Abstract
Leaves of two barley (Hordeum vulgare L.) isolines, Alg-R, which has the dominant Mla1 allele conferring hypersensitive race-specific resistance to avirulent races of Blumeria graminis, and Alg-S, which has the recessive mla1 allele for susceptibility to attack, were inoculated with B. graminis f. sp. hordei. Total leaf and apoplastic antioxidants were measured 24 h after inoculation when maximum numbers of attacked cells showed hypersensitive death in Alg-R. Cytoplasmic contamination of the apoplastic extracts, judged by the marker enzyme glucose-6-phosphate dehydrogenase, was very low (less than 2%) even in inoculated plants. Dehydroascorbate, glutathione, superoxide dismutase, catalase, ascorbate peroxidase, glutathione reductase, monodehydroascorbate reductase, and dehydroascorbate reductase were present in the apoplast. Inoculation had no effect on the total foliar ascorbate pool size or the redox state. The glutathione content of Alg-S leaves and apoplast decreased, whereas that of Alg-R leaves and apoplast increased after pathogen attack, but the redox state was unchanged in both cases. Large increases in foliar catalase activity were observed in Alg-S but not in Alg-R leaves. Pathogen-induced increases in the apoplastic antioxidant enzyme activities were observed. We conclude that sustained oxidation does not occur and that differential strategies of antioxidant response in Alg-S and Alg-R may contribute to pathogen sensitivity.
The mechanisms by which plant cells sense the presence of foreign organisms and transduce this information to the nucleus to elicit an appropriate response are largely unresolved. Appropriate contacts with useful organisms, such as mycorrhizal fungi or N2-fixing bacteria, must evoke a rapid response to establish liaisons that facilitate mutual benefit. In contrast, identification of a harmful pathogen must cause adaptive responses that prevent the spread and limit the sustainability of the invasive agent. In recent years it has become clear that redox signals are integral to the transduction sequences by which changes in the environment modify metabolism and gene transcription.
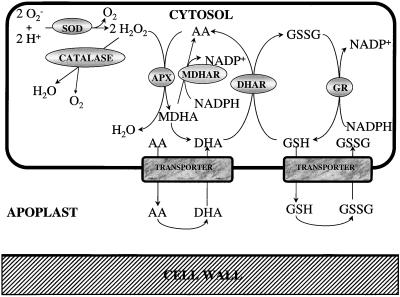
The plasmalemma of plant cells produces bursts of H2O2 in response to both biotic and abiotic stimuli (Doke et al., 1994; Doke, 1997; Wojtaszek, 1997). The mechanisms involved in the regulation of the initiation, intensity, and duration of these bursts are largely unknown but it is clear that plants, like animals, use active oxygen species such as H2O2 to perturb the redox state of cells and allow controlled oxidation that may have an immediate antimicrobial effect (Segal and Abo, 1993; Groom et al., 1996; Lamb and Dixon, 1997). The damage caused to the metabolism and delicate fabric of the plant cells per se depends on the efficiency of the endogenous antioxidant defense system (Fig. 1). Although plant cells contain glutathione peroxidases similar to those present in animal cells, these enzymes in plants appear to catalyze the glutathione-dependent destruction of lipid peroxides rather than H2O2 (Eshdat et al., 1997; Roxas et al., 1997). In plants H2O2 is destroyed predominantly by APXs and catalases (Asada, 1997; Willekens et al., 1997). Although the catalases are restricted to the peroxisomes, and perhaps mitochondria, APXs have been found in every compartment of the plant cell in which they have been sought (Asada, 1997; Jiménez et al., 1997). Confirmation of the role of redox signals in the elicitation of defense reactions has come from studies on transformed plants; for example tobacco antisense transformants containing only 10% of the foliar catalase activity of the untransformed controls show enhanced expression of defense-related proteins and increased accumulation of the antioxidant glutathione (Chamnongpol et al., 1996).
Figure 1.
Reduction of H2O2 by the ascorbate-glutathione cycle.
Incompatible plant-pathogen interactions lead to the phenomenon known as programmed cell death (Jones and Dangl, 1996; Mittler and Lam, 1996). Activation of programmed cell death during HR results in the formation of a zone of dead cells around the infection site. H2O2 produced around the developing papillae and surrounding halos of barley (Hordeum vulgare L.) leaves infected with the powdery mildew fungus (Thordal-Christensen et al., 1997) is considered to be involved in the orchestration of HR (Levine et al., 1994; Mehdy, 1994).
The production of H2O2 during the oxidative burst is involved in the integration of cellular processes and the adaptation to environmental stimuli. First, it leads to rapid cell wall reinforcement because it is involved in oxidative cross-linking (Bradley et al., 1992) and insolubilization of Hyp-rich proteins (Bradley et al., 1992; Wojtaszek et al., 1995; Otte and Barz, 1996). Second, it causes release of calcium into the cellular matrix, which may be central to the signal transduction process (Price et al., 1994). Third, it alters the concentrations and redox status of intracellular antioxidants, such as ascorbate and glutathione, which are also signal-transducing molecules (Foyer et al., 1997). Fourth, it is implicated in the induction of defense genes (Wu et al., 1997). Stimuli that induce defense-gene expression cause a reciprocal repression of cell-cycle-related genes (Logemann et al., 1995). The cellular redox state is a critical factor in the regulation of the G1/S transition in the eukaryotic cell cycle (Russo et al., 1995). The ability to reduce cell division under stress conditions allows conservation of energy and reduces the risk of heritable damage.
Sustained H2O2 production by the plasmalemma appears to be an integral feature of incompatible plant-pathogen interactions (Chai and Doke, 1987; Baker et al., 1991, 1993; Levine et al., 1994). The measurement of H2O2 production in whole-plant tissues or organs in situ is fraught with technical difficulties. However, using various cytochemical detection techniques, prolonged bursts of H2O2 production have been measured between 5 and 8 h after inoculation of lettuce by the phytopathogenic bacterium Pseudomonas syringae (Bestwick et al., 1997). Similarly, in barley inoculated with Blumeria graminis (originally called Erysiphe graminis), H2O2 production was detected in the epidermal cell wall adjacent to the mesophyll cells and subjacent to the primary germ tube from 6 h after inoculation, and subjacent to the appressorium after 15 h (Thordal-Christensen et al., 1997). In contrast, accumulation of H2O2 was not detected in bean leaf discs infected with Botrytis cinerea until 48 h after inoculation, when the infection was spreading rapidly (Bestwick et al., 1997).
The oxidative burst is considered to produce such large quantities of H2O2 that the antioxidative defenses of the cell are overwhelmed at least temporarily (Lamb and Dixon, 1997; Wojtaszek, 1997). An early response to H2O2 addition, however, is the transcription of enzymes involved in antioxidant defense. Induction of glutathione S-transferase gene expression, for example, has been observed 30 to 60 min after addition of exogenous H2O2 to soybean cell cultures (Levine et al., 1994). The time required to modify gene expression in isolated cell cultures in response to H2O2 addition or pathogen infection may be quite different, however, from that in intact tissues and organs under pathogen attack. In tobacco leaves inoculated with tobacco mosaic virus, increases in antioxidant capacity were observed only after the onset of necrosis (Fodor et al., 1997). The temporal distribution of responses and the dynamic changes in the concentrations of active oxygen species within the cellular and extracellular compartments of intact tissues are key factors governing the outcome of plant-pathogen interactions (Tiedemann, 1997).
In leaves fungal infections have been shown to induce different components of the ascorbate-glutathione cycle (Fig. 1) and other antioxidant defenses (Gönner and Schlösser, 1993; El-Zahaby et al., 1995; Fodor et al., 1997). HR increases transcription of specific SOD and catalase genes, in addition to glutathione S-transferases and glutathione peroxidases, to ensure that maximal protection is maintained within the appropriate cellular compartments (Bowler et al., 1994). Glutathione, a major low-molecular-weight thiol in plant cells, is increased after pathogen infection (Edwards et al., 1991). Exogenous application of GSH increased Phe ammonia-lyase and chalcone synthase transcripts in bean cell suspensions (Wingate et al., 1988). In addition, GSH-responsive elements on the promoters of these genes have been identified (Dron et al., 1988). GSH was found to accumulate in bean and alfalfa cell-suspension cultures treated with fungal elicitor (Edwards et al., 1991) and in elicitor-treated liverwort cells (Nakagawara et al., 1993). In carrot inhibition of glutathione synthesis triggers phytoalexin accumulation, whereas the addition of H2O2 mimics this response (Guo et al., 1993).
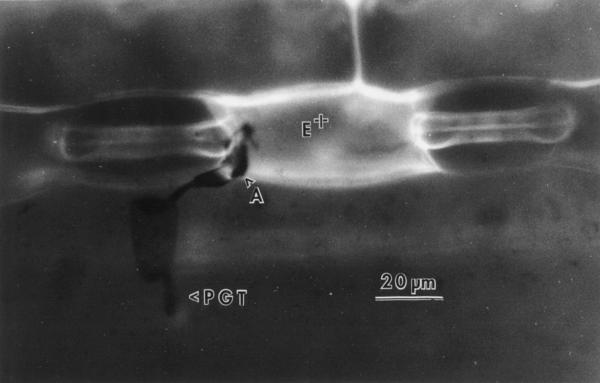
B. graminis D.C. f. sp. hordei Marchal is a biotrophic fungal pathogen that causes barley powdery mildew. Recently, the processes of B. graminis development and host-cell response to attack have been reviewed (Aist and Bushnell, 1991; Carver et al., 1995). Epidermal cells of barley and other cereals attacked by appressoria of B. graminis may express two different forms of response correlating to resistance that prevent establishment of a biotrophic relationship: (a) infection is arrested before penetration of the attacked cells, and this is correlated to the deposition of papillae (wall appositions) by the living cell (Carver et al., 1996); (b) the host cell dies (HR), thus preventing establishment of biotrophy, and autofluorogens accumulate throughout the dead cell (Fig. 2) (Koga et al., 1990). In the present study two near-isogenic lines of barley, developed by J.G. Moseman (Agricultural Research Service, U.S. Department of Agriculture, Beltsville, MD) and differing at the Mla locus, were used to study the antioxidant response to pathogen attack 24 h after inoculation. Algerian/4* (F14) Man. (R) (hereafter referred to as Alg-R) has race-specific resistance to B. graminis conferred by the dominant Mla1 allele, and associated with hypersensitive cell death (HR) (Johnson et al., 1979; Zeyen and Bushnell, 1979; Koga et al., 1990; Hippe-Sanwald et al., 1992). Algerian/4* (F14) Man. (S) (hereafter referred to as Alg-S) carries the recessive mla1 allele for susceptibility to B. graminis. In Alg-R leaves about 50% of attacked cells express HR and show whole-cell autofluorescence as a result of attack by an avirulent fungal isolate (Zeyen et al., 1995). By contrast, Alg-S shows cell death at a very low frequency (about 1% of attacked cells).
Figure 2.
Incident fluorescence micrograph of a B. graminis f. sp. hordei germling attacking Alg-R barley 24 h after inoculation. The germling produced a primary germ tube (PGT) and an appressorial germ tube (A). The epidermal cell attacked by the appressorium shows intense whole-cell autofluorescence (E+) indicative of cell death typical of the HR.
Previous studies with B. graminis infection of barley have demonstrated that maximum induction of genes coding for peroxidases, pathogenesis-related proteins, and enzymes associated with phytoalexin production occurred 24 h after inoculation (Boyd et al., 1994; Clark et al., 1994). To determine whether the induction of defense responses was associated with sustained oxidation, the oxidation states of the ascorbate and glutathione pools of whole barley leaves were examined 24 h after inoculation and compared with those of uninoculated leaves. Because the apoplast is the site of H2O2 production during the oxidative burst, our principle objective was to determine pathogen-induced changes in the antioxidant status of the apoplast. We examined the distribution of glutathione and ascorbate and associated antioxidant enzymes in whole-leaf and apoplastic extracts from inoculated leaves of resistant and susceptible barley. We documented pathogen-induced changes in the activities of antioxidant enzymes and the total pool sizes of ascorbate and glutathione and their degree of oxidation in the cellular and extracellular compartments of barley leaves 24 h after inoculation, when the infection peg had emerged from beneath the appressorium and attempted to penetrate the host epidermal cells. No extensive oxidation of either the foliar ascorbate or glutathione pool was observed, however, suggesting that H2O2 produced as a result of the plant-pathogen interaction was efficiently destroyed either during or before the 24-h time point.
MATERIALS AND METHODS
Plant and Pathogen Material
Seedlings of Alg-R (resistant; Mla1 allele) and Alg-S (susceptible; mla1 allele) were grown for 9 d in seed trays of John Innes No. 3 compost (J and P Peat, Ltd., Tollund House, Carlisle, UK) in controlled-environment chambers with a 16-h photoperiod at 340 μmol m−2 s−1, at 20°C day/15°C night, and at a constant (70%) RH.
Blumeria graminis f. sp. hordei, isolate CC1 (avirulent to Mla1), was maintained on susceptible barley (Hordeum vulgare L.) seedlings in a spore-proof greenhouse. One day before the inoculum was required for experimentation, heavily sporulating plants were shaken to remove older conidia and to ensure a supply of vigorous young spores for experimentation.
Inoculation and Incubation of Experimental Material
To inoculate experimental plants, trays of 9-d-old seedlings were taken at 9 am to the spore-proof greenhouse, where they were inoculated with B. graminis conidia by shaking heavily infected plants over them. It was impossible to ensure total uniformity of inoculum distribution, but glass slides placed among the seedlings showed an average density of approximately 10 conidia mm−2. Inoculations were completed within 15 min. Seed trays were immediately returned to the controlled-environment chambers and incubated for 24 h before harvesting for biochemical analyses. Equivalent trays of uninoculated seedlings provided control material. In Alg-R, the HR can be first detected about 18 h after inoculation (e.g. Bushnell and Liu, 1994), and increases in frequency up to 30 h. Therefore, it was assumed that in the present study, in which material was harvested for biochemical analyses 24 h after inoculation, most cells destined to express the HR would have done so. To check this assumption, three inoculated leaves of each isoline were fixed for light microscopy 24 and 48 h after inoculation for assessment of pathogen development and epidermal host-cell responses to attack.
Fixation and Clearing of Leaf Tissue for Microscopy
Leaves were fixed and prepared for microscopy by a procedure that avoids displacement of the fungus (Carver et al., 1991). They were mounted without a coverslip and observed with a “no-coverslip” 40× objective lens (Carver et al., 1991). To assess the success of attempted primary infection by B. graminis, on each leaf 25 germlings with appressoria were examined by transmitted light microscopy to determine whether they had penetrated the host epidermal cell successfully to form a primary haustorium. Autofluorescent host-cell responses to B. graminis attack have been described many times previously (e.g. see Carver et al., 1995). For each germling, the presence at appressorium contact of whole-cell fluorescence, indicative of HR, was determined using incident fluorescence microscopy (blue exciter filter, maximum transmittance 400 nm; dichroic mirror and barrier filter transmittance, 500–800 nm).
Extraction of Soluble Apoplastic Components (EWF)
Soluble apoplastic enzymes and metabolites were extracted by vacuum infiltration by a method similar to that described by Polle et al. (1990). Freshly cut leaves (5 g) were washed three times with distilled water, placed in aluminum foil dishes containing 30 mL of infiltration solution consisting of either 50 mm Mes/KOH buffer (pH 6.0), 40 mm KCl, and 2 mm CaCl2 (for the extraction of enzymes), or 50 mm acetate buffer (pH 4.5), 100 mm KCl, and 2 mm CaCl2 (for the extraction of metabolites). To ensure immersion in the infiltration solution, a second perforated aluminum dish was placed on top of the leaves. The infiltration dishes were placed in a vacuum desiccator and the leaves were infiltrated for 20 periods of 30 s at −70 kPa. They were then blotted gently, loaded into a perforated centrifuge tube (9 mL, 1.5 cm in diameter), and placed in an Eppendorf tube (1.5 mL). EWF was recovered by centrifugation (10 min, 2900g, 4°C). For the extraction of the metabolites, EWF was centrifuged directly in Eppendorf tubes containing 500 μL of 0.1 m HClO4 to immediately stop metabolism. Before analysis, sufficient K2CO3 (5 m) was added to each sample of EWF to adjust the pH to either 4.0 to 5.0 (for ascorbate determination) or 6.0 to 7.0 (for glutathione determination). The exact conditions required for extraction of EWF from barley leaves were optimized. Before the studies reported here appropriate recovery experiments were performed with known quantities of metabolites to ensure that any oxidation arising from extraction procedures was taken into account.
Between 1.1 and 1.2 mL of EWF was obtained from 5 g of leaves (fresh weight). EWF was used immediately after isolation for the determination of enzymic activities or for metabolite measurements. In all cases the samples were kept at 4°C until assay.
Extraction of Enzymes from Whole Leaves
Freshly cut leaves (0.3 g) were weighed, immersed in liquid N2, and ground to a fine powder in the same buffer as that used for EWF extractions of enzymes. When the mixtures had thawed, they were ground again. Because SOD and APX have membrane-bound isoenzyme forms, the extracts were not centrifuged and assays were performed on the crude leaf homogenates. The extracts were analyzed for the cytoplasmic marker enzyme G6PDH, and for the antioxidant enzymes SOD, GR, APX, MDHAR, DHAR, and catalase.
Extraction of Metabolites from Whole Leaves
Leaves (3.0 g) were ground in liquid N2 to a fine powder and 1 mL of cold HClO4 (2.5 m) was added. After the homogenates had thawed they were ground again. The crude extracts were centrifuged at 16,000g for 5 min at 4°C, and the supernatant was divided into two aliquots of 400 μL and stored on ice before neutralization. For ascorbate determinations, 100 μL of 0.1 m NaH2PO4/NaOH buffer (pH 5.6) and sufficient 5 m K2CO3 were added to bring the pH to 4.0 to 5.0 at 4°C. For glutathione determination, 100 μL of 0.1 m Hepes/KOH buffer (pH 7.0) was added and the pH was adjusted with 5 m K2CO3 to 6.0 to 7.0. The mixtures were centrifuged (5 min, 16,000g, 4°C) to remove insoluble potassium perchlorate and the clear supernatants were used for assay.
Determination of Enzyme Activities
All measurements were made at 25°C (except for measurement of catalase, which was done at 20°C) and were performed four times for each sample. G6PDH, which was used as a cytoplasmic marker, was determined as described by Weimar and Rothe (1986). GR was measured spectrophotometrically at 340 nm by a modification of the method of Foyer and Halliwell (1976). The assay contained 50 mm Hepes (pH 8.0), 0.5 mm EDTA, 500 μm GSSG, 100 μL of extract, and 250 μm NADPH. Control rates were obtained in the absence of GSSG.
MDHAR was assayed at 340 nm by a modification of the method of Miyake and Asada (1992). Monodehydroascorbate was generated via the action of ascorbate oxidase (0.4 unit; 1 unit = 1 μmol of ascorbate oxidized per min) in a reaction mixture (1 mL) containing 100 mm Hepes/KOH (pH 7.6), 25 μm NADPH, 2.5 mm ascorbate, and 100 μL of extract.
DHAR was assayed in a reaction mixture (1 mL) consisting of 50 mm Hepes/KOH buffer (pH 7.0), 2.5 mm GSH, 0.2 mm DHA, 0.1 mm EDTA, and 10 to 50 μL of extract. Reaction rates were measured by monitoring the change in A265 as ascorbate was generated (Miyake and Asada, 1992). DHAR activity was calculated using an extinction coefficient of 7.0 mm−1 cm−1.
APX was measured spectrophotometrically by a modification of the method of Nakano and Asada (1987). The reaction mixture (1 mL) contained 50 mm KH2PO4/K2HPO4 buffer (pH 7.0), 250 μm ascorbate, 1 mm H2O2, and 100 μL of extract. The decrease in A290 was measured as ascorbate was oxidized. APX activity was calculated using an extinction coefficient of 2.8 mm−1 cm−1 for ascorbate at 290 nm.
SOD activity was measured as described by McCord and Fridovich (1969) by the inhibition of color formation at 560 nm in the presence of the extract in a reaction mixture (1 mL) containing 50 m Hepes/KOH buffer (pH 7.8), 0.5 mm EDTA buffer, 0.05 unit of xanthine oxidase, 0.5 mm nitroblue tetrazolium, and 4 mm xanthine. A concentration curve was produced for each sample to calculate activity.
Catalase was measured at 20°C in a liquid-phase O2 electrode (Hansatech, Kings Lynn, UK). Total extractable catalase activity was measured via O2 evolution in a reaction medium (1 mL) containing 100 mm Hepes/KOH (pH 7.4), 0.5 m H2O2, and 10 to 50 μL of extract (Clairborne, 1985).
Determination of Ascorbate and Glutathione
Ascorbate and DHA were measured as described by Foyer et al. (1983) via the decrease in A265 after the addition of ascorbate oxidase. GSH was measured by the method of Griffiths (1980). GSSG was determined as described by Griffiths (1980) except that endogenous GSH was measured before GSSG in the same cuvette; total glutathione (GSH plus GSSG) was estimated via the increase in A412 after the addition of GR and NADPH. GSSG was determined by the difference of the two values.
Statistical Analysis
The significance of differences between mean values obtained from four samples produced in two independent experiments was determined by one-way analysis of variance.
RESULTS
Host Epidermal Cell Responses and B. graminis Development
Table I shows the proportions of B. graminis appressoria that stimulated whole-cell autofluorescence indicative of the HR in leaves of the resistant Alg-R and the susceptible Alg-S barley isolines. In both isolines there was little change between 24 and 48 h after inoculation in the proportions of appressoria that caused cell death. However, there were very great differences between the isolines in the proportions of appressoria that stimulated this response. In Alg-R, at 24 h more than 60% of the cells attacked by B. graminis appressoria showed whole-cell autofluorescence indicative of the HR, and this proportion did not increase in the later sample. In Alg-S, less than 3% of cells died in response to attack. Thus, near-maximal expression of the HR had been achieved by 24 h after inoculation, which is when tissues were harvested for biochemical analyses.
Table I.
Meana percentages of B. graminis f. sp. hordei appressoria that were associated with a localized autofluorescent host-cell response, cell death (HR) indicated by whole-cell autofluorescence, and that penetrated epidermal cells successfully to form haustoria in barley fixed 24 and 48 h after inoculation
| Parameter Measured | Time after Inoculation | Barley Isoline
|
|
|---|---|---|---|
| Alg-R | Alg-S | ||
| h | % | ||
| Hypersensitive cell death | 24 | 62.70 | 0.00 |
| 48 | 61.30 | 2.70 | |
| Fungal penetration success | 24 | 2.70 | 88.00 |
| 48 | 6.70 | 96.00 | |
Data are based on observations of 25 germlings on three replicate leaves in each case.
The proportion of appressoria that formed haustoria was also quite different between Alg-R and Alg-S (Table I). In Alg-R, only approximately 3% had formed haustoria at 24 h, and this increased to only about 7% by 48 h. In Alg-S, 88% of appressoria had formed haustoria at 24 h, and this increased only slightly to 96% by 48 h. Thus, in both isolines success of attempted epidermal cell infection was largely determined by 24 h after inoculation.
Determination of Contamination in Apoplastic Extracts by Cytoplasmic Components and Calculation of Corrected Values for Apoplastic Components
G6PDH, a cytoplasmic enzyme, was used to calculate cytoplasmic contamination of the apoplastic extracts. On average for all of the samples collected, less than 2% of the total extractable foliar G6PDH activity was found in the apoplast fluid of both inoculated and control leaves (Table II). Exact values for the contamination of each sample were obtained, and this allowed an accurate determination of cytoplasmic contamination in each apoplastic sample. From this, all of the following data relating to analysis of apoplastic constituents were corrected to allow for cytoplasmic contamination of each sample.
Table II.
Determination of the contamination of the apoplastic extracts (means ± se, n = 4)
| Leaf Type | G6PDH Activity in the
Total Leaf and in the Apoplast (%) of Tissue from Barley
|
|
|---|---|---|
| Alg-R | Alg-S | |
| μmol min−1 g−1 fresh wt | ||
| Control | 0.15 ± 0.02 | 0.20 ± 0.04 |
| 0.25% | 0.16% | |
| Inoculated | 0.34 ± 0.01 | 0.27 ± 0.02 |
| 0.66% | 1.24% | |
Ascorbate and Glutathione Contents
Ascorbate and glutathione were measured in whole-leaf and apoplastic extracts from control (noninoculated) and inoculated leaves (Fig. 3).
Figure 3.
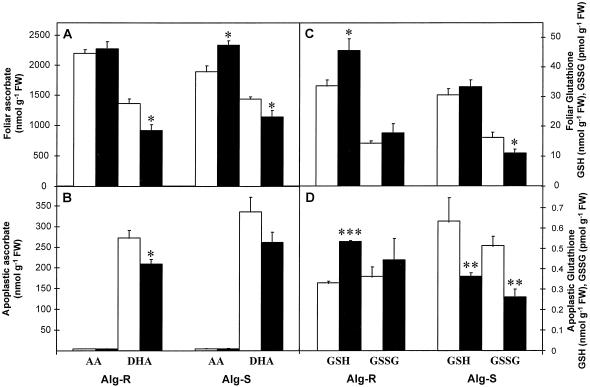
The effect of powdery mildew attack on ascorbate (A and B) and glutathione (C and D) contents of barley leaves (A and C) and apoplastic extracts (B and D) 24 h after inoculation. Black bars, Inoculated leaves; white bars, noninoculated controls. Bars represent se of means (n = 4, four repetitions from two independent experiments). *, **, and *** indicate values that differ significantly from the control at P < 0.05, P < 0.01, and P < 0.001, respectively. FW, Fresh weight.
Ascorbate
Total foliar ascorbate content (reduced plus oxidized) was similar in both barley lines (Fig. 3A). In both lines the total ascorbate pool was largely reduced (57%–62%) in inoculated and in control leaves. A significant (P < 0.05) increase (23%) in AA was found in inoculated Alg-S leaves compared with the controls; in Alg-R the increase was slight and insignificant. In both lines a significant (P < 0.05) decrease (20%–30%) in DHA was found in inoculated leaves compared with the controls.
The apoplast contained 7% to 10% of the total leaf (oxidized plus reduced) ascorbate pool. In all cases the apoplastic ascorbate pool consisted almost entirely of DHA (Fig. 3B). To determinate whether this was the situation in vivo or whether ascorbate in the apoplast was oxidized upon extraction, recovery experiments were performed in which a known quantity of AA was infiltrated into the apoplast before extraction. DHA and AA were determined before and after extraction. After extraction, all of the added AA was recovered. Importantly, no DHA was found in the buffer after extraction. These results confirmed that AA was not oxidized during the extraction procedures. Furthermore, in the apoplastic extracts obtained from AA-infiltrated leaves, after correcting values for endogenous ascorbate, DHA was four times higher than AA, suggesting that AA was oxidized to DHA in the apoplast. This is in agreement with previous observations (Horemans, 1997).
The apoplast of inoculated barley leaves contained 7.0% ± 0.5% of the total leaf (oxidized plus reduced) ascorbate. This was slightly lower than in the apoplast of controls. After inoculation, a significant (P < 0.05) decrease (23%) in DHA was found in the apoplast of Alg-R but not in the apoplast of Alg-S (Fig. 3B).
Glutathione
Noninoculated leaves of Alg-R and Alg-S had similar total glutathione (Fig. 3C). The glutathione pool was largely (>99%) reduced in noninoculated and inoculated leaves of both lines. Inoculation caused a significant (P < 0.05) increase (36%) in GSH in Alg-R but not in Alg-S. In contrast, inoculation caused a significant (P < 0.05) decrease (37%) in GSSG only in Alg-S (Fig. 3C).
Glutathione was a minor component of the apoplast of both lines (Fig. 3D). In noninoculated Alg-S and Alg-R, 2.05% and less than 1%, respectively, of the total glutathione pool was found in the apoplast. Both GSH and GSSG were found in the apoplast (Fig. 3D), but the percentage reduction state of the apoplastic pool was less than in whole leaves. Inoculation caused a significant (P < 0.001) increase (61%) of apoplastic GSH in Alg-R. By contrast, inoculation caused a significant (P < 0.01) decrease of both apoplastic GSH (42%) and GSSG (46%) in Alg-S.
Enzyme Activities
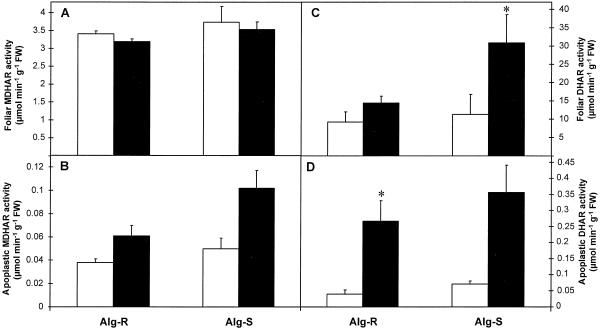
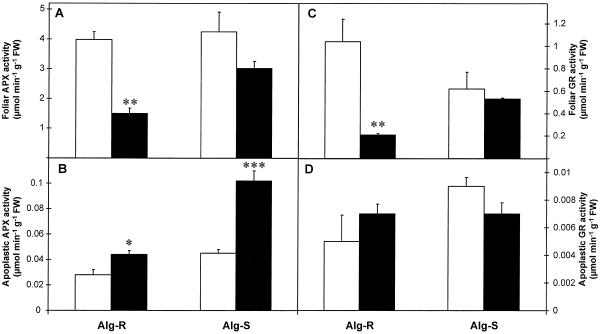
The maximum extractable activities of the antioxidant enzymes SOD, APX, MDHAR, DHAR, GR, and catalase measured in whole-leaf and apoplastic extracts of noninoculated and inoculated leaves are shown in Figures 4–6.
Figure 4.
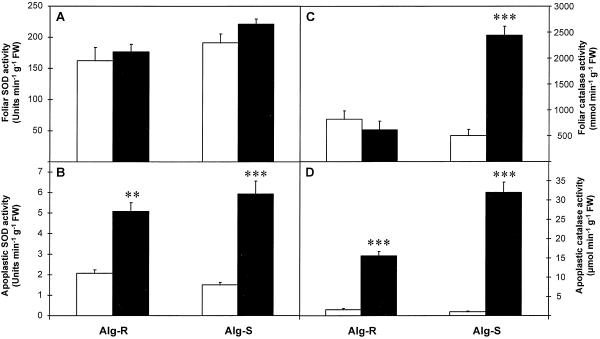
The effect of powdery mildew attack on SOD (A and B) and catalase (C and D) activities of barley leaves (A and C) and apoplastic extracts (B and D) 24 h after inoculation. Black bars, Inoculated leaves; white bars, noninoculated controls. Bars represent se of means (n = 4, four repetitions from two independent experiments). *, **, and *** indicate values that differ significantly from the control at P < 0.05, P < 0.01, and P < 0.001, respectively. FW, Fresh weight.
Figure 6.
The effect of powdery mildew attack on MDHAR (A and B) and DHAR (C and D) activities of barley leaves (A and C) and apoplastic extracts (B and D) 24 h after inoculation. Black bars, Inoculated leaves; white bars, noninoculated controls. Bars represent se of means (n = 4, four repetitions from two independent experiments). *, **, and *** indicate values that differ significantly from the control at P < 0.05, P < 0.01, and P < 0.001, respectively. FW, Fresh weight.
Total SOD
Total SOD activities were similar in whole-leaf and apoplastic extracts from noninoculated leaves of both lines (Fig. 4, A and B). After inoculation, whole-leaf SOD activity did not change (Fig. 4A), but a significant increase in apoplastic SOD was found in both resistant (150%) and susceptible (300%) lines compared with noninoculated controls (Fig. 4B). The apoplast of inoculated leaves contained 2.5% to 3% of the total foliar SOD activity, but only 0.8% to 1.3% was found in the controls.
Catalase
Noninoculated whole-leaf extracts of both resistant and susceptible lines had similar catalase activities (Fig. 4C). In Alg-S inoculation caused a massive (400%) and significant (P < 0.001) increase in catalase activity in these extracts. In contrast, there was no significant change in Alg-R after inoculation (Fig. 4C).
Less than 0.5% of the total catalase activity was found in the apoplast of noninoculated leaves (Fig. 4D). Inoculation caused a significant (P < 0.001) increase in apoplastic catalase activity in Alg-R (900%) and in Alg-S (2230%) (Fig. 4D). The apoplast of inoculated Alg-R and Alg-S leaves contained 2.52% and 1.31%, respectively, of the total leaf catalase activity.
APX
Noninoculated whole-leaf extracts of both resistant and susceptible lines had similar APX activities (Fig. 5A). Inoculation caused a significant (P < 0.01) decrease (62%) of APX activity in whole-leaf Alg-R extracts, but caused no significant change in inoculated Alg-S (Fig. 5A). The apoplast of noninoculated leaves contained 0.7% to 1% of the total foliar APX activity. Inoculation caused a significant increase of apoplastic APX activity in Alg-R (56%; P < 0.05) and Alg-S (130%; P < 0.001) (Fig. 4B). After inoculation, the apoplast of both lines contained 3% to 3.5% of the total foliar APX activity.
Figure 5.
The effect of powdery mildew attack on APX (A and B) and GR (C and D) activities of barley leaves (A and C) and apoplastic extracts (B and D) 24 h after inoculation. Black bars, Inoculated leaves; white bars, noninoculated controls. Bars represent se of means (n = 4, four repetitions from two independent experiments). *, **, and *** indicate values that differ significantly from the control at P < 0.05, P < 0.01, and P < 0.001, respectively. FW, Fresh weight.
GR
In noninoculated whole-leaf extracts, GR activity in Alg-R was double that in Alg-S (Fig. 5C). Inoculation caused a significant (P < 0.01) decrease (80%) only in the resistant Alg-R line, whereas no significant change was found in Alg-S (Fig. 5C).
The apoplast of noninoculated leaves contained 0.5% (Alg-R) and 1.4% (Alg-S) of the total foliar APX activity. No significant change was found in apoplastic GR activity in either line after inoculation (Fig. 5D). As a consequence of the large inoculation-dependent decrease in total foliar GR in Alg-R, the percentage of GR in the apoplast of Alg-R increased by 3% but no change was observed in Alg-S.
MDHAR
MDHAR activity was similar in whole-leaf extracts of both lines and no significant changes resulted from inoculation (Fig. 6A). The apoplast of barley contained 2% to 3% of the total foliar MDHAR activity. As for whole-leaf extracts, in apoplastic extracts MDHAR activity was similar between both lines and was not affected significantly by inoculation (Fig. 6B).
DHAR
DHAR activity was similar in whole-leaf extracts of both lines and no significant changes resulted from inoculation (Fig. 6C). About 0.5% of total foliar DHAR activity was found in the apoplast of noninoculated leaves. In both lines inoculation appeared to cause an increase in apoplastic DHAR activity, but the variation between samples was high; thus, although the increase in apoplastic DHAR was significant (P < 0.05) in Alg-R, it was not significant in Alg-S (Fig. 6D).
DISCUSSION
The development of B. graminis and host epidermal responses, shown in Figure 2 and Table I, were comparable with those observed in previous studies of Alg-R and Alg-S (Carver et al., 1994; Zeyen et al., 1995). Conidia of B. graminis germinate within 1 h of inoculation, producing first a short, aseptate primary germ tube, which attaches to the host epidermal cell surface and engenders localized host-cell responses (approximately 1–6 h). A second germ tube emerges (about 2–4 h), elongates, and differentiates a specialized infection structure, the appressorium (approximately 8–10 h). An infection peg emerges from beneath the appressorium and attempts to penetrate the host epidermal cell (about 12–15 h). If penetration succeeds, a feeding structure, the haustorium, is formed within the epidermal cell (approximately 15–18 h) and absorbs nutrients from the living host cell to supply the developing colony. The response of Alg-R to attack by the avirulent B. graminis isolate was extreme, leading to a very high frequency of epidermal cell HR (Table I). By contrast, in Alg-S cell death was extremely infrequent. The proportion of HR cells did not increase between 24 and 48 h, indicating that the majority of important plant cell responses were under way or accomplished by the time the biochemical assays were performed. The antioxidant status of the whole leaves and the leaf apoplast, therefore, were determined 24 h after inoculation, i.e. when near-maximal HR had been achieved (Table I). At this time the plasmalemma was no more “leaky” in any of the inoculated leaves than in the healthy leaves, as indicated by the low contamination (less than 2%) of the apoplast by the cytoplasmic marker (G6PDH) in EWF extracts from either Alg-S or Alg-R leaves. The changes in antioxidant status observed in this study, therefore, are a consequence of the reaction of the plant to penetration by the fungus.
The pathogen-induced responses observed in this study result not only from phenomena occurring in cells undergoing the HR, but also from those occurring in surrounding tissue alerted by signals derived from the cells undergoing the HR. No inoculation-dependent decreases in either the AA-to-DHA or GSH-to-GSSG ratios were observed in either the resistant or the susceptible line. The AA-to-DHA ratio was increased in Alg-S leaves 24 h after inoculation. This suggests that if general oxidation of the mesophyll tissues, attributable to H2O2 generation, had occurred, it was transient and reversed by 24 h after inoculation in both lines.
The total ascorbate pool size was decreased in inoculated Alg-R leaves relative to controls, suggesting that a change in turnover had occurred in the resistant isoline. This was not observed in the susceptible isoline. Catalase activity increased and GSH accumulation occurred after inoculation with B. graminis. Clear differences in the responses of these antioxidants to attack were observed in the resistant and susceptible lines. Total extractable catalase activity had dramatically increased (by about 400%) 24 h after inoculation in Alg-S, whereas catalase activity was unchanged in Alg-R. This suggests that there is an inverse relationship between catalase induction in barley leaves and resistance to B. graminis. Although the temporal sequence of events cannot be deduced from the present studies, it is interesting to note that H2O2-induced oxidation was found to cause expression of pathogenesis-related genes in transformed tobacco plants deficient in the major catalase isoform, Cat 1 (Chamnongpol et al., 1996). H2O2 per se did not accumulate in these transformants, but the total glutathione pool increased, and there was a strong decrease in the GSH-to-GSSG ratio (Willekens et al., 1997). In the present study the induction of catalase activity in the susceptible line may have occurred too late in the response sequence to afford protection. Alternatively, catalase induction may have limited signal transduction by effectively removing H2O2 as it was formed.
No inoculation-dependent increases in the activities of any of the other antioxidant enzymes were observed. Foliar APX activity decreased in Alg-R but not in Alg-S after inoculation, whereas DHAR activity was unchanged. Slight changes in APX and DHAR activities have been observed in susceptible barley lines 4 d after inoculation (El-Zahaby et al., 1995).
Foliar glutathione was increased in Alg-R but not in Alg-S after inoculation, indicating a positive relationship between glutathione accumulation and pathogen resistance, as has been observed previously (Dron et al., 1988; Wingate et al., 1988; Edwards et al., 1991). Glutathione synthesized in leaf cells is transported throughout the plant (Noctor et al., 1997a, 1997b) and therefore has been identified as a putative long-distance signaling molecule (Foyer et al., 1997). Glutathione accumulation must occur after engagement of Ca2+-dependent signal transduction pathways, such as have been described after H2O2 addition to aquorin-containing cells (Price et al., 1994). Differential antioxidant deployment, however, between Alg-R and Alg-S may be central to resistance strategies. Foliar APX and GR activities had decreased (62% and 80%, respectively) in Alg-R but not in Alg-S 24 h after inoculation. The GSH-to-GSSG ratio was high in both Alg-R and Alg-S at this time, but a decrease in GR activity could sensitize the system to later bursts of H2O2 production to allow perturbations in the GSH-to-GSSG ratio.
There has been an upsurge of interest in the antioxidant defenses of the apoplast in recent years as the importance of this compartment has become apparent (Penal and Castillo, 1991; Arrigoni, 1994; Luwe, 1996). Because ascorbate is a substrate for cell wall peroxidases, it may play a role in the regulation of cell wall lignification, particularly during the HR, through its capacity to inhibit the oxidation of phenolic compounds by peroxidases (Takahama and Oniki, 1992; de Cabo et al., 1996; Mehlhorn et al., 1996). The pathogen-induced increase in the peroxidase activity of the cell wall would be effective only in the absence of AA. In the barley leaves used in the present study, which were obtained from 9-d-old seedlings, only DHA was found in the apoplast. Similar results have been obtained with dicotyledonous leaves during the early stages of development (Luwe, 1996). The absence of AA from the apoplast may reflect the requirement for efficient functioning of cell wall peroxidases during leaf expansion. A strong plasmalemma-bound, ascorbate-oxidizing activity has been detected (Horemans et al., 1997).
Apoplastic DHA decreased after inoculation in Alg-R but not in Alg-S. This may suggest that more rapid import of DHA into the cytosol occurred after inoculation in Alg-R compared with Alg-S. In contrast, the net loss of total ascorbate observed in Alg-R leaves after inoculation is consistent with severe previous experience of oxidative stress during HR.
Although a substantial proportion (about 8%) of the total foliar (reduced plus oxidized) ascorbate pool was found in the apoplast, there was little or no glutathione (only 1%–2%). The differences in the apoplastic contents of ascorbate and glutathione are striking and may reflect the differential roles of these two antioxidants. It is interesting that the glutathione pool increased in the apoplast of the Alg-R leaves and decreased in Alg-S after inoculation, perhaps implying that increased synthesis and export of glutathione are resistance responses.
The activities of antioxidant enzymes in the apoplast are low compared with the activities in whole leaves, but because the volume of the aqueous apoplastic phase is only 4.5% of the total cell volume (Winter et al., 1993), these percentages reflect large activities per unit volume. Substantial amounts of SOD, APX, MDHAR, and GR were found in the apoplast of healthy leaves (1%–4%), suggesting that all of the enzymes required for destruction of superoxide and H2O2 (i.e. the ascorbate-glutathione cycle) were present in this compartment. The apoplastic antioxidant enzyme activities showed an almost universal increase in response to inoculation and were much greater (at least double) in the susceptible Alg-S line compared with Alg-R. This may suggest that increased apoplastic antioxidant defenses were a feature of the establishment of biotrophy in the susceptible host. The ascorbate-glutathione cycle, however, requires a source of reducing power in the form of NADPH to sustain detoxification. Although NADPH may be present in the apoplast, at least some DHA and GSSG produced by the action of the ascorbate-glutathione cycle may be returned to the cytosol for reduction, since transporters for these oxidized forms have been described (Foyer and Lelandais, 1996; Jamaï et al., 1996; Horemans et al., 1997).
ACKNOWLEDGMENTS
We thank Dr. Andrea Polle (Universität Göttingen, Germany) for her invaluable advice concerning the technique used to extract the apoplast. We also thank Dr. William Bushnell (U.S. Department of Agriculture, Cereal Rust Laboratory, St. Paul, MN) for providing the barley seed.
Abbreviations:
- AA
reduced ascorbate
- Alg
Algerian
- APX
ascorbate peroxidase
- DHA
dehydroascorbate
- DHAR
dehydroascorbate reductase
- EWF
extracellular washing fluid
- G6PDH
Glc-6-P dehydrogenase
- GR
glutathione reductase
- GSSG
glutathione disulfide
- HR
hypersensitive response
- MDHAR
monodehydroascorbate reductase
- SOD
superoxide dismutase
LITERATURE CITED
- Aist JR, Bushnell WR (1991) Invasion of plants by powdery mildew fungi, and cellular mechanisms of resistance. In GT Cole, HC Hoch, eds, The Fungal Spore and Disease Initiation in Plants and Animals. Plenum Press, New York, pp 321–345
- Arrigoni O. Ascorbate system in plant development. J Bioenerg Biomembr. 1994;26:407–419. doi: 10.1007/BF00762782. [DOI] [PubMed] [Google Scholar]
- Asada K. The role of ascorbate peroxidase and monodehydroascorbate reductase in H2O2scavenging in plants. In: Scandalios JG, editor. Oxidative Stress and the Molecular Biology of Antioxidant Defences. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 715–735. [Google Scholar]
- Baker CJ, Mock NM, Glazener JA, Orlandi EW. Recognition responses in pathogen/non-host and race/cultivar interactions involving soybean (Glycine max) and Pseudomonas syringaepathovars. Physiol Mol Plant Pathol. 1993;43:81–94. [Google Scholar]
- Baker CJ, O'Neill NR, Keppler LD, Orlandi EW. Early responses during plant bacteria interactions in tobacco cell suspensions. Phytopathology. 1991;81:1504–1507. [Google Scholar]
- Bestwick CS, Brown IR, Bennett MHR, Mansfield JW. Localisation of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv. phaseolicola. Plant Cell. 1997;9:209–221. doi: 10.1105/tpc.9.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler C, Van Camp W, Van Montagu M, Inzé D. Superoxide dismutase in plants. Crit Rev Plant Sci. 1994;13:199–218. [Google Scholar]
- Boyd LA, Smith PH, Green RM, Brown JKM. The relationship between the expression of defense-related genes and mildew development in barley. Mol Plant Microbe Interact. 1994;7:401–410. [Google Scholar]
- Bradley DJ, Kjellbom P, Lamb CJ. Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall structural protein: a novel, rapid plant defence response. Cell. 1992;70:21–30. doi: 10.1016/0092-8674(92)90530-p. [DOI] [PubMed] [Google Scholar]
- Bushnell WR, Liu Z. Incompatibility conditioned by the Mlagene in powdery mildew of barley: timing of the effect of cordycepin on hypersensitive cell-death. Physiol Mol Plant Pathol. 1994;44:389–402. [Google Scholar]
- Carver TLW, Ingerson-Morris SM, Thomas GJ, Zeyen RJ. Early interactions during powdery mildew infection. Can J Bot. 1995;73:632–639. [Google Scholar]
- Carver TLW, Robbins MP, Zeyen RJ. Effects of two PAL inhibitors on the susceptibility and localized autofluorescent host cell responses of oat leaves attacked by Erysiphe graminisD.C. Physiol Mol Plant Pathol. 1991;39:269–287. [Google Scholar]
- Carver TLW, Zeyen RJ, Bushnell WR, Robbins MP. Inhibition of phenylalanine ammonia lyase and cinnamyl alcohol dehydrogenase increases quantitative susceptibility of barley to powdery mildew (Erysiphe graminisD.C.) Physiol Mol Plant Pathol. 1994;44:261–272. [Google Scholar]
- Carver TLW, Zhang L, Zeyen RJ, Robbins MP. Phenolic biosynthesis inhibitors suppress adult plant resistance to Erysiphe graminisin oat at 20°C and 10°C. Physiol Mol Plant Pathol. 1996;49:121–141. [Google Scholar]
- Chai HB, Doke N. Activation of the potential of potato leaf tissue to react hypersensitively to Phytophthora infestansby cytospore germination fluid and the enhancement of this potential by calcium ions. Physiol Mol Plant Pathol. 1987;30:27–37. [Google Scholar]
- Chamnongpol S, Willekens H, Langebartels C, Van Montagu M, Inzé D, Van Camp W. Transgenic tobacco with a reduced catalase activity develops necrotic lesions and induces pathogenesis-related expression under high light. Plant J. 1996;10:491–503. [Google Scholar]
- Clairborne A. Catalase activity. In: Greenwald EA, editor. Handbook of Methods for Oxygen Radical Research. Boca Raton, FL: CRC Press; 1985. pp. 283–284. [Google Scholar]
- Clark TA, Zeyen RJ, Smith AG, Carver TLW, Vance CP. Phenylalanine ammonia lyase mRNA accumulation, enzyme activity and cytoplasmic responses in barley isolines, differing at MI-a and MI-o loci, attacked by Erysiphe graminis f. sp. hordei. Physiol Mol Plant Pathol. 1994;44:171–185. [Google Scholar]
- de Cabo RC, González-Reyes JA, Córdola F, Navas P. Rooting hastened in onions by ascorbate and ascorbate free radical. J Plant Growth Regul. 1996;15:53–56. [Google Scholar]
- Doke N. The oxidative burst: roles in signal transduction and plant stress. In: Scandalios JG, editor. Oxidative Stress and the Molecular Biology of Antioxidant Defences. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 785–813. [Google Scholar]
- Doke N, Miura Y, Sanchez L, Kawakita K. Involvement of superoxide in signal transduction: responses to attack by pathogens, physical and chemical shocks, and UV irradiation. In: Foyer CH, Mullineaux PM, editors. Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants. Boca Raton, FL: CRC Press; 1994. pp. 177–198. [Google Scholar]
- Dron M, Clouse SD, Dixon RA, Lawton MA, Lamb CJ. Glutathione and fungal elicitor regulation of a plant defense promoter in electroporated protoplasts. Proc Natl Acad Sci USA. 1988;85:6738–6742. doi: 10.1073/pnas.85.18.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R, Blount JW, Dixon RA. Glutathione and elicitation of the phytoalexin response in legume cell cultures. Planta. 1991;184:403–409. doi: 10.1007/BF00195343. [DOI] [PubMed] [Google Scholar]
- El-Zahaby HM, Gullner G, Kiràly Z. Effects of powdery mildew infection of barley on the ascorbate-glutathione cycle and other antioxidants in different host-pathogen interactions. Phytopathology. 1995;85:1225–1230. [Google Scholar]
- Eshdat Y, Holland D, Faltin Z, Ben-Hayyim G. Plant glutathione peroxidases. Physiol Plant. 1997;100:234–240. [Google Scholar]
- Fodor J, Gullner G, Adam AL, Barna B, Kömives T, Kiraly Z. Local and systemic responses of antioxidants to tobacco mosaic virus infection and to salicylic acid in tobacco. Plant Physiol. 1997;114:1443–1451. doi: 10.1104/pp.114.4.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Lelandais M. A comparison of the relative rates of transport of ascorbate and glucose across the thylakoid, chloroplast and plasmalemma membranes of pea leaf mesophyll cells. J Plant Physiol. 1996;148:391–398. [Google Scholar]
- Foyer CH, Lopez-Delgado H, Dat JF, Scott IM. Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol Plant. 1997;100:241–254. [Google Scholar]
- Foyer CH, Rowell J, Walker D. Measurements of the ascorbate content of spinach leaf protoplasts and chloroplasts during illumination. Planta. 1983;157:239–244. doi: 10.1007/BF00405188. [DOI] [PubMed] [Google Scholar]
- Gönner MV, Schlösser E. Oxidative stress in interactions between Avena sativa L. and Drechsleraspp. Physiol Mol Plant Pathol. 1993;42:221–234. [Google Scholar]
- Griffiths OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Groom QJ, Torres MA, Fordham-Skelton AP, Hammond-Kosack KE, Robinson NJ, Jones JDG. RbohA, a rice homologue of the mammalian gp91phoxrespiratory burst oxidase gene. Plant J. 1996;10:515–522. doi: 10.1046/j.1365-313x.1996.10030515.x. [DOI] [PubMed] [Google Scholar]
- Guo Z-J, Nakagawara S, Sumitani K, Ohta Y. Effect of intracellular glutathione level on the production of 6-methoxymellein in cultured carrot (Daucus carota) cells. Plant Physiol. 1993;102:45–51. doi: 10.1104/pp.102.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippe-Sanwald S, Hermanns M, Somerville SC. Ultrastructural comparison of incompatible and compatible interactions in the barley powdery mildew disease. Protoplasma. 1992;168:27–40. [Google Scholar]
- Horemans N (1997) Ascorbate-mediated functions at the plasma membrane of higher plants. Thesis. Antwerpen University, Antwerp, Belgium
- Horemans N, Asard H, Caubergs RJ. Transport of dehydroascorbate into purified plasma membrane vesicles of Phaseolus vulgarisL. Plant Physiol. 1997;114:1247–1253. doi: 10.1104/pp.114.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamaï A, Tommasini R, Martinoia E, Delrot S. Characterization of glutathione uptake in broad bean leaf protoplasts. Plant Physiol. 1996;111:1145–1152. doi: 10.1104/pp.111.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez A, Hernández JA, de Rio LA, Sevilla F. Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol. 1997;114:275–284. doi: 10.1104/pp.114.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LEB, Bushnell WR, Zeyen RJ. Binary pathways for analysis of primary infection and host response in populations of powdery mildew fungi. Can J Bot. 1979;57:497–511. [Google Scholar]
- Jones AM, Dangl JL. Logjam at the Styx: programmed cell death in plants. Trends Plant Sci. 1996;1:114–119. [Google Scholar]
- Koga H, Busnell WR, Zeyen RJ. Specificity of cell type and timing of events associated with papilla formation and the hypersensitive reaction in leaves of Hordeum vulgare attacked by Erysiphe graminis f. sp. hordei. Can J Bot. 1990;68:2344–2352. [Google Scholar]
- Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Logemann E, Wu S, Schröder J, Schmeltzer E, Somssich IE, Hahlbrock K. Gene activation by U.V. light, fungal elicitor or fungal infection in Petroselinum crispumis correlated with repression of cell-cycle-related genes. Plant J. 1995;8:865–876. doi: 10.1046/j.1365-313x.1995.8060865.x. [DOI] [PubMed] [Google Scholar]
- Luwe M. Antioxidants in the apoplast and symplast of beech (Fagus sylvaticaL.) leaves, seasonal variations and responses to changing ozone concentrations in air. Plant Cell Environ. 1996;19:321–328. [Google Scholar]
- McCord JM, Fridovich I. Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- Mehdy MC. Active oxygen species in plant defense against pathogens. Plant Physiol. 1994;105:467–472. doi: 10.1104/pp.105.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlhorn H, Lelandais M, Korth HG, Foyer CH. Comparison of ascorbate-dependent peroxidase activity in horseradish peroxidase types I and II and in leaf extracts. FEBS Letts. 1996;378:203–206. [Google Scholar]
- Mittler R, Lam E. Sacrifice in the face of foes: pathogen-induced programmed cell death in higher plants. Trends Microbiol. 1996;4:10–15. doi: 10.1016/0966-842x(96)81499-5. [DOI] [PubMed] [Google Scholar]
- Miyake C, Asada K. Thylakoid-bound ascorbate peroxidase in spinach chloroplasts and photoreduction of its primary oxidation product monodehydroascorbate radicals in thylakoids. Plant Cell Physiol. 1992;33:541–553. [Google Scholar]
- Nakagawara S, Nakamura N, Guo Z-J, Sumitani K, Katoh K, Ohta Y. Enhanced formation of a constitutive sesquiterpenoid in culture cells of liverwort, Calypogeia granulataInoue, during elicitation: effects of vanadate. Plant Cell Physiol. 1993;34:421–429. [Google Scholar]
- Nakano Y, Asada K. Purification of ascorbate peroxidase in spinach chloroplasts: its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 1987;28:131–140. [Google Scholar]
- Noctor G, Jouanin L, Arisi A-CM, Valadier M-H, Roux Y, Foyer CH. Light-dependent modulation of foliar glutathione synthesis and associated amino acid metabolism in transformed poplar. Planta. 1997a;202:357–369. [Google Scholar]
- Noctor G, Jouanin L, Foyer CH. The biosynthesis of glutathione explored in transformed plants. In: Hatzios KK, editor. Regulation of Enzymatic Systems Detoxifying Xenobiotics in Plants. NATO ASI Series. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1997b. pp. 109–124. [Google Scholar]
- Otte O, Barz W. The elicitor-induced oxidative burst in cultured chickpea cells drives the rapid insolubilization of two cell wall structural proteins. Planta. 1996;200:238–246. [Google Scholar]
- Penal C, Castillo FJ. Peroxidases of plant plasma membranes, apoplastic ascorbate and relation of redox activities to plant physiology. In: Crane FL, Morre DJ, Low JE, editors. Oxidoreduction at the Plasma Membrane (Relation to Growth and Transport), Vol 2. Boca Raton, FL: CRC Press; 1991. pp. 111–120. [Google Scholar]
- Polle A, Chakrabarti K, Schürmann W, Rennenberg H. Composition and properties of hydrogen peroxide decomposing systems in extracellular and total extracts from needles of Norway spruce (Picea abiesL. Karst.) Plant Physiol. 1990;94:312–319. doi: 10.1104/pp.94.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AH, Taylor A, Ripley S, Griffiths A, Trewavas AJ, Knight MR. Oxidative signals in tobacco increase cytosolic calcium. Plant Cell. 1994;6:1301–1310. doi: 10.1105/tpc.6.9.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roxas VP, Smith RK, Allen ER, Allen RD. Overexpression of glutathione S-transferase/glutathione peroxidase enhances the growth of transgenic tobacco seedlings during stress. Nature Biotech. 1997;15:988–991. doi: 10.1038/nbt1097-988. [DOI] [PubMed] [Google Scholar]
- Russo T, Zambrano N, Esposito F, Ammendola R, Climino F, Fiscella M, Jackman J, O'Connor M, Anderson CW, Apella E. A p53-independent pathway for activation of WAF1/CIP1expression following oxidative stress. J Biol Chem. 1995;270:29386–29391. doi: 10.1074/jbc.270.49.29386. [DOI] [PubMed] [Google Scholar]
- Segal AW, Abo A. The biochemical basis of the NADPH oxidase of phagocytes. Trends Biochem Sci. 1993;18:43–47. doi: 10.1016/0968-0004(93)90051-n. [DOI] [PubMed] [Google Scholar]
- Takahama U, Oniki T. Regulation of peroxidase-oxidation of phenolics in the apoplast of spinach leaves by ascorbate. Plant Cell Physiol. 1992;33:379–387. [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. Subcellular localisation of H2O2 in plants, H2O2accumulation in papillae and hypersensitive response during barley-powdery mildew interaction. Plant J. 1997;11:1187–1194. [Google Scholar]
- Tiedemann AV. Evidence for a primary role of active oxygen species in induction of host cell death during infection of bean leaves with Botrytis cinerea. Physiol Mol Plant Pathol. 1997;50:151–166. [Google Scholar]
- Weimar M, Rothe G. Preparation of extracts from mature spruce needles for enzymatic analysis. Physiol Plant. 1986;69:692–698. [Google Scholar]
- Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, Van Montagu M, Inzé D, Van Camp W. Catalase is a sink for H2O2 and is indispensable for stress defense in C3plants. EMBO J. 1997;16:4806–4816. doi: 10.1093/emboj/16.16.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingate VPM, Lawton MA, Lamb CJ. Glutathione causes a massive and selective induction of plant defense genes. Plant Physiol. 1988;87:206–210. doi: 10.1104/pp.87.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H, Robinson DG, Heldt HW. Subcellular volumes and metabolite concentrations in barley leaves. Planta. 1993;191:180–190. [Google Scholar]
- Wojtaszek P. Oxidative burst: an early plant response to pathogen infection. Biochem J. 1997;322:681–692. doi: 10.1042/bj3220681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtaszek P, Trethowan J, Bolwell GP. Specificity in the immobilisation of cell wall proteins in response to different elicitor molecules in suspension-cultured cells of French bean (Phaseolus vulgarisL.) Plant Mol Biol. 1995;28:1075–1087. doi: 10.1007/BF00032668. [DOI] [PubMed] [Google Scholar]
- Wu G, Shortt BJ, Lawrence JL, Fitzsimmons KC, Levine EB, Raskin I, Shah DM. Activation of host defense mechanisms by elevated production of H2O2in transgenic plants. Plant Physiol. 1997;115:427–435. doi: 10.1104/pp.115.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyen RJ, Bushnell WR. Papillae response of barley epidermal cells caused by Erysiphe graminis: rate and method of deposition determined by microcinematography and transmission electron microscopy. Can J Bot. 1979;57:898–913. [Google Scholar]
- Zeyen RJ, Bushnell WR, Carver TLW, Robbins MP, Clark TA, Boyles DA, Vance CP. Inhibiting phenylalanine ammonia lyase and cinnamyl-alcohol dehydrogenase suppresses Mla1 (HR) but not mlo5(non-HR) barley powdery mildew resistances. Physiol Mol Plant Pathol. 1995;47:119–140. [Google Scholar]