (FF) were the same in the 3 groups as well as FF volumes (5.03 ± 1.91 vs 5.25 ± 2.78 vs 5.12 ± 2.02 respectively).
ICSI and FISH results
Of the 313 oocytes diagnosed, 35.78 % (112 out of 313) were found to be abnormal in tests for chromosomes 13, 16, 18, 21 and 22. A total of 201 normal oocytes were injected and 179 were injected without a prior diagnosis (technical failure: n = 27; slightly immature, with a cytoplasmic bridge: n = 41; completely immature with no PB at the time of the biopsy and then matured at the time of the ICSI procedure: n = 111). Of the 27 technical failures, 21 PBs were lost because the cytoplasmic membrane burst during extraction and 6 were not interpretable because of a hybridization anomaly.
Groups 1, 2 and 3 did not differ significantly in terms of the first PB abnormality rates (35.25 % (55 out of 156), 31.68 % (32 out of 101) and 44.64 % (25 out of 56), respectively). The overall fertilization rate was 72.14 % (145 out of 201), with a cleavage rate of 91.72 % (133 out of 145) and a per-transfer clinical pregnancy rate of 21.12 % (15 out of 71) (Table 2).
The mechanism of the observed aneuploidies was evaluated according to the indication for PCS. Figure 1 shows different types of FISH results (normal, PCS, ND) in a polar body. In the “advanced maternal age” group (group 1), PSSC affected 74.5 % of the abnormal samples (41 out of 55) and ND affected the remaining 25.5 % (14 out of 55). In the “implantation failure” group (group 2), PSSC affected 62.5 % of the abnormal samples (20 out of 32) and ND affected 37.5 % (12 out of 32). Lastly, in group 3 (“implantation failure + advanced maternal age”), PSSC affected 56 % of the abnormal samples (14 out of 25) and ND affected 44 % (11 out of 25).
Follicular fluid and FISH results
For the study population as a whole, we found a significant difference between follicular FSH levels in normal oocytes (n = 201) and those in abnormal oocytes (n = 112) i.e. 4.85 ± 1.75 IU/L vs. 5.41 ± 2.47 IU/L, respectively; p = 0.021. In within-group analyses, this difference was only statistically significant for group 1, the group of aged woman without implantation failure and usually (80 %) with severe male infertility (4.79 ± 1.68 for 101 normal oocytes vs. 5.45 ± 1.81 IU/L for 55 abnormal oocytes; p = 0.024) (Fig. 2).
For the population as a whole, we found that a greater number of chromosomal abnormalities per oocyte (increasing from 0 to 3) was associated with a higher follicular FSH level. This trend became statistically significant for the comparison of oocytes with 0 (n = 201) and with 2 (n = 26) abnormalities per oocyte (4.85 ± 1.75 IU/L vs. 5.71 ± 1.96 IU/L, respectively; p = 0.037) in the total population. Within-group analysis primarily confirmed this elevation in group 1, where there was a significant difference in follicular FSH levels when comparing oocytes with 0 (n = 101) and 2 (n = 13) abnormalities (4.79 ± 1.68 IU/L vs. 6.01 ± 1.83 IU/L, respectively; p = 0.016). Though the number of oocytes with 3 anomalies was low (n = 7) in group 1 there was also an increased FSH level comparing oocytes with 0 (n = 101) and 3 (n = 7) abnormalities (4.97 ± 1.68 IU/L vs. 6.64 ± 2.10 IU/L, respectively; p = 0.0059) as well as comparing oocytes with 1 (n = 35) and 3 (n = 7) abnormalities (5.0 ± 1.72 IU/L vs. 6.64 ± 2.10 IU/L, respectively; p = 0.021). Due to the low number of oocytes with 3 abnormalities we grouped all the oocytes with 2 or 3 abnormalities per oocyte. This analysis presented in Fig. 2 confirmed that a greater number abnormalities per oocyte (increasing from 0 to 2 or 3) was associated with higher follicular FSH levels (Fig. 3).
Patient’s characteristics, ovarian stimulation and FISH results
As shown in Table 3 for the study as a whole we found no difference between normal and abnormal oocytes dealing with patient’s characteristics (basal FSH and indication of PCS) and dealing with stimulation characteristics (number of FSH units administrated and E2 levels 36 h before retrieval and number of oocytes). The ages of the patients were different between the normal oocytes and abnormal oocytes (p = 0.036). The follicular fluid volume was lower for the normal oocytes than the abnormal oocytes (p = 0.027)
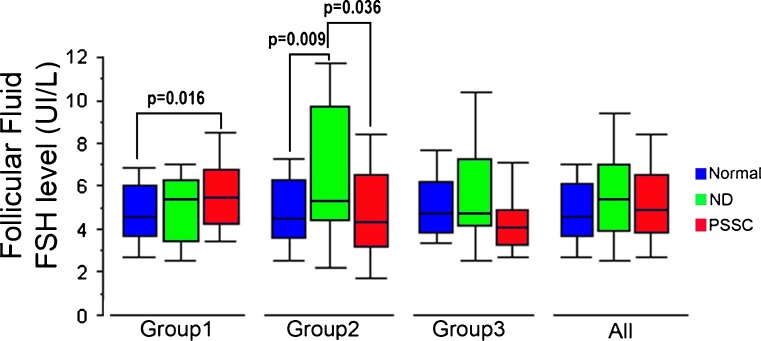
In the within-group analysis, the elevation of FSH levels in abnormal oocytes resulting from PSSC was only found in group 1 (in which the PSSC rate was highest, at 74.5 %), with 5.57 ± 1.89 for the 41 PSSC oocytes vs. 4.79 ± 1.68 for the 101 normal oocytes, respectively (p = 0.016). In group 2, follicular FSH levels for the 12 abnormal oocytes with ND (6.93 ± 4.57 IU/L) were found to be greater than those for the 69 normal oocytes (4.76 ± 1.86 IU/L; p = 0.009) or for the 20 oocytes with PSSC (4.96 ± 2.34 IU/L; p = 0.036). In group 3, the FSH level did not appear to depend on the aetiology of the abnormality (Fig. 4).
Fig. 4.
Follicular fluid FSH levels and the mechanisms of aneuploidy (PSSC and ND) in the 3 study subgroups and the study population as a whole (Group 1: Advanced maternal age; Group 2: Implantation failure; Group 3: Implantation failure + advanced maternal age)
We did not find any other significant difference between normal and abnormal oocytes in terms of the other FF assays (E2, P4, AMH, Testosterone and total protein)(Table 4). LH was undetectable (<0.2 IU/l) in follicular fluid
Discussion
The objective of the present study was to investigate the relationship between first PB aneuploidies and the levels of various molecules in the FF (i.e. the oocyte’s microenvironment) by studying groups of women at a high risk of chromosomal abnormalities. Older patients [5, 15, 22, 25, 44–46, 69] and patients with implantation failure [7, 13, 14, 16, 70, 71] tend to have particularly high oocyte aneuploidy rates. Indeed, these two criteria are recognised as indications for pre-implantation genetic screening (PGS) for aneuploidy in many countries (but not in France, where PGD is restricted to couples in which one of the partners has a known genetic anomaly). Hence, in France, these patients undergo PCS after biopsy of the first PB. According to the French regulations, PCS must be performed on a gamete and must take place before ICSI, thus allowing only the first PB to be diagnosed.
One disadvantage of this strategy is that some oocytes cannot be screened. The main reasons are immaturity (i.e. the presence of a cytoplasmic bridge at the time of PB removal) and late maturation (between the biopsy time and ICSI time). In the present study, these factors concerned 152 oocytes (41 with a bridge and 111 without a PB at the time of the biopsy). There were also few technical failures (loss of PB and/or membrane rupture: n = 21; hybridization defects: n = 6).
These factors limit the benefit provided by this technique in terms of an increased likelihood of pregnancy. Although the safety issue must also be considered, the literature data [9, 38] and our own results are reassuring in this respect [20]. In the present study, we obtained 15 pregnancies from 71 attempts with fresh embryo transfer, i.e. a pregnancy rate of 21.12 % per transfer. This value is similar to those obtained with PGS in the same indications [37, 53, 60]. Our pregnancy rate may appear to be rather low but it must be borne in mind that our patients had a poor overall IVF prognosis.
Several randomized studies of PGS for aneuploidy screening have reported that older (over-38) patients had much the same pregnancy rate as non-screened patients [8, 22, 55, 56]. This finding may have been due to (a) technical difficulties related to mosaicism in embryos and FISH errors, (b) the potential toxicity of removal of the two blastomers (which does not seem to be the case for the PB) and/or (c) genetic normalization after diagnosis (resulting in normal blastocysts and foetuses) [6]
Polar body analysis appears to be a good strategy for overcoming these problems. Analysing the full set of chromosomes (and thus more efficient “sorting”) is possible [21, 33, 58, 73, 74] but is not technically feasible in oocytes in the few hours between follicle puncture and ICSI. Polar body array CGH was found to predict zygote ploidy with acceptable accuracy [15, 18]. A critical step seems to be the timing of the biopsy [33] and the accurate, morphological identification of the first and second polar bodies. In a recent study, the morphology of simultaneously biopsied polar bodies (though used in most studies) was not found to be predictive of the cell division origin [59]. This could explain some of the disparities in the literature concerning the origin of meiotic abnormalities. Using our five- chromosome FISH technique, the first PB abnormality rates were 35.78 % in our overall population, 35.25 % in group 1 (advanced maternal age), 31.7 % in group 2 (implantation failure) and 44.6 % in group 3 (advanced maternal age and implantation failure). These values are similar to those obtained in over-35 or over-38 patients in various series studying the first PB [65], 1998 and [30, 33, 39, 66, 69] or in patients having undergone PGS for other indications [53, 70]. Our results confirm that chromosomal abnormalities have a significant impact on implantation failure, with a frequency of 31.7 % (for the five chromosomes tested here) in a group of women with a mean age of 32 years and 1 month.
Nevertheless, the aetiology of the chromosomal abnormality appeared to differ according to the patient group - particularly if one compares patients with implantation failure with those included in the programme because of advanced maternal age only. In our series, PSSC caused 74.5 % of chromosomal abnormalities in the older patients. This finding agrees with previous reports of PSSC in 71 % of non-fertilized oocytes [43], 70 % of fertilized oocytes in women aged 35 or over [66] and 70 % of mature oocytes in women aged 38 or over [69].
In contrast, the two aetiologies had more similar frequencies in group 2 (PSSC: 62.5 %; ND: 37.5 %). These results are also similar to those reported by Pellestor [43] for under-35 patients (PSSC: 47 %; ND: 53 %) and in our previous work [70].
In the present study, we probed the relationship between oocyte aneuploidy, FSH levels in the FF and levels of AMH and the other hormones controlling ovarian function (i.e. LH, E2, P4, testosterone and inhibin B).
In order to determine hormone concentrations in the FF, we prepared dilution ranges. Total protein, FSH, LH and AMH could be assayed on pure samples, thus illustrating the need to have samples with as little blood contamination as possible. In contrast, the oestradiol and progesterone assays required prior dilution of the FF (by factors of 1:2000 and 1:1000, respectively). The sample’s appearance (ranging from yellow to haemorrhagic) prompted us to consider the problem of blood contamination and its potential impact on the hormone assays. In order to assess the degree of contamination, we evaluated the red blood cell count for each of the different aspects of FF. It turned out that contamination was almost non-existent for yellow or orange samples (with blood dilutions of 1:400 and 1:100, respectively), moderate for haematic samples and high for haemorrhagic samples. It thus makes sense to exclude haemorrhagic samples, which accounted for only 3 % of the samples.
Our results confirm the link between the age of the patient and the risk of oocyte aneuploïdy but on the other hand no predictive value of day 3 basal FSH of the patient was found (7.13 ± 2.75 vs 6.75 ± 2.41 for respectively abnormal and normal oocytes). The number of oocytes retrieved was the same for normal and abnormal oocytes. It has been suggested that elevated basal levels of endogenous FSH (associated with age and/or ovarian failure) are associated with meiotic errors [29, 42, 64]. However, Massie [35] reported that basal FSH was not predictive of fetal aneuploidy reducing the importance of endogenous FSH as a prediction of oocyte aneuploidy. Our results agree with the hypothesis that basal FSH is not predictive factor of oocyte aneuploidy. Moreover, our results suggest a link between exogenous FSH (which is required for controlled ovarian hyperstimulation before ICSI) and oocyte aneuploidy. We found significantly higher FSH levels in FF from oocytes with aneuploid PBs than in normal oocytes. We also found that the greater the number of chromosomal abnormalities per oocyte, the higher the follicular FSH level. It is clear that FSH plays a crucial role in normal follicular development. An increased volume of follicular fluid which is an indicator of the size of the follicle was also associated to an increased risk of aneuploïdy (p = 0.027) (Table 3). The hormone is used at supraphysiological high doses during ovarian hyperstimulation prior to IVF and ICSI and some authors have proposed that the high doses of gonadotropins used in ART cause oocyte aneuploidies [4, 12, 32, 40] but others have came to the opposite conclusion [10, 36, 68]. Indeed aneuploidy after ovarian controlled hyperstimulation seems to have specific characteristics. In natural conceptions aneuploidies occur mainly during the first meiosis division and in IVF over half of aneuploidies in oocytes from aged women result from second meiosis division according to [21]. The supraphysiological follicular FSH levels found in our study suggest that we were measuring exogenous hormone that was related to the quantity of FSH administered and this exogenous FSH might induce more aneuploidies than endogenous FSH.
Elevated FSH levels in FF were found associated with aneuploidy in oocyte. This could be explained if FSH either promotes the generation of abnormalities or hinder the elimination of abnormal oocytes (by blocking apoptosis, for example). Indeed, another aspect of this issue relates to the fact that FSH blocks the selection of the dominant follicle, has an anti-apoptotic effect and may thus indirectly block the putative selection of aneuploid oocytes by apoptosis leading several oocytes together to maturation. This potential effect of exogenous FSH may be visible in our group 1 (aged women), in which elevated follicular FSH may be associated with poor chromatid cohesion (i.e. PSSC, the main aetiology in group 1, at 74.5 %). This is also a group with 80 % male infertility suggesting that the women of this group have no fertility problem excepted their age. Moreover, the number of chromosomal abnormalities per PB increased with follicular FSH levels in the total population and especially so in older women - confirming the hypothesis of a harmful effect in this group.
Conversely (and somewhat surprisingly), FSH appeared to be associated with ND in women with implantation failure. In patients with implantation failure, FSH is probably a less important parameter in aneuploidy than other individual genetic or epigenetic risk factors [72]. In summary, we consider that elevated FSH may promote both oocyte abnormalities (PSSC and ND) in particularly fragile cases at risk of aneuploidy.
The other parameters tested here had little or no value as aneuploidy markers. In particular, AMH (which has been presented as a predictive marker of pregnancy [27, 54, 57]) was not significantly associated with chromosomal abnormalities.
We believe that our present results are of value in 3 main respects. First in practical terms, our results argue against excessive ovarian stimulation in ART, with the possible induction of high FSH levels in FF. In aged patients with high plasma FSH levels, it may be prejudicial to perform hyperstimulation; mild stimulation would be more appropriate. It might of course be useful to recommend PGD or prenatal diagnosis. Second measuring FSH in FF could become a non-invasive test of oocyte quality. However, the high variability in the FF from one aneuploid oocyte to another decreases this marker’s predictive value for aneuploidy. And last from a more fundamental point of view, the association of high follicular fluid FSH level and oocyte aneuploïdy suggests a role of FSH either in generating those aneuploïdies or in blocking their selection.
In terms of our understanding of the causes of chromosomal abnormalities, it may be worth focusing on the importance of hormonal changes that regulate or disrupt meiosis and thus favour aneuploidy.
Glossary
- PSSC
Premature separation of sister chromatids
- ND
Non-disjunction
- PB
Polar body
- CGH
Comparative genomic hybridization
- FF
Follicular fluid
- IVF
In vitro fertilization
- ICSI
Intra-cytoplasmic sperm injection
- PGD
Pre-implantation genetic diagnosis
- FISH
Fluorescence in situ hybridization
- PGS
Pre-implantation genetic screening
- PCS
Preconception screening
Footnotes
Capsule The higher follicular fluid FSH levels found in aneuploid oocytes (versus euploid oocytes) suggest that ovarian hyperstimulation has an effect on errors of meiosis.
References
- 1.Assidi M, Montag M, Ven K, Sirard MA. Biomarkers of human oocyte developmental competence expressed in cumulus cells before ICSI: a preliminary study. J Assist Reprod Genet. 2011;28(2):173–188. doi: 10.1007/s10815-010-9491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assou S, Haouzi D, Vos J, Hamamah S. Human cumulus cells as biomarkers for embryo and pregnancy outcomes. Mol Hum Reprod. 2010;16:531–538. doi: 10.1093/molehr/gaq032. [DOI] [PubMed] [Google Scholar]
- 3.Baart EB, Martini E, Berg I, Macklon NS, Galjaard RJ, Fauser BC, Opstal D. Preimplantation genetic screening reveals a high incidence of aneuploidy and mosaicism in embryos from young women undergoing IVF. Hum Reprod. 2006;21:223–233. doi: 10.1093/humrep/dei291. [DOI] [PubMed] [Google Scholar]
- 4.Baart EB, Martini E, Eijkemans MJ, Opstal D, Beckers NG, Verhoeff A, Macklon NS, Fauser BC. Milder ovarian stimulation for in-vitro fertilization reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Hum Reprod. 2007;22:980–988. doi: 10.1093/humrep/del484. [DOI] [PubMed] [Google Scholar]
- 5.Benzacken B, Martin-Pont B, Bergère M, Hugues JN, Wolf JP, Selva J. Chromosome 21 detection in human oocyte fluorescence in situ hybridization: possible effect of maternal age. J Assist Reprod Genet. 1998;15(3):105–110. doi: 10.1023/A:1023056502731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brezina P, Nguyen KHD, Benner AT, Du L, Ross R, Barker A, Anchan RM, Richter K, Kearns WG. Aneuploid blastomeres may undergo a process of genetic normalization resulting in euploid blastocysts. ESHRE. 2011;26(sup 1):i53. [Google Scholar]
- 7.Caglar GS, Asimakopoulos B, Nikolettos N, Diedrich K, Al-Hasani S. Preimplantation genetic diagnosis for aneuploidy screening in repeated implantation failure. Reprod Biomed Online. 2005;10(3):381–388. doi: 10.1016/S1472-6483(10)61800-7. [DOI] [PubMed] [Google Scholar]
- 8.Checa MA, Alonso-Coello P, Solà I, Robles A, Carreras R, Balasch J. IVF/ICSI with or without preimplantation genetic screening for aneuploidy in couples without genetic disorders: a systematic review and meta-analysis. J Assist Reprod Genet. 2009;26(5):273–283. doi: 10.1007/s10815-009-9328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clement-Sengewald A, Buchholz T, Schutze K. Laser microdissection as a new approach to prefertilization genetic diagnosis. Pathobiology. 2000;68:232–236. doi: 10.1159/000055929. [DOI] [PubMed] [Google Scholar]
- 10.Delhanty JD, Penketh RJ. Cytogenetic analysis of unfertilized oocytes retrieved after treatment with the LHRH analogue, buserelin. Hum Reprod. 1990;5(6):699–702. doi: 10.1093/oxfordjournals.humrep.a137171. [DOI] [PubMed] [Google Scholar]
- 11.Ebner T, Sommergruber M, Moser M, Shebl O, Schreier-Lechner E, Tews G. Basal level of anti-Mullerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006;21:2022–2026. doi: 10.1093/humrep/del127. [DOI] [PubMed] [Google Scholar]
- 12.Elbling L, Colot M. Abnormal development and transport and increased sister-chromatid exchange in preimplantation embryos following superovulation in mice. Mutat Res. 1985;147(4):189–195. doi: 10.1016/0165-1161(85)90057-3. [DOI] [PubMed] [Google Scholar]
- 13.Findikli N, Kahraman S, Saglam Y, Beyazyurek C, Sertyel S, Karlikaya G, Karagozoglu H, Aygun B. Embryo aneuploidy screening for repeated implantation failure and unexplained recurrent miscarriage. Reprod Biomed Online. 2006;13(1):38–46. doi: 10.1016/S1472-6483(10)62014-7. [DOI] [PubMed] [Google Scholar]
- 14.Fragouli E, Katz-Jaffe M, Alfarawati S, Stevens J, Colls P, Goodall NN, Tormasi S, Gutierrez-Mateo C, Prates R, Schoolcraft WB, Munne S, Wells D. Comprehensive chromosome screening of polar bodies and blastocysts from couples experiencing repeated implantation failure. Fertil Steril. 2010;94(3):875–887. doi: 10.1016/j.fertnstert.2009.04.053. [DOI] [PubMed] [Google Scholar]
- 15.Fragouli E, Alfarawati S, Goodall NN, Sánchez-García JF, Colls P, Wells D. The cytogenetics of polar bodies: insights into female meiosis and the diagnosis of aneuploidy. Mol Hum Reprod. 2011. [DOI] [PubMed]
- 16.Gianaroli L, Magli MC, Ferraretti AP, Munne S. Preimplantation diagnosis for aneuploidies in patients undergoing in vitro fertilization with a poor prognosis: identification of the categories for which it should be proposed. Fertil Steril. 1999;72:837–844. doi: 10.1016/S0015-0282(99)00377-5. [DOI] [PubMed] [Google Scholar]
- 17.Gebhardt KM, Feil DK, Dunning KR, Lane M, Russell DL. Human cumulus cell gene expression as a biomarker of pregnancy outcome after single embryo transfer. Fertil Steril. 2011;96(1):47–52.e2. doi: 10.1016/j.fertnstert.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 18.Geraedts J, Montag M, Magli MC, Repping S, Handyside A, Staessen C, Harper J, Schmutzler A, Collins J, Goossens V, Ven H, Vesela K, Gianaroli L. Polar body array CGH for prediction of the status of the corresponding oocyte. Part I: clinical results. Hum Reprod. 2011;26(11):3173–3180. doi: 10.1093/humrep/der294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gleicher N, Weghofer A, Barad DH. Dehydroepiandrosterone (DHEA) reduces embryo aneuploidy: direct evidence from preimplantation genetic screening (PGS) Reprod Biol Endocrinol. 2010;8:140–145. doi: 10.1186/1477-7827-8-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammoud I, Molina-Gomes D, Albert M, Bergere M, Bailly M, Wainer R, Selva J, Vialard F. Are zona pellucida laser drilling and polar body biopsy safe for in vitro matured oocytes? J Assist Reprod Genet. 2010;27:423–427. doi: 10.1007/s10815-010-9422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Handyside AH, Montag M, Magli MC, Repping S, Harper J, Schmutzler A, Vesela K, Gianaroli L, Geraedts J. Multiple meiotic errors caused by predivision of chromatids in women of advanced maternal age undergoing in vitro fertilisation. Eur J Hum Genet. 2012. doi:10.1038/ejhg.2011.272 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 22.Hardarson T, Hanson C, Lundin K, Hillensjö T, Nilsson L, Stevic J, Reismer E, Borg K, Wikland M, Bergh C. Preimplantation genetic screening in women of advanced maternal age caused a decrease in clinical pregnancy rate: a randomized controlled trial. Hum Reprod. 2008;23(12):2806–2812. doi: 10.1093/humrep/den217. [DOI] [PubMed] [Google Scholar]
- 23.Hassold T, Hall H, Hunt P. The origin of human aneuploidy: where we have been, where we are going. Hum Mol Genet. 2007;16(2):R203–R208. doi: 10.1093/hmg/ddm243. [DOI] [PubMed] [Google Scholar]
- 24.Hassold T, Hunt P. Maternal age and chromosomally abnormal pregnancies: what we know and what we wish we knew. Curr Opin Pediatr. 2009;21(6):703–708. doi: 10.1097/MOP.0b013e328332c6ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heffner LJ. Advanced maternal age–how old is too old? N Engl J Med. 2004;4:1927–1929. doi: 10.1056/NEJMp048087. [DOI] [PubMed] [Google Scholar]
- 26.Hodges CA, Ilagan A, Jennings D, Keri R, Nilson J, Hunt PA. Experimental evidence that changes in oocyte growth influence meiotic chromosome segregation. Hum Reprod. 2002;17:1171–1180. doi: 10.1093/humrep/17.5.1171. [DOI] [PubMed] [Google Scholar]
- 27.Irez T, Ocal P, Guralp O, Cetin M, Aydogan B, Sahmay S. Different serum anti-Müllerian hormone concentrations are associated with oocyte quality, embryo development parameters and IVF-ICSI outcomes. Arch Gynecol Obstet. 2011;284(5):1295–1301. doi: 10.1007/s00404-011-1979-6. [DOI] [PubMed] [Google Scholar]
- 28.Kan A, Ng EH, Yeung WS, Ho PC. Perifollicular vascularity in poor ovarian responders during IVF. Hum Reprod. 2006;21:1539–1544. doi: 10.1093/humrep/del021. [DOI] [PubMed] [Google Scholar]
- 29.Kline JK, Kinney AM, Levin B, Kelly AC, Ferin M, Warburton D. Trisomic pregnancy and elevated FSH: implications for the oocyte pool hypothesis. Hum Reprod. 2011;26(6):1537–1550. doi: 10.1093/humrep/der091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuliev A, Cieslak J, Ilkevitch Y, Verlinsky Y. Chromosomal abnormalities in a series of 6,733 human oocytes in preimplantation diagnosis for age-related aneuploidies. Reprod Biomed Online. 2003;6:54–59. doi: 10.1016/S1472-6483(10)62055-X. [DOI] [PubMed] [Google Scholar]
- 31.Leridon H. Human fertility: the basics components. USA: Chicago University Press; 1977. [Google Scholar]
- 32.Magli MC, Ferraretti AP, Crippa A, Lappi M, Feliciani E, Gianaroli L. First meiosis errors in immature oocytes generated by stimulated cycles. Fertil Steril. 2006;86(3):629–635. doi: 10.1016/j.fertnstert.2006.02.083. [DOI] [PubMed] [Google Scholar]
- 33.Magli MC, Montag M, Köster M, Muzi L, Geraedts J, Collins J, Goossens V, Handyside AH, Harper J, Repping S, Schmutzler A, Vesela K, Gianaroli L. Polar body array CGH for prediction of the status of the corresponding oocyte. Part II: technical aspects. Hum Reprod. 2011;26(11):3181–3185. doi: 10.1093/humrep/der295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majumder K, Gelbaya TA, Laing I, Nardo LG. The use of anti-Müllerian hormone and antral follicle count to predict the potential of oocytes and embryos. Eur J Obstet Gynecol Reprod Biol. 2010;150:166–170. doi: 10.1016/j.ejogrb.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 35.Massie JA, Burney RO, Milki AA, Westphal LM, Lathi RB. Basal follicle-stimulating hormone as a predictor of fetal aneuploidy. Fertil Steril. 2008;90(6):2351–2355. doi: 10.1016/j.fertnstert.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 36.Massie JA, Shahine LK, Milki AA, Westphal LM, Lathi RB. Ovarian stimulation and the risk of aneuploid conceptions. Fertil Steril. 2011;95(3):970–972. doi: 10.1016/j.fertnstert.2010.07.1088. [DOI] [PubMed] [Google Scholar]
- 37.Mastenbroek S, Twisk M, Veen F, Repping S. Preimplantation genetic screening: a systematic review and meta-analysis of RCTs. Hum Reprod Update. 2011;17(4):454–466. doi: 10.1093/humupd/dmr003. [DOI] [PubMed] [Google Scholar]
- 38.Montag M, Ven K, Delacretaz G, Rink K, Ven H. Laser-assisted microdissection of the zona pellucida facilitates polar body biopsy. Fertil Steril. 1998;69:539–542. doi: 10.1016/S0015-0282(97)00538-4. [DOI] [PubMed] [Google Scholar]
- 39.Montag M, Ven K, Dorn C, Ven H. Outcome of laser-assisted polar body biopsy and aneuploidy testing. Reprod Biomed Online. 2004;9:425–429. doi: 10.1016/S1472-6483(10)61278-3. [DOI] [PubMed] [Google Scholar]
- 40.Munne S, Magli C, Adler A, Wright G, Boer K, Mortimer D, Tucker M, Cohen J, Gianaroli L. Treatment-related chromosome abnormalities in human embryos. Hum Reprod. 1997;12(4):780–784. doi: 10.1093/humrep/12.4.780. [DOI] [PubMed] [Google Scholar]
- 41.Munné S, Sepulveda S, Balmaceda J, Fernandez E, Fabres C, Mackenna A, Lopez T, Crosby JA, Zegers-Hochschild F. Selection of the most common chromosome abnormalities in oocytes prior to ICSI. Prenat Diagn. 2000;20(7):582–586. doi: 10.1002/1097-0223(200007)20:7<582::AID-PD872>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 42.Nasseri A, Mukherjee T, Grifo JA, Noyes N, Krey L, Copperman AB. Elevated day 3 serum follicle stimulating hormone and/or estradiol may predict fetal aneuploidy. Fertil Steril. 1999;71:715–718. doi: 10.1016/S0015-0282(98)00525-1. [DOI] [PubMed] [Google Scholar]
- 43.Pellestor F, Andreo B, Arnal F, Humeau C, Demaille J. Mechanisms of non-disjunction in human female meiosis: the co-existence of two modes of malsegregation evidenced by the karyotyping of 1397 in-vitro unfertilized oocytes. Hum Reprod. 2002;17:2134–2145. doi: 10.1093/humrep/17.8.2134. [DOI] [PubMed] [Google Scholar]
- 44.Pellestor F, Andréo B, Arnal F, Humeau C, Demaille J. Maternal aging and chromosomal abnormalities: new data drawn from in vitro unfertilized human oocytes. Hum Genet. 2003;112(2):195–203. doi: 10.1007/s00439-002-0852-x. [DOI] [PubMed] [Google Scholar]
- 45.Pellestor F. Maternal age and chromosomal abnormalities in human oocytes. Med Sci (Paris) 2004;20(6–7):691–696. doi: 10.1051/medsci/2004206-7691. [DOI] [PubMed] [Google Scholar]
- 46.Pellestor F, Andréo B, Anahory T, Déchaud H, Hédon B, Hamamah S. The cytogenetics of human oocytes: 40 years of progress. Gynecol Obstet Fertil. 2005;33(5):283–292. doi: 10.1016/j.gyobfe.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Penarrubia J, Fabregues F, Manau D, Creus M, Casals G, Casamitjana R, et al. Basal and stimulation day 5 anti-Mullerian hormone serum concentrations as predictors of ovarian response and pregnancy in assisted reproductive technology cycles stimulated with gonadotropin-releasing hormone agonist—gonadotropin treatmen. Hum Reprod. 2005;20:915–922. doi: 10.1093/humrep/deh718. [DOI] [PubMed] [Google Scholar]
- 48.Plachot M. Chromosomal abnormalities in oocytes. Mol Cell Endocrinol. 2001;183:59–63. doi: 10.1016/S0303-7207(01)00577-9. [DOI] [PubMed] [Google Scholar]
- 49.Roberts R, Iatropoulou A, Ciantar D, Stark J, Becker DL, Franks S, et al. Follicle-stimulating hormone affects metaphase I chromosome alignment and increases aneuploidy in mouse oocytes matured in vitro. Biol Reprod. 2005;72:107–118. doi: 10.1095/biolreprod.104.032003. [DOI] [PubMed] [Google Scholar]
- 50.Rossi G, Macchiarelli G, Palmerini MG, Canipari R, Cecconi S. Meiotic spindle configuration is differentially influenced by FSH and epidermal growth factor during in vitro maturation of mouse oocytes. Hum Reprod. 2006;21:1765–1770. doi: 10.1093/humrep/del074. [DOI] [PubMed] [Google Scholar]
- 51.Selesniemi K, Lee HJ, Muhlhauser A, Tilly JL. From the cover: prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc Natl Acad Sci USA. 2011;108:12319–12324. doi: 10.1073/pnas.1018793108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Selva J, Bergere M, Molina Gomes D, Hammoud I, Lombroso R, Vialard F. Contribution de l’analyse du premier globule polaire à la compréhension du mécanisme des aneuploïdies humaines. Bull Acad Natle Méd. 2005;189:1761–1772. [PubMed] [Google Scholar]
- 53.Sermon K, Moutou C, Harper J, Geraedts J, Scriven P, Wilton L, et al. ESHRE PGD Consortium data collection IV: May–December 2001. Hum Reprod. 2005;20:19–34. doi: 10.1093/humrep/deh552. [DOI] [PubMed] [Google Scholar]
- 54.Silberstein T, MacLaughlin DT, Shai I, Trimarchi JR, Lambert-Messerlian G, Seifer DB, et al. Mullerian inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod. 2006;21:159–163. doi: 10.1093/humrep/dei270. [DOI] [PubMed] [Google Scholar]
- 55.Staessen C, Platteau P, Assche E, Michiels A, Tournaye H, Camus M, et al. Comparison of blastocyst transfer with or without preimplantation genetic diagnosis for aneuploidy screening in couples with advanced maternal age: a prospective randomized controlled trial. Hum Reprod. 2004;19:2849–2858. doi: 10.1093/humrep/deh536. [DOI] [PubMed] [Google Scholar]
- 56.Staessen C, Verpoest W, Donoso P, Haentjens P, Elst J, Liebaers I, Devroey P. Preimplantation genetic screening does not improve delivery rate in women under the age of 36 following single-embryo transfer. Hum Reprod. 2008;23(12):2818–2825. doi: 10.1093/humrep/den367. [DOI] [PubMed] [Google Scholar]
- 57.Takahashi C, Fujito A, Kazuka M, Sugiyama R, Ito H, Isaka K. Anti-Müllerian hormone substance from follicular fluid is positively associated with success in oocyte fertilization during in vitro fertilization. Fertil Steril. 2008;89(3):586–591. doi: 10.1016/j.fertnstert.2007.03.080. [DOI] [PubMed] [Google Scholar]
- 58.Treff NR, Su J, Kasabwala N, Tao X, Miller KA, Scott RT., Jr Robust embryo identification using first polar body single nucleotide polymorphism microarray-based DNA fingerprinting. Fertil Steril. 2010;93(7):2453–2455. doi: 10.1016/j.fertnstert.2009.08.070. [DOI] [PubMed] [Google Scholar]
- 59.Treff NR, Scott RT Jr, Su J, Campos J, Stevens J, Schoolcraft W, Katz-Jaffe M. Polar body morphology is not predictive of its cell division origin. J Assist Reprod Genet. 2011 Dec 6. [DOI] [PMC free article] [PubMed]
- 60.Twisk M, Mastenbroek S, Wely M, Heineman MJ, Veen F, Repping S. Preimplantation genetic screening for abnormal number of chromosomes (aneuploidies) in in vitro fertilisation or intracytoplasmic sperm injection. Cochrane Database Syst Rev. 2006;25(1):CD005291. doi: 10.1002/14651858.CD005291.pub2. [DOI] [PubMed] [Google Scholar]
- 61.Blerkom J, Davis P. Differential effects of repeated ovarian stimulation on cytoplasmic and spindle organization in metaphase II mouse oocytes matured in vivo and in vitro. Hum Reprod. 2001;16:757–764. doi: 10.1093/humrep/16.4.757. [DOI] [PubMed] [Google Scholar]
- 62.Blerkom J. The influence of intrinsic and extrinsic factors on the developmental potential and chromosomal normality of the human oocyte. J Soc Gynecol Investig. 1996;3:3–11. doi: 10.1016/1071-5576(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 63.Blerkom J, Antczak M, Schrader R. The developmental potential of the human oocyte is related to the dissolved oxygen content of follicular fluid: association with vascular endothelial growth factor levels and perifollicular blood flow characteristics. Hum Reprod. 1997;12:1047–1055. doi: 10.1093/humrep/12.5.1047. [DOI] [PubMed] [Google Scholar]
- 64.Montfrans JM, Hooff MH, Martens F, Lambalk CB. Basal FSH, estradiol and inhibin B concentrations in women with a previous Down’s syndrome affected pregnancy. Hum Reprod. 2002;17(1):44–47. doi: 10.1093/humrep/17.1.44. [DOI] [PubMed] [Google Scholar]
- 65.Verlinsky Y, Cieslak J, Ivakhnenko V, Lifchez A, Strom C, Kuliev A. Birth of healthy children after preimplantation diagnosis of common aneuploidies by polar body fluorescent in situ hybridization analysis. Preimplantation Genetics Group. Fertil Steril. 1996;66(1):126–129. doi: 10.1016/s0015-0282(16)58399-x. [DOI] [PubMed] [Google Scholar]
- 66.Verlinsky Y, Cieslak J, Ivakhnenko V, Evsikov S, Wolf G, White M, et al. Prevention of age-related aneuploidies by polar body testing of oocytes. J Assist Reprod Genet. 1999;16:165–169. doi: 10.1023/A:1020304621338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verlinsky Y, Cohen J, Munne S, Gianaroli L, Simpson JL, Ferraretti AP. Over a decade of experience with preimplantation genetic diagnosis: a multicenter report. Fertil Steril. 2004;82:292–294. doi: 10.1016/j.fertnstert.2003.09.082. [DOI] [PubMed] [Google Scholar]
- 68.Verpoest W, Fauser BC, Papanikolaou E, Staessen C, Landuyt L, Donoso P, Tournaye H, Liebaers I, Devroey P. Chromosomal aneuploidy in embryos conceived with unstimulated cycle IVF. Hum Reprod. 2008;23(10):2369–2371. doi: 10.1093/humrep/den269. [DOI] [PubMed] [Google Scholar]
- 69.Vialard F, Petit C, Bergere M, Molina Gomes D, Petit-Martel V, Lombroso R, et al. Evidence of a high proportion of unbalanced premature sister chromatid separation in the first polar bodies of women of advanced age. Hum Reprod. 2006;21:1172–1178. doi: 10.1093/humrep/dei484. [DOI] [PubMed] [Google Scholar]
- 70.Vialard F, Lombroso R, Bergere M, Gomes DM, Hammoud I, Bailly M, Selva J. Oocyte aneuploidy mechanisms are different in two situations of increased chromosomal risk: older patients and patients with recurrent implantation failure after in vitro fertilization. Fertil Steril. 2007;87(6):1333–1339. doi: 10.1016/j.fertnstert.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 71.Vialard F, Hammoud I, Molina-Gomes D, Wainer R, Bergere M, Albert M, Bailly M, Mazancourt P, Selva J. Gamete cytogenetic study in couples with implantation failure: aneuploidy rate is increased in both couple members. J Assist Reprod Genet. 2008;25(11–12):539–545. doi: 10.1007/s10815-008-9258-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vialard F, Boitrelle F, Molina-Gomes D, Selva J. Predisposition to aneuploidy in the oocyte. Cytogenet Genome Res. 2011;133(2–4):127–135. doi: 10.1159/000324231. [DOI] [PubMed] [Google Scholar]
- 73.Voullaire L, Slater H, Williamson R, Wilton L. Chromosome analysis of blastomeres from human embryos by using comparative genomic hybridization. Hum Genet. 2000;106:210–217. doi: 10.1007/s004390051030. [DOI] [PubMed] [Google Scholar]
- 74.Wells D, Delhanty JD. Comprehensive chromosomal analysis of human preimplantation embryos using whole genome amplification and single cell comparative genomic hybridization. Mol Hum Reprod. 2000;6:1055–1062. doi: 10.1093/molehr/6.11.1055. [DOI] [PubMed] [Google Scholar]
- 75.Xu YW, Peng YT, Wang B, Zeng YH, Zhuang GL. Zhou CQ High follicle-stimulating hormone increases aneuploidy in human oocytes matured in vitro. Fertil Steril. 2011;95:99–104. doi: 10.1016/j.fertnstert.2010.04.037. [DOI] [PubMed] [Google Scholar]
- 76.Zenzes MT, Wang P, Casper RF. Cigarette smoking may affect meiotic maturation of human oocytes. Hum Reprod. 1995;10:3213–3217. doi: 10.1093/oxfordjournals.humrep.a135891. [DOI] [PubMed] [Google Scholar]