Abstract
Background
Developmental hip disorders (DHDs), eg, developmental dysplasia of the hip, slipped capitis femoris epiphysis, and femoroacetabular impingement, can be considered morphology variants of the normal hip. The femoroacetabular morphology of DHD is believed to induce osteoarthritis (OA) through local cumulative mechanical overload acting on genetically controlled patterning systems and subsequent damage of joint structures. However, it is unclear why hip morphology differs between individuals with seemingly comparable load histories and why certain hips with DHD progress to symptomatic OA whereas others do not.
Questions/Purposes
We asked (1) which mechanical factors influence growth and development of the proximal femur; and (2) which genes or genetic mechanisms are associated with hip ontogenesis.
Methods
We performed a systematic literature review of mechanical and genetic factors of hip ontogeny. We focused on three fields that in recent years have advanced our knowledge of adult hip morphology: imaging, evolution, and genetics.
Where Are We Now?
Mechanical factors can be understood in view of human evolutionary peculiarities and may summate to load histories conducive to DHD. Genetic factors most likely act through multiple genes, each with modest effect sizes. Single genes that explain a DHD are therefore unlikely to be found. Apparently, the interplay between genes and load history not only determines hip morphotype, but also joint cartilage robustness (“cartilotype”) and resistance to symptomatic OA.
Where Do We Need to Go?
We need therapies that can improve both morphotype and cartilotype.
How Do We Get There?
Better phenotyping, improving classification systems of hip morphology, and comparative population studies can be done with existing methods. Quantifying load histories likely requires new tools, but proof of principle of modifying morphotype in treatment of DDH and of cartilotype with exercise is available.
Introduction
Hip ontogenesis, or morphogenesis, describes the development of the hip from its fetal origin to the adult form. Developmental hip disorders (DHDs) such as developmental dysplasia of the hip (DDH), slipped capitis femoris epiphysis (SCFE), or femoroacetabular impingement (FAI) may cause symptoms and disability in adulthood or earlier as a result of altered joint morphology [24, 49, 85, 106]. Regardless of being primarily acetabular or femoral, DHDs share a common mechanism of local cumulative mechanical overload and damage of joint structures that may cause osteoarthritis (OA) [24, 31]. Interventions that decrease DHD incidence may thus decrease OA disease burden. However, such interventions should be based on good explanations of hip ontogeny and OA development. Current explanations for skeletogenesis and bone morphology imply an important role of mechanical loading, explicitly [15, 35], or more implicitly [79], acting on genetically controlled patterning systems [25, 54, 110]. Although detailed explanations are available from animal models [25, 68, 79], this knowledge is as yet not very useful to decrease DHD prevalence nor applicable for individual patients. For example, it is currently unclear why important hip morphology differences exist between individuals or populations with comparable load histories [37, 112] (nor why these differences appear larger for hips than knees). Clearly, genetic differences may account for these morphological differences. However, genes have only recently been linked to hip morphology [5, 53, 103] and genetic architecture for hip morphogenesis is apparently complex and polygenic with modest effect size for individual genes (ie, no genes specific for each DHD have been found). Furthermore, it is currently unclear why certain hips with DHD progress to symptomatic OA, whereas others do not [6, 106]. We propose integrating observations from several fields that have recently advanced understanding of adult hip morphology (ie, imaging, evolution, and genetics) can improve our explanations of hip ontogeny and OA development.
Imaging studies using new parameters and image analysis allow comprehensive quantification of proximal femoral morphology [27, 65, 99] and have strengthened the association between morphology and OA development or prevalence [19, 28, 29, 55, 64].
Observations from evolution studies comparing humans with other large apes indicate the growing human lower limbs with open physes and long moment arms can undergo high loading for several years (eg, in sports). When compared with other animals (eg, mice and chickens), experimental models have begun to elucidate the fundamental molecular, mechanical, and genetic mechanisms and their interactions in skeletogenesis.
Genetic studies, in the last decade, have begun to explore the relation between loading and gene expression [7, 67, 79] and, more recently, between genes and hip morphology [5, 103]. These studies indicate that the same genes active in skeletogenesis, for example through regulation of growth plate chondrocytes, may also play a role in OA development in later life.
We therefore performed a systematic literature review of (1) mechanical factors that influence growth and development of the proximal femur in animals and humans; and (2) genes or genetic mechanisms associated with hip ontogenesis. We specifically sought to identify information on the (potential) interaction between mechanical and genetic factors. Furthermore, we sought information from the three fields described previously that, in recent years, have advanced our knowledge of adult hip morphology: imaging, evolution, and genetics. Because OA development is related to morphology variants, we discussed our findings with respect to DHD.
Methods: Search Strategy and Criteria
We performed three searches in Medline, Embase, and Web of Science summarizing the literature on mechanical and genetic factors of hip growth and development. For these searches we formed six groups of search terms and one group with exclusion terms composed by the first three authors in joint discussion. The first group, Group A, referred to terms related to “the hip”, Group B to “growth and development”, Group C to “mechanical factors”, Group D to “genetic factors”, Group E to “DHD”, and Group F referred to “prevalence”. Group G consisted of exclusion terms and was composed of irrelevant title words found during pilot searches (see Appendix).
For each search we combined three groups of terms. For example, to investigate the influence of mechanical factors on hip growth and development, we combined Groups A, B, and C. They were connected using the Boolean operator AND. In addition, Group G was added using the Boolean operator NOT. Terms within a group were combined using the Boolean operator OR. All search terms and group combinations are reported in the Appendix. The search field was “title and abstract” combined with MESH terms when using Medline. The search field for exclusion terms was “title” only. The three searches resulted in three lists of articles for each database. These lists were then searched based on titles and abstracts and had to contain specific reference to mechanical or genetic aspects of hip ontogeny, or imaging and image analysis, or evolution, embryology, or genes. Articles that did not contain any of these subjects were excluded. Also, articles written in languages other than English, German, French, or Dutch were excluded. After selection, further articles were added from reference lists of included articles.
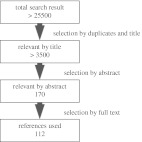
The first search, regarding the effect of mechanical phenomena on growth and development of the proximal femur in both humans and animals, yielded more than 13,500 results. The second search for genes and genetic mechanisms associated with skeletogenesis and the hip resulted in more than 8500 articles. The third search was focused on prevalence of DDH, SCFE, and FAI with regard to different populations, twin studies, and sex. More than 3500 articles were found.
Of the more than 25,500 publications found in total, 25,330 were irrelevant based on duplicates, title, and abstract, leaving 170 publications for evaluation (Fig. 1).
Fig. 1.
Flowchart for evaluation of literature.
Results
Imaging and Quantification of Hip Morphology
Parameters for measuring hip morphology currently relate to concavity, a compound measure determined by femoral head sphericity, relative neck width, and offset (femoral head position relative to the neck) [99]. Previously hip morphology was mostly described without quantifying the sphericity of the femoral head or its relationship to the femoral neck. Goodman et al. [26] introduced the concept of concavity of the femoral head-neck junction in 1997 followed by its quantification with the alpha angle in 2002 [65]. Similar angles characterize concavity in other planes [8, 22, 38, 99]. Concavity is a measure of the potential ROM of the proximal femur in the acetabulum before bony contact occurs and mostly used to describe the cam morphotype of FAI. Acetabular measurements of version and center-edge angle [107] currently quantify the pincer morphotype of FAI [24]. Clearly, imaging for the next decade needs to integrate femoral and acetabular parameters to further our understanding of hip morphology and function.
Methods for image analysis of hip morphology now include statistical shape models (SSMs) [29]. SSMs can be used to compare complex three-dimensional morphology without the need to assume ideal geometry, eg, a spherical femoral head. An SSM of the hip can be built by placing a large number of points (eg, 70) on AP pelvis radiographs on designated locations of the femur, acetabulum, and pelvis [103]. Statistical methods then construct an average hip shape from all radiographic points in all patients, and a computer algorithm (principle component analysis) recombines these into “shape modes,” which constitute the SSM. Each shape mode describes a distinct change in hip morphology, a number of SDs away from the cohort’s average shape. SSMs are used increasingly to describe complex morphology [29, 55, 88], but as yet there are no studies that show how shape modes correlate to FAI morphotypes (cam and pincer).
We found no quantitative imaging studies for any mammal, including humans, of femoral head and head-neck morphology development from embryo to adult. Nevertheless, qualitative comparison of intrauterine and perinatal hip morphology with adult hip morphology shows the relatively unloaded intrauterine femoral head is round in fairly uniform degree but that morphology develops during postnatal locomotor development into more or less spherical femoral heads. Examples can be found for the rabbit [108], cow [90], and primates [2, 4]. Postnatal diversion of hip morphology in itself does not imply it is attributable only to the loading of locomotor development; it can also be the expression of genetic pattern formation [54, 110]. These issues can be further explored in animal models in which the limb muscles are absent or paralyzed in utero.
Mechanical Factors and Embryology
Mechanical factors in early hip morphogenesis can be studied by blocking muscle contraction in experimental embryo models. The mouse embryo is the best studied animal model for mammalian development [68] with many genetic manipulations and molecular tools available, eg, genetically modified mice with altered, reduced, or absent muscles. Another model is the chick embryo paralyzed with a neuromuscular blocking agent [79]. Although long bone development is endochondral in mice, and intramembranous in chicks, reduced or absent mechanical loading affects bone and joint formation in both. Absence of muscle contractions does not alter the first phase in joint development (interzone formation [21]). However, the next phase, joint space development (cavitation), is characterized by changes in gene expression and histology of the developing joint tissues. Gene activation in response to loading has recently been documented in both animal models [7, 41, 42, 67]. In muscle-paralyzed chicks, all bones and synovial joints are affected in the absence of muscle contraction, but in mice, some early bone and joint structures are unaffected [68]. This difference is likely the result of the uterus wall that generates mechanical forces affecting the embryo as opposed to the rigid nongrowing eggshell of birds [68]. Thus, local mechanical stimuli appear to provide positional information, guiding genetic patterning and morphogenesis. Further clues indicate static loading is needed for bone modeling, whereas motion would be mainly involved in joint development [70].
Mechanical Factors and Evolution
Mechanical factors in human hip ontogeny can also be studied by comparing with quadrupedal mammals and the other large apes. The human fetus has a very large head, long legs, and is positioned in an upright mother. In quadrupedal mammals, the abdomen and uterus hang under a horizontal spine like a hammock. Early 20th century authors [13, 48] proposed the uterus wall hyperflexes the human hip, levering the long femur against the prominent anterosuperior iliac spine. This levering is assumed to lower femoral head pressure in the acetabulum, decreasing its relative depth, and to create a torsional moment on the femur increasing anteversion [48]. The large apes, having smaller heads, shorter legs, and a flat ilium without prominent iliac spines, are indeed without hip dysplasia (except one single gorilla [91]). Thus, mechanical factors may adequately explain human neonatal/infant hip dysplasia. Several studies have corroborated these earlier pathomechanical concepts [13, 18], for increasing femoral version [11, 40, 73, 105] and decreasing relative acetabular depth [73, 104]. Furthermore, anteversion increases in rabbits splinted in flexion-external rotation; flexion-internal rotation produces retroversion [108]. Likewise, postnatal femoral detorsion is a consistent finding in normal hip development [69].
Humans walk with approximately 5° hip extension at toe-off while prone extension is approximately 10° to 20° [77, 78]. Active hip flexion is 120°, whereas walking flexion is approximately 35° and running 50° [66]. Thus, the human weightbearing range of hip motion shifts close to its extension limit during bipedal gait development. Quadrupeds bear weight closer to midrange hip flexion [3], and femoral neck anteversion then acts to align the capital growth plate more perpendicular to the vertical gait forces [96]. The human extended hip position diminishes this mechanical advantage anteversion can have on shear forces on the capital physis. Furthermore, the human capital physis, nearly horizontal in neonates, tilts to approximately 30° more vertical in adolescence. Both mechanisms render the capital physis more vulnerable to shear forces.
The large apes walk bipedally but do not run bipedally [20]. Moreover, human growth and development is 5 to 6 years longer compared with chimpanzees, which reach adulthood at 11 to 12 years [84, 95]. Human lower limbs have much longer moment arms than the large apes [82]. Corrected for body weight, peak hip forces in humans are much higher than quadrupeds, increasing further with running or sports [9, 10]. These factors can summate to a load history of enduring high loads on the growing hip.
Thus, species-specific mechanical factors in human hip ontogeny can be interpreted to explain neonatal/infant hip dysplasia, to increase shear forces on the capital physis that may induce SCFE, and to create a load history that may induce morphologic changes in the growing hip.
Genetic Factors, Human Hip Morphogenesis, and Osteoarthritis Development
Genetic factors orchestrate hip morphogenesis. Nonetheless, specific underlying genes have thus far not been identified for the three common DHDs [53]. Genetic architecture for hip morphogenesis is most likely complex and polygenic with modest effect size for individual genes [53], similar perhaps to genetic factors for height [46]. Further indications of the polygenic nature of hip morphogenesis are associations of DDH to other skeletal abnormalities, eg, facial, whether part of an established syndrome [47] or not [32].
Morphology variants such as DDH and FAI are known morphological risk factors for onset of hip OA [45, 94]. Therefore, many researchers used a genetic approach to elucidate underlying OA mechanisms, but only recently have such studies begun to incorporate hip morphology analysis. Genetic methods include genomewide association studies (GWASs), testing many common genetic variants in different individuals for their association with OA [101]) and candidate gene studies that test specific genes, ie, those involved in skeletogenesis (Table 1).
Table 1.
Human genetic studies for association with hip and knee osteoarthritis
| Gene | Study design, population | Protein | Function | Mechanism | Study outcome | Comment |
|---|---|---|---|---|---|---|
| ASPN [81, 86] | Asians | Asporin | Extracellular matrix macromolecule from proteoglycan protein family | Asporin may inhibit TGFß | ASPN is associated with DDH in Chinese | Effects shown in Asian populations |
| DIO2 [56, 103] | Genomewide linkage Europeans Hip OA | Deiodinase 2 (D2) | DIO2 encodes enzyme D2 | D2 regulates availability of active thyroid hormone T3, important for long bone formation | DIO2 relates to both morphotype and cartilotype of the hip | First to relate gene to form to OA; SSM not related to cam/pincer? |
| GDF5 [57, 100] | Candidate gene Asian European |
GDF5 (from TGFß superfamily and related to BMP) | GDF5 is regulator of cell growth and differentiation in both embryonic and adult tissues | GDF5 is involved in bone and joint formation and also expressed in soft tissue joint structures | Decreased GDF5 expression may lead to increased OA susceptibility [57, 76] GDF5 is associated with 17% risk increase of knee OA (OR, 1.17; 95% CI, 1.12–1.23) |
GDF5 insufficient mice develop OA [16] |
| FRZB [5] | White women ≥ 65 years | sFRP3 | Encodes sFRP3, and inhibits WNT signaling in both embryos and adults | WNT signaling in chondrocytes and osteoblasts is important in cartilage and bone homeostasis and during skeletal patterning in embryogenesis | FRZB influences both morphotype and cartilotype of the hip | FRZB knockout mice develop OA [52] Shape Mode 2 relates to lack of head-neck offset? [5] |
| SMAD3 [102] | European | SMAD3 protein, member of TGFß superfamily |
Smad3 is a key intracellular messenger in TGF signaling pathway | TGFß/Smad3 signaling has been shown to be essential for maintaining articular cartilage | Genetic variation in the SMAD3 gene has a role in the risk of large-joint OA OA hip OR, 1.22 OA knee OR, 1.12 |
OA = osteoarthritis; TGF = tumor growth factor; BMP = bone morphogenic protein; DDH = developmental dysplasia of the hip; OR = odds ratio.
Indeed, the majority of best-confirmed OA susceptibility genes appear involved in skeletal morphogenesis and/or cartilage and bone homeostasis (Table 1) [5, 16, 52, 56, 57, 76, 81, 86, 100, 102, 103]. This raises the question whether genetic variants cause subtle skeletal malformations that increase mechanical stress on articular cartilage surfaces, initiating OA [12].
Pollard and coworkers [71] found that siblings of patients with FAI have a higher prevalence of cam morphotype than control subjects (relative risk 2.8). Moreover, siblings had more clinical signs of FAI (eg, positive impingement test) than control subjects with the same FAI morphology (relative risk 2.5). This suggests an additional genetic component, beyond the increased risk of abnormal morphology, may be involved in the development of OA [71].
Using a statistical shape model to analyze hip morphology in sibling pairs, Waarsing and coworkers [103] found high heritability estimates for four hip shape modes, ie, hip morphology was under strong genetic influence. Exploring the association between OA susceptibility genes and hip morphology further, they found carrier status of one OA susceptibility variant in the deiodinase-iodothyronine, type 2 gene was associated with OA but more likely through increasing the cartilage vulnerability to mechanical stress by nonoptimal hip morphology [103]. A similar study found variant alleles of the FRZB genes influence both hip morphology and the relationship between hip morphology and OA [5]. Thus, all three studies that examined the relation between hip morphology, genes and OA, find it is not morphology alone that is associated with OA, but likely a combination with a genetically determined cartilage vulnerability.
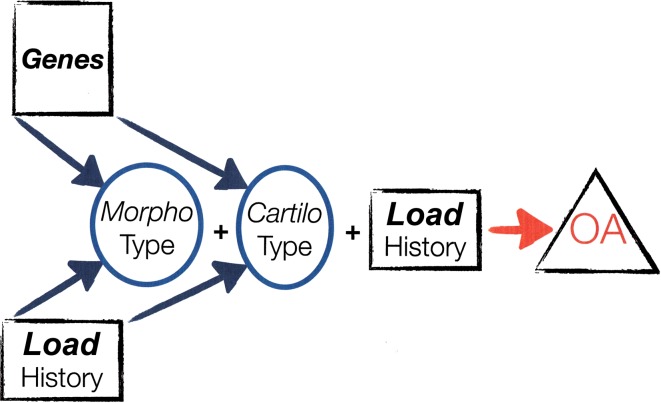
In analogy to morphotype, this cartilage vulnerability or robustness (including subchondral bone) can be conceptualized as cartilotype, ie, the ability of cartilage to withstand mechanical stress. Thus, a hip can have unfavorable morphotype, but favorable cartilotype, and may not develop progressive OA. Conversely, it may have only minor suboptimal morphology, but unfavorable cartilotype, and develop OA. For both scenarios, OA development will be influenced by load history determined by frequency and magnitude of all loads (Fig. 2). This concept may explain prospective studies with 10 to 40 years followup that show a substantial proportion of subjects with an FAI or DDH morphotype does not develop progressive OA [6, 34, 106].
Fig. 2.
Interplay of genes, load history produces morphotype, interplay of morphotype, cartilotype (see text), and load history may or may not lead to OA.
Developmental Dysplasia of the Hip
Compared with life after birth, the hip experiences a more uniform load history in utero. Prevalence differences in healthy single-birth primiparous neonate DDH therefore suggest genetic differences explain the occurrence of DDH rather than in utero positioning. (This may not apply to twin pregnancies in which clear differences in load history may exist when one fetus has its knees extended and the other flexed.)
Indeed, neonate DDH varies widely between ethnic groups, from 0.87 per 1000 live births in Hong Kong Chinese [98] to 10.5 per 1000 in southern Australia [111]. A recent twin study [17] confirms a notion proposed earlier [109] that flexed knees in utero decrease DDH prevalence. Taken together, these findings indicate a strong genetic factor in DDH. Correspondingly, large population studies show a 12-fold increase in risk for first-degree relatives and siblings [92]. Prevalence of neonatal and infant DDH has been reduced considerably by education, screening, and early treatment programs [61].
Slipped Capital Femoris Epiphysis
Incidence of SCFE varies widely between ethnic groups, ie, approximately 4.5 per 100,000 in Polynesian children and 0.1 (girls) to 0.5 (boys) in Korean children [50]. Further genetic influences have been shown in several ethnic groups and within families [50, 51, 63]. Increasing prevalence has been documented repeatedly over the last 20 years in each study related to increasing obesity [58, 63, 89].
The cam, pistol grip, or head tilt morphotype has been interpreted as a subclinical slip of the femoral epiphysis [26, 33, 59], but none of these authors examined the age cohort in which this slip supposedly occurs. Recently, two studies (discussed subsequently) reported cam morphotype prevalence in a total of 256 men aged 9 to 25 years [87] and 12 to 19 years [1] but found no clinical, MRI [87], or radiographic [1] signs of SCFE. Furthermore, Siebenrock and coworkers [87] showed earlier that the direction of tilt of the capital epiphysis in SCFE, posteroinferior, differs from the anterosuperior extension of the physis in cam morphotype.
Femoroacetabular Impingement
FAI morphotypes are unknown as yet in childhood (before the age of 10 years) [69]. This is unknown as yet, because this may also be related to the present lack of hip morphology studies of large populations of children using multiple plane or three-dimensional imaging techniques. We found no longitudinal studies demonstrating development of FAI morphotypes. Neither are there longitudinal studies demonstrating the effect of loading on hip morphology in children or adolescents. However, two cross-sectional studies in adolescents indeed suggest such a relation.
In 1971, Murray and Duncan [60] proposed a relation between athletic activity and hip morphology. However, the characteristics of study and control groups were not adequately defined to allow clear statements about the effect of load history on the hip. What the study did show, however, was high prevalence of cam morphotype (“tilt deformity”) in an adolescent English population, particularly considering that only AP radiographs were used, making underestimation of cam prevalence likely [22].
Forty years later, Siebenrock and coworkers used radial MRI scans to compare cam prevalence in 37 competitive basketball players, aged 9 to 25 years, with 38 weight- and age-matched nonsports control subjects. They found higher alpha angles in the athletes than control subjects (average 60.5° ± 9° and 47.4° ± 4°, respectively). Intriguingly, these differences were more pronounced in players with a closed capital physis, indicating an ongoing effect of load history after physeal closure.
Agricola et al. [1] compared hip morphology in 89 preprofessional soccer players, aged 12 to 19 years, with 92 age-matched control subjects. In this study, sports activity was not systematically documented in the control subjects, and 84% of control subjects were seen initially for hip complaints, although none revisited this hospital during the next 2 years. Cam morphotype prevalence was increased in the soccer players when assessed by shape of the femoral head-neck junction (ie, normal, flat, bump), but not when only radiographic alpha angles > 60° were compared (26 % versus 17% for soccer players and control subjects, respectively).
Thus, high-intensity sports during adolescence may be associated with a higher prevalence of cam morphotype. However, these are findings in white European populations. In other populations, for example Asian populations, cam morphotype prevalence appears to be very low (Table 2) [28, 30, 43, 44, 59, 62, 65, 72, 75, 93, 99]. Accordingly, studies comparing Asian and white populations with identical methods found rounder femoral heads, ie, higher proximal femoral concavity in Asians [23, 36]. Therefore, it appears unlikely it is only sports participation that causes cam morphotype. More likely, genetic factors also influence susceptibility to a given load history.
Table 2.
Prevalence of cam deformity in white male control subjects and Asian control subjects and Asian patients undergoing THA: cam morphotype prevalence varies with ethnicity, age, and imaging parameters
| Study | Population | Age | Imaging | Parameter measured | Percent cam morphotype |
|---|---|---|---|---|---|
| Murray, 1965 [59] | 25 English male control subjects | – | AP pelvic radiographs | “Head tilt deformity” femoral head ratio (FHR) > 1.35 | 10% |
| Nötzli et al., 2002 [65] | 17 Swiss male control subjects with ≥ 20° internal rotation in 90° flexion | 30 ± 5 years | MRI | Alpha angle > 50° at 3:00 position | 0% |
| Gosvig et al., 2010 [28] | 1332 Danish male control subjects population study | 60 ± 13.6 years | AP pelvic radiographs | “Pistol grip deformity” triangular index | 19.6% |
| Reichenbach et al., 2010 [75] | 244 Swiss male control subjects 18-year-old army recruits | 19.9 years | Radial MRI | Decreased offset head-neck junction anterior quadrant (0–3:00) | 24% |
| Hack et al., 2010 [30] | 90 Canadian male control subjects (79% whites) | 29.4 years | Radial MRI | Alpha angle > 55° at 1:30 position | 51.6% |
| Pollard et al., 2010 [72] | 39 English male control subjects without hip signs or symptoms | 47.5 ± 12 years | Crosstable lateral radiographs | Alpha angle > 62° | 2.6% |
| Laborie et al., 2011 [44] | 874 Norwegian male control subjects population study | 18.6 ± 0.6 years | AP pelvic radiographs | Pistol-grip deformity focal femoral neck prominence flattening of lateral femoral head | 21.5% pistol-grip deformity; 10.3% focal neck prominence; 14.4% flattening of femoral head |
| Toogood ≤ 50 years [99] | 140 US (Cleveland) cadaver femora; 50% whites and 50% blacks |
37 years | Photographs | Alpha angle > 55° at 3:00 position | 18.6% |
| Toogood et al., 2009 (> 50 years) [99] | 48 US (Cleveland) cadaver femora; 50% whites and 50% blacks | 63 years | Photographs | Alpha angle > 55° at 3:00 position | 43.8% |
| Kim, 1989 [43] | 172 Korean fetuses; 67 male adult cadavers; 244 male Korean adults hospitalized for other reasons than hip |
14–38 weeks 60.6 years 58.4 years |
Caliper measurement/244 AP pelvic radiographs | Femoral head sphericity (caliper); femoral head sphericity (caliper); femoral head sphericity (Mose ring on radiograph) |
“Spherical femoral heads in fetal and adult cadavers”; spherical femoral heads on radiographs, “no pistol grip” |
| Takeyama et al., 2009 [93] | 158 Japanese male patients undergoing THA | – | Preoperative crosstable lateral radiographs | Alpha angle > 60° | 1.6% |
| Nakahara et al., 2011 [62] | 21 Japanese male control subjects (36 hips) | 72.7 ± 5.7 years | Three-dimensional CT | Alpha angle > 55° at 1:00 position | 25% |
Discussion
In a systematic literature review we examined three fields that may add information to the explanation of hip ontogeny and OA development: imaging, evolution, and genetics. Incorporating advances in these fields in existing mechanical and genetic explanations may further our understanding of hip ontogeny and OA development. Because OA development is related to morphology variants of DHD, we discussed our findings with respect to DDH, SCFE, and FAI.
Where Are We Now?
During the course of our literature review, we identified a number of limitations in approaches and the literature that precludes a better understanding of the relationship of load history and genetics in hip development. First, as a result of the human evolutionary peculiarities, we currently lack an appropriate animal model that mimics human hip ontogeny. Second, to study load histories in hip morphogenesis, we likely need new and precise tools to quantify these loads. Furthermore, evidence shows tissues ignore the large majority of a loading experience, but our understanding about which part of a load history primarily triggers morphological change is limited [14]. Third, experimental data, particularly genetic, are expanding rapidly, but translation to therapeutic studies in humans awaits better phenotyping of patients.
An unresolved issue, but an important one to guide future clinical studies, is whether hip morphology is primarily determined by genetic or mechanical factors. Genetic factors, although evident in DDH, SCFE, and FAI, most likely act through several or numerous genes, each with modest effect sizes. In other words, single genes that explain a DHD have not been identified nor is it likely they will be [53]. Mechanical factors can be understood in view of human evolutionary peculiarities. Starting in utero, and continuing with development of upright gait and slow skeletal maturation, they may summate to load histories conducive to DHD. Conversely, an emerging body of evidence documents an interplay between genetic and mechanical factors in the development of hip morphotype.
Whether a given hip morphotype will lead to progressive OA is, again, influenced by mechanical factors, ie, load history, but appears influenced also by the ability of cartilage to withstand mechanical stress, ie, “cartilotype” (Fig. 2).
Where Do We Need to Go?
We need therapies that can improve both morphotype and cartilotype. In guiding future studies, we can distinguish what can be done using currently available tools and those that likely require development of new tools or technology.
How Do We Get There?
Studies That Can Be Done Using Current Tools
Further identification of genes involved in the complex interactions of morphogenesis and development of OA, ie, morphotype and cartilotype, can be done with existing tools but requires a multidisciplinary approach with GWASs in thousands of cases and control subjects. GWASs can only identify variation in DNA that is relatively common in a population. Newer tools such as exome sequencing can help find causative genes for hip morphotypes. Exome sequencing narrows down the search because it examines only the (1.5%) portion of the genome that is expressed as protein [97], allowing a much larger number of samples to be sequenced. A combination of methods will likely yield the most interesting results.
Imaging for the next decade needs to integrate femoral and acetabular parameters, allow motion simulation, and analysis of large patient populations. The first two depend on improved analysis of CT or MRI and the latter on (semi-)automated analysis (eg, SSM). Currently, most prospective data of hip development and morphology are available only in conventional radiographs. Reconstructing the three-dimensional shape of the pelvis and hip from these two-dimensional images would be highly valuable. SSM-based techniques for this have already been described and await clinical use [83].
A combination of these genetic and imaging data can clarify how (defects of) implicated genes cause the actual disease phenotype. This can lead to more prognostic classification systems that may improve and individualize patient care.
Studies That May Need New Technology/Tools
Meaningful quantification of load history requires new tools for both experimental and clinical measurement but also depends on better understanding of which part of a load history triggers morphological change. Ultimately, integrating information on morphotype, cartilotype, and load history may allow us to better predict the future hip function for an individual patient.
Nonetheless, proof of principle of modifying morphotype is already available in the effectiveness of early treatment programs for infant DHD. Similar programs can be envisaged for FAI, but guiding hip morphogenesis may be a very different and more difficult task in adolescents than infants. Screening for FAI may already be done using a ROM testing apparatus [74].
Proof of principle of modification of cartilotype and its assessment has been given for the knee, in which an exercise program led to improved cartilage quality as assessed by delayed gadolinium-enhanced MRI (dGEMRIC quantifies cartilage glycosaminoglycan [GAG] concentrations; T2 [transverse relaxation time] mapping evaluates cartilage hydration and collagen fiber integrity [39]). This study only examined the effect of exercise on cartilage quality [80], but biochemical or genetic modification of cartilotype may also become therapeutic options.
Appendix: search strategy and criteria
We performed three searches in Medline, Embase, and Web of Science summarizing the literature on mechanical and genetic factors of hip growth and development. For these searches we formed six groups of search terms and one group with exclusion terms composed of the first three authors in joint discussion. The first group, Group A, referred to terms related to “the hip”, Group B to “growth and development”, Group C to “mechanical factors”, Group D to “genetic factors”, Group E to “DHD”, and Group F referred to “prevalence”. Group G consisted of exclusion terms and was composed of selecting irrelevant title words found during pilot searches.
For each search we combined three groups of terms. For example, to investigate the influence of mechanical factors on hip growth and development, we combined Group A, B, and C. They were connected using the Boolean operator AND. In addition, Group G was added using the Boolean operator NOT. Terms within a group were combined with the Boolean operator OR. All used search terms and group combinations are reported in the Appendix. The search field was “title and abstract” combined with MESH terms when using Medline. The search field for exclusion terms was “title” only. The three searches resulted in three lists of articles for each database. These lists were then searched based on titles and abstracts and had to contain specific reference to mechanical or genetic aspects of hip ontogeny, or imaging and image analysis, or evolution, embryology, or genes. Articles that did not contain any of these subjects were excluded. Also, articles written in other languages than English, German, French, or Dutch were excluded. After selection, further articles were added from reference lists of included articles.
The first search, regarding the effect of mechanical phenomena on growth and development of the proximal femur in both humans and animals, yielded over 13,500 results. The second search for genes and genetic mechanisms associated with skeletogenesis and the hip resulted in more than 8500 articles. The third search was focused on the prevalence of DDH, SCFE, and FAI with regard to different populations, twin studies, and sex. Over 3500 articles were found.
Of the more than 25,500 publications found in total, 25,330 were irrelevant based on duplicates, title, and abstract, leaving 170 publications for evaluation (Fig. 2).
|
A Terms related to the hip |
B Terms related to growth and development |
C Terms related to mechanical factors |
D Terms related to genetic factors |
E Terms related to DDH, SCFE, FAI |
F Terms related to prevalence |
G Exclusion terms |
|---|---|---|---|---|---|---|
| hip femur head femoral head femur neck femoral neck femoral torsion proximal femur proximal femoral acetabulum Epiphyses epiphysis growth plate |
development growth ontogeny fetus fetal intrauterine intra-uterine intra uterine prenatal pregnancy trimester antenatal young adolescent child infant children childhood puberty neonatal neonate neonates toddler toddlers schoolchildren schoolchild infants youth evolution |
biomechanics stress sports sporting athletic activity weight-Bearing weight Bearing exercise movement posture load loading gait mechanical running motor activity locomotion pressure stability shape |
genetic genes gene aetiology etiology genetics polymorphism twins twin family gdf5 frzb dio2 calm1 smad3 biological evolution |
impingement FAI dislocation hip dysplasia disease hip slipped epiphysiolysis epiphysiolyses SCFE DDH cam pincer SUFE morphology morphometric |
ethnic prevalence incidence gender population asian asia japanese japan africa european britain french north american north america german germany Spanish spain racial race caucasians caucasian chinese china korea korean united kingdom france |
cerebral palsy prostheses osteotomy fracture fractures arthroplasty replacement implant implants obesity diabetes blood pressure adiposity component components obese metabolic syndrome liver cardiovascular |
List of the terms used in the searches categorized in seven groups. Terms within a group were combined using the Boolean operator OR.
To find publications on the effect of mechanical factors on hip growth and development, we combined search terms A AND B AND C NOT G.
To find publications regarding genetic factors associated with hip growth and development, we combined A AND B AND D NOT G.
To find publications on prevalence of DDH, SCFE, and FAI in different populations, we combined search terms A AND E AND F NOT G.
Footnotes
Each author certifies that he or she, or a member of their immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
References
- 1.Agricola R, Bessems JH, Ginai AZ, Heijboer MP, Heijden RA, Verhaar JA, Weinans H, Waarsing JH. The development of cam-type deformity in adolescent and young male soccer players. Am J Sports Med. 2012;40:1099–1106. doi: 10.1177/0363546512438381. [DOI] [PubMed] [Google Scholar]
- 2.Anemone RL, The VCL. hypothesis revisited: patterns of femoral morphology among quadrupedal and saltatorial prosimian primates. Am J Phys Anthropol. 1990;83:373–393. doi: 10.1002/ajpa.1330830310. [DOI] [PubMed] [Google Scholar]
- 3.Back W, Schamhardt HC, Savelberg HH, Bogert AJ, Bruin G, Hartman W, Barneveld A. How the horse moves: 2. Significance of graphical representations of equine hind limb kinematics. Equine Vet J. 1995;27:39–45. doi: 10.1111/j.2042-3306.1995.tb03030.x. [DOI] [PubMed] [Google Scholar]
- 4.Baker JJ, Searight KJ, Atzeva Stump M, Kehrer MB, Shanafelt C, Graham E, Smith TD. Hip Anatomy and Ontogeny of Lower Limb Musculature in Three Species of Nonhuman Primates. Anatomy Research International, Hindawi Publishing Corporation; 2011. Available at: http://www.hindawi.com/journals/ari/2011/580864/. Accessed July 1, 2012. [DOI] [PMC free article] [PubMed]
- 5.Baker-Lepain JC, Lynch JA, Parimi N, McCulloch CE, Nevitt MC, Corr M, Lane NE. Variant alleles of the WNT antagonist FRZB are determinants of hip shape and modify the relationship between hip shape and osteoarthritis. Arthritis Rheum. 2012;64:1457–1465. doi: 10.1002/art.34526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bardakos NV, Villar RN. Predictors of progression of osteoarthritis in femoroacetabular impingement: a radiological study with a minimum of ten years follow-up. J Bone Joint Surg Br. 2009;91:162–169. doi: 10.1302/0301-620X.91B2.21137. [DOI] [PubMed] [Google Scholar]
- 7.Bastow ER, Lamb KJ, Lewthwaite JC, Osborne AC, Kavanagh E, Wheeler-Jones CP, Pitsillides AA. Selective activation of the MEK-ERK pathway is regulated by mechanical stimuli in forming joints and promotes pericellular matrix formation. J Biol Chem. 2005;280:11749–11758. doi: 10.1074/jbc.M414495200. [DOI] [PubMed] [Google Scholar]
- 8.Beaule PE, Zaragoza E, Motamedi K, Copelan N, Dorey FJ. Three-dimensional computed tomography of the hip in the assessment of femoroacetabular impingement. J Orthop Res. 2005;23:1286–1292. doi: 10.1016/j.orthres.2005.03.011.1100230608. [DOI] [PubMed] [Google Scholar]
- 9.Bergmann G, Graichen F, Rohlmann A. Hip joint loading during walking and running, measured in two patients. J Biomech. 1993;26:969–990. doi: 10.1016/0021-9290(93)90058-M. [DOI] [PubMed] [Google Scholar]
- 10.Bergmann G, Graichen F, Rohlmann A. Hip joint forces in sheep. J Biomech. 1999;32:769–777. doi: 10.1016/S0021-9290(99)00068-8. [DOI] [PubMed] [Google Scholar]
- 11.Bonneau N, Simonis C, Seringe R, Tardieu C. Study of femoral torsion during prenatal growth: interpretations associated with the effects of intrauterine pressure. Am J Phys Anthropol. 2011;145:438–445. doi: 10.1002/ajpa.21521. [DOI] [PubMed] [Google Scholar]
- 12.Bos SD, Slagboom PE, Meulenbelt I. New insights into osteoarthritis: early developmental features of an ageing-related disease. Curr Opin Rheumatol. 2008;20:553–559. doi: 10.1097/BOR.0b013e32830aba48. [DOI] [PubMed] [Google Scholar]
- 13.Browne D. Congenital Deformities of mechanical origin: section for the study of disease in children. Proc Royal Soc Med. 1936;29:1409–1431. doi: 10.1177/003591573602901109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burr DB, Robling AG, Turner CH. Effects of biomechanical stress on bones in animals. Bone. 2002;30:781–786. doi: 10.1016/S8756-3282(02)00707-X. [DOI] [PubMed] [Google Scholar]
- 15.Carter DR, Orr TE, Fyhrie DP, Schurman DJ. Influences of mechanical stress on prenatal and postnatal skeletal development. Clin Orthop Relat Res. 1987;219:237–250. [PubMed] [Google Scholar]
- 16.Daans M, Luyten FP, Lories RJ. GDF5 deficiency in mice is associated with instability-driven joint damage, gait and subchondral bone changes. Ann Rheum Dis. 2011;70:208–213. doi: 10.1136/ard.2010.134619. [DOI] [PubMed] [Google Scholar]
- 17.Pellegrin M, Moharamzadeh D. Developmental dysplasia of the hip in twins: the importance of mechanical factors in the etiology of DDH. J Pediatr Orthop. 2010;30:774–778. doi: 10.1097/BPO.0b013e3181fc35c0. [DOI] [PubMed] [Google Scholar]
- 18.Dickson JW. Pierre Le Damany on congenital dysplasia of the hip. Proc Royal Soc Med. 1969;62:575–577. doi: 10.1177/003591576906200618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doherty M, Courtney P, Doherty S, Jenkins W, Maciewicz RA, Muir K, Zhang W. Nonspherical femoral head shape (pistol grip deformity), neck shaft angle, and risk of hip osteoarthritis: a case-control study. Arthritis Rheum. 2008;58:3172–3182. doi: 10.1002/art.23939. [DOI] [PubMed] [Google Scholar]
- 20.Doran DM. Ontogeny of locomotion in mountain gorillas and chimpanzees. J Hum Evol. 1997;32:323–344. doi: 10.1006/jhev.1996.0095. [DOI] [PubMed] [Google Scholar]
- 21.Drachman DB, Sokoloff L. The role of movement in embryonic joint formation. Dev Biol. 1966;14:401–420. doi: 10.1016/0012-1606(66)90022-4. [DOI] [Google Scholar]
- 22.Dudda M, Albers C, Mamisch TC, Werlen S, Beck M. Do normal radiographs exclude asphericity of the femoral head-neck junction? Clin Orthop Relat Res. 2009;467:651–659. doi: 10.1007/s11999-008-0617-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dudda M, Kim YJ, Zhang Y, Nevitt MC, Xu L, Niu J, Goggins J, Doherty M, Felson DT. Morphologic differences between the hips of Chinese women and white women: could they account for the ethnic difference in the prevalence of hip osteoarthritis? Arthritis Rheum. 2011;63:2992–2999. doi: 10.1002/art.30472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganz R, Leunig M, Leunig-Ganz K, Harris WH. The etiology of osteoarthritis of the hip: an integrated mechanical concept. Clin Orthop Relat Res. 2008;466:264–272. doi: 10.1007/s11999-007-0060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldring MB, Tsuchimochi K, Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97:33–44. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- 26.Goodman DA, Feighan JE, Smith AD, Latimer B, Buly RL, Cooperman DR. Subclinical slipped capital femoral epiphysis. Relationship to osteoarthrosis of the hip. J Bone Joint Surg Am. 1997;79:1489–1497. doi: 10.2106/00004623-199710000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Gosvig KK, Jacobsen S, Palm H, Sonne-Holm S, Magnusson E. A new radiological index for assessing asphericity of the femoral head in cam impingement. J Bone Joint Surg Br. 2007;89:1309–1316. doi: 10.1302/0301-620X.89B10.19405. [DOI] [PubMed] [Google Scholar]
- 28.Gosvig KK, Jacobsen S, Sonne-Holm S, Palm H, Troelsen A. Prevalence of malformations of the hip joint and their relationship to sex, groin pain, and risk of osteoarthritis: a population-based survey. J Bone Joint Surg Am. 2010;92:1162–1169. doi: 10.2106/JBJS.H.01674. [DOI] [PubMed] [Google Scholar]
- 29.Gregory JS, Waarsing JH, Day J, Pols HA, Reijman M, Weinans H, Aspden RM. Early identification of radiographic osteoarthritis of the hip using an active shape model to quantify changes in bone morphometric features: can hip shape tell us anything about the progression of osteoarthritis? Arthritis Rheum. 2007;56:3634–3643. doi: 10.1002/art.22982. [DOI] [PubMed] [Google Scholar]
- 30.Hack K, Primio G, Rakhra K, Beaule PE. Prevalence of cam-type femoroacetabular impingement morphology in asymptomatic volunteers. J Bone Joint Surg Am. 2010;92:2436–2444. doi: 10.2106/JBJS.J.01280. [DOI] [PubMed] [Google Scholar]
- 31.Hadley NA, Brown TD, Weinstein SL. The effects of contact pressure elevations and aseptic necrosis on the long-term outcome of congenital hip dislocation. J Orthop Res. 1990;8:504–513. doi: 10.1002/jor.1100080406. [DOI] [PubMed] [Google Scholar]
- 32.Harila V, Valkama M, Sato K, Tolleson S, Hanis S, Kau CH, Pirttiniemi P. Occlusal asymmetries in children with congenital hip dislocation. Eur J Orthod. 2012;34:307–311. doi: 10.1093/ejo/cjr004. [DOI] [PubMed] [Google Scholar]
- 33.Harris WH. Etiology of osteoarthritis of the hip. Clin Orthop Relat Res. 1986;213:20–33. [PubMed] [Google Scholar]
- 34.Hartofilakidis G, Bardakos NV, Babis GC, Georgiades G. An examination of the association between different morphotypes of femoroacetabular impingement in asymptomatic subjects and the development of osteoarthritis of the hip. J Bone Joint Surg Br. 2011;93:580–586. doi: 10.2106/JBJS.J.00875. [DOI] [PubMed] [Google Scholar]
- 35.Heegaard JH, Beaupre GS, Carter DR. Mechanically modulated cartilage growth may regulate joint surface morphogenesis. J Orthpo Res. 1999;17:509–517. doi: 10.1002/jor.1100170408. [DOI] [PubMed] [Google Scholar]
- 36.Hoaglund FT, Shiba R, Newberg AH, Leung KY. Diseases of the hip. A comparative study of Japanese Oriental and American white patients. J Bone Joint Surg Am. 1985;67:1376–1383. [PubMed] [Google Scholar]
- 37.Inoue K, Wicart P, Kawasaki T, Huang J, Ushiyama T, Hukuda S, Courpied J. Prevalence of hip osteoarthritis and acetabular dysplasia in French and Japanese adults. Rheumatology (Oxford). 2000;39:745–748. doi: 10.1093/rheumatology/39.7.745. [DOI] [PubMed] [Google Scholar]
- 38.Ito K, Minka MA, 2nd, Leunig M, Werlen S, Ganz R. Femoroacetabular impingement and the cam-effect. A MRI-based quantitative anatomical study of the femoral head-neck offset. J Bone Joint Surg Br. 2001;83:171–176. doi: 10.1302/0301-620X.83B2.11092. [DOI] [PubMed] [Google Scholar]
- 39.Jazrawi LM, Alaia MJ, Chang G, Fitzgerald EF, Recht MP. Advances in magnetic resonance imaging of articular cartilage. J Am Acad Orthop Surg. 2011;19:420–429. doi: 10.5435/00124635-201107000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Jouve JL, Glard Y, Garron E, Piercecchi MD, Dutour O, Tardieu C, Bollini G. Anatomical study of the proximal femur in the fetus. J Pediatr Orthop B. 2005;14:105–110. doi: 10.1097/01202412-200503000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Kahn J, Shwartz Y, Blitz E, Krief S, Sharir A, Breitel DA, Rattenbach R, Relaix F, Maire P, Rountree RB, Kingsley DM, Zelzer E. Muscle contraction is necessary to maintain joint progenitor cell fate. Dev Cell. 2009;16:734–743. doi: 10.1016/j.devcel.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 42.Kavanagh E, Church VL, Osborne AC, Lamb KJ, Archer CW, Francis-West PH, Pitsillides AA. Differential regulation of GDF-5 and FGF-2/4 by immobilisation in ovo exposes distinct roles in joint formation. Dev Dyn. 2006;235:826–834. doi: 10.1002/dvdy.20679. [DOI] [PubMed] [Google Scholar]
- 43.Kim YH. Relationship between the sphericity of femoral head-acetabulum and the low incidence of primary osteoarthritis of the hip joint in Koreans. Yonsei Med J. 1989;30:280–287. doi: 10.3349/ymj.1989.30.3.280. [DOI] [PubMed] [Google Scholar]
- 44.Laborie LB, Lehmann TG, Engesaeter IO, Eastwood DM, Engesaeter LB, Rosendahl K. Prevalence of radiographic findings thought to be associated with femoroacetabular impingement in a population-based cohort of 2081 healthy young adults. Radiology. 2011;260:494–502. doi: 10.1148/radiol.11102354. [DOI] [PubMed] [Google Scholar]
- 45.Lane NE, Lin P, Christiansen L, Gore LR, Williams EN, Hochberg MC, Nevitt MC. Association of mild acetabular dysplasia with an increased risk of incident hip osteoarthritis in elderly white women: the study of osteoporotic fractures. Arthritis Rheum. 2000;43:400–404. doi: 10.1002/1529-0131(200002)43:2<400::AID-ANR21>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 46.Lango Allen H, Estrada K, Lettre G, Berndt SI. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature. 2010;467:832–838. [DOI] [PMC free article] [PubMed]
- 47.Larsen LJ, Schottstaedt ER, Bost FC. Multiple congenital dislocations associated with characteristic facial abnormality. J Pediatr. 1950;37:574–581. doi: 10.1016/S0022-3476(50)80268-8. [DOI] [PubMed] [Google Scholar]
- 48.Damany P. [The Congenital Hip Dislocation] [in French] Paris, France: Masson; 1923. [Google Scholar]
- 49.Leunig M, Casillas MM, Hamlet M, Hersche O, Notzli H, Slongo T, Ganz R. Slipped capital femoral epiphysis: early mechanical damage to the acetabular cartilage by a prominent femoral metaphysis. Acta Orthop Scand. 2000;71:370–375. doi: 10.1080/000164700317393367. [DOI] [PubMed] [Google Scholar]
- 50.Loder RT. The demographics of slipped capital femoral epiphysis. An international multicenter study. Clin Orthop Relat Res. 1996;322:8–27. [PubMed] [Google Scholar]
- 51.Loder RT, Nechleba J, Sanders JO, Doyle P. Idiopathic slipped capital femoral epiphysis in Amish children. J Bone Joint Surg Am. 2005;87:543–549. doi: 10.2106/JBJS.D.01773. [DOI] [PubMed] [Google Scholar]
- 52.Lories RJ, Peeters J, Bakker A, Tylzanowski P, Derese I, Schrooten J, Thomas JT, Luyten FP. Articular cartilage and biomechanical properties of the long bones in Frzb-knockout mice. Arthritis Rheum. 2007;56:4095–4103. doi: 10.1002/art.23137. [DOI] [PubMed] [Google Scholar]
- 53.Loughlin J. Genetics of osteoarthritis. Curr Opin Rheumatol. 2011;23:479–483. doi: 10.1097/BOR.0b013e3283493ff0. [DOI] [PubMed] [Google Scholar]
- 54.Lovejoy CO, McCollum MA, Reno PL, Rosenman BA. Developmental biology and human evolution. Annu Rev Anthropol. 2003;32:85–109. doi: 10.1146/annurev.anthro.32.061002.093223. [DOI] [Google Scholar]
- 55.Lynch JA, Parimi N, Chaganti RK, Nevitt MC, Lane NE. The association of proximal femoral shape and incident radiographic hip OA in elderly women. Osteoarthritis Cartilage. 2009;17:1313–1318. doi: 10.1016/j.joca.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meulenbelt I, Min JL, Bos S, Riyazi N, Houwing-Duistermaat JJ, Wijk HJ, Kroon HM, Nakajima M, Ikegawa S, Uitterlinden AG, Meurs JB, Deure WM, Visser TJ, Seymour AB, Lakenberg N, Breggen R, Kremer D, Duijn CM, Kloppenburg M, Loughlin J, Slagboom PE. Identification of DIO2 as a new susceptibility locus for symptomatic osteoarthritis. Hum Mol Genet. 2008;17:1867–1875. doi: 10.1093/hmg/ddn082. [DOI] [PubMed] [Google Scholar]
- 57.Miyamoto Y, Mabuchi A, Shi D, Kubo T, Takatori Y, Saito S, Fujioka M, Sudo A, Uchida A, Yamamoto S, Ozaki K, Takigawa M, Tanaka T, Nakamura Y, Jiang Q, Ikegawa S. A functional polymorphism in the 5’ UTR of GDF5 is associated with susceptibility to osteoarthritis. Nat Genet. 2007;39:529–533. doi: 10.1038/2005. [DOI] [PubMed] [Google Scholar]
- 58.Murray AW, Wilson NI. Changing incidence of slipped capital femoral epiphysis: a relationship with obesity? J Bone Joint Surg Br. 2008;90:92–94. doi: 10.1302/0301-620X.90B1.19502. [DOI] [PubMed] [Google Scholar]
- 59.Murray RO. The aetiology of primary osteoarthritis of the hip. Br J Radiol. 1965;38:810–824. doi: 10.1259/0007-1285-38-455-810. [DOI] [PubMed] [Google Scholar]
- 60.Murray RO, Duncan C. Athletic activity in adolescence as an etiological factor in degenerative hip disease. J Bone Joint Surg Br. 1971;53:406–419. [PubMed] [Google Scholar]
- 61.Myers J, Hadlow S, Lynskey T. The effectiveness of a programme for neonatal hip screening over a period of 40 years: a follow-up of the New Plymouth experience. J Bone Joint Surg Br. 2009;91:245–248. doi: 10.1302/0301-620X.91B2.21300. [DOI] [PubMed] [Google Scholar]
- 62.Nakahara I, Takao M, Sakai T, Nishii T, Yoshikawa H, Sugano N. Gender differences in 3D morphology and bony impingement of human hips. J Orthop Res. 2011;29:333–339. doi: 10.1002/jor.21265. [DOI] [PubMed] [Google Scholar]
- 63.Nguyen AR, Ling J, Gomes B, Antoniou G, Sutherland LM, Cundy PJ. Slipped capital femoral epiphysis: rising rates with obesity and aboriginality in South Australia. J Bone Joint Surg Br. 2011;93:1416–1423. doi: 10.2106/JBJS.K.00077. [DOI] [PubMed] [Google Scholar]
- 64.Nicholls AS, Kiran A, Pollard TC, Hart D, Arden CP, Spector T, Gill HS, Murray DW, Carr AJ, Arden NK. The association between hip morphology parameters and 19-year risk of end-stage osteoarthritis in the hip: a nested case-control study. Arthritis Rheum. 2011. [DOI] [PMC free article] [PubMed]
- 65.Nötzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84:556–560. doi: 10.1302/0301-620X.84B4.12014. [DOI] [PubMed] [Google Scholar]
- 66.Novacheck TF. Walking, running, and sprinting: a three-dimensional analysis of kinematics and kinetics. Instr Course Lect. 1995;44:497–506. [PubMed] [Google Scholar]
- 67.Nowlan NC, Prendergast PJ, Murphy P. Identification of mechanosensitive genes during embryonic bone formation. PLoS Comp Biol. 2008;4:e1000250. doi: 10.1371/journal.pcbi.1000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nowlan NC, Sharpe J, Roddy KA, Prendergast PJ, Murphy P. Mechanobiology of embryonic skeletal development: insights from animal models. Birth Defects Res C Embryo Rev. 2010;90:203–213. doi: 10.1002/bdrc.20184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ogden JA. Development and growth of the hip. In: Katz JF, Siffert RS, editors. Managment of Hip Disorders in Children. Philadelphia, PA, USA: JB Lippincott; 1983. pp. 1–32. [Google Scholar]
- 70.Palacios J, Rodriguez JI, Ruiz A, Sanchez M, Alvarez I, DeMiguel E. Long bone development in extrinsic fetal akinesia: an experimental study in rat fetuses subjected to oligohydramnios. Teratology. 1992;46:79–84. doi: 10.1002/tera.1420460111. [DOI] [PubMed] [Google Scholar]
- 71.Pollard TC, Villar RN, Norton MR, Fern ED, Williams MR, Murray DW, Carr AJ. Genetic influences in the aetiology of femoroacetabular impingement: a sibling study. J Bone Joint Surg Br. 2010;92:209–216. doi: 10.2106/JBJS.I.01200. [DOI] [PubMed] [Google Scholar]
- 72.Pollard TC, Villar RN, Norton MR, Fern ED, Williams MR, Simpson DJ, Murray DW, Carr AJ. Femoroacetabular impingement and classification of the cam deformity: the reference interval in normal hips. Acta Orthop. 2010;81:134–141. doi: 10.3109/17453671003619011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ralis Z, McKibbin B. Changes in shape of the human hip joint during its development and their relation to its stability. J Bone Joint Surg Br. 1973;55:780–785. [PubMed] [Google Scholar]
- 74.Reichenbach S, Juni P, Nuesch E, Frey F, Ganz R, Leunig M. An examination chair to measure internal rotation of the hip in routine settings: a validation study. Osteoarthritis Cartilage. 2010;18:365–371. doi: 10.1016/j.joca.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 75.Reichenbach S, Juni P, Werlen S, Nuesch E, Pfirrmann CW, Trelle S, Odermatt A, Hofstetter W, Ganz R, Leunig M. Prevalence of cam-type deformity on hip magnetic resonance imaging in young males: a cross-sectional study. Arthritis Care Res (Hoboken). 2010;62:1319–1327. doi: 10.1002/acr.20198. [DOI] [PubMed] [Google Scholar]
- 76.Reynard LN, Bui C, Canty-Laird EG, Young DA, Loughlin J. Expression of the osteoarthritis-associated gene GDF5 is modulated epigenetically by DNA methylation. Hum Mol Genet. 2011;20:3450–3460. doi: 10.1093/hmg/ddr253. [DOI] [PubMed] [Google Scholar]
- 77.Roaas A, Andersson GB. Normal range of motion of the hip, knee and ankle joints in male subjects, 30–40 years of age. Acta Orthop Scand. 1982;53:205–208. doi: 10.3109/17453678208992202. [DOI] [PubMed] [Google Scholar]
- 78.Roach KE, Miles TP. Normal hip and knee active range of motion: the relationship to age. Phys Ther. 1991;71:656–665. doi: 10.1093/ptj/71.9.656. [DOI] [PubMed] [Google Scholar]
- 79.Roddy KA, Prendergast PJ, Murphy P. Mechanical influences on morphogenesis of the knee joint revealed through morphological, molecular and computational analysis of immobilised embryos. PloS One. 2011;6:e17526. doi: 10.1371/journal.pone.0017526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roos EM, Dahlberg L. Positive effects of moderate exercise on glycosaminoglycan content in knee cartilage: a four-month, randomized, controlled trial in patients at risk of osteoarthritis. Arthritis Rheum. 2005;52:3507–3514. doi: 10.1002/art.21415. [DOI] [PubMed] [Google Scholar]
- 81.Sakao K, Takahashi KA, Arai Y, Saito M, Honjyo K, Hiraoka N, Kishida T, Mazda O, Imanishi J, Kubo T. Asporin and transforming growth factor-beta gene expression in osteoblasts from subchondral bone and osteophytes in osteoarthritis. J Orthop Sci. o2009;14:738–747. [DOI] [PubMed]
- 82.Schultz AH. The physical distinctions of man. Proc Am Phil Soc. 1950;94:428–449. [Google Scholar]
- 83.Schumann S, Tannast M, Nolte LP, Zheng G. Validation of statistical shape model based reconstruction of the proximal femur—a morphology study. Med Eng Phys. 2010;32:638–644. doi: 10.1016/j.medengphy.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 84.Shea BT. Allometry and heterochrony in the African apes. Am J Phys Anthropol. 1983;62:275–289. doi: 10.1002/ajpa.1330620307. [DOI] [PubMed] [Google Scholar]
- 85.Shefelbine SJ, Carter DR. Mechanobiological predictions of growth front morphology in developmental hip dysplasia. J Orthop Res. 2004;22:346–352. doi: 10.1016/j.orthres.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 86.Shi D, Dai J, Zhu P, Qin J, Zhu L, Zhu H, Zhao B, Qiu X, Xu Z, Chen D, Yi L, Ikegawa S, Jiang Q. Association of the D repeat polymorphism in the ASPN gene with developmental dysplasia of the hip: a case-control study in Han Chinese. Arthritis Res Ther. 2011;13:R27. doi: 10.1186/ar3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Siebenrock KA, Ferner F, Noble PC, Santore RF, Werlen S, Mamisch TC. The cam-type deformity of the proximal femur arises in childhood in response to vigorous sporting activity. Clin Orthop Relat Res. 2011;469:3229–3240. doi: 10.1007/s11999-011-1945-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smyth PP, Taylor CJ, Adams JE. Vertebral shape: automatic measurement with active shape models. Radiology. 1999;211:571–578. doi: 10.1148/radiology.211.2.r99ma40571. [DOI] [PubMed] [Google Scholar]
- 89.Song KS, Oh CW, Lee HJ, Kim SD. Epidemiology and demographics of slipped capital femoral epiphysis in Korea: a multicenter study by the Korean Pediatric Orthopedic Society. J Pediatr Orthop. 2009;29:683–686. doi: 10.1097/BPO.0b013e3181b769d3. [DOI] [PubMed] [Google Scholar]
- 90.Starke A, Herzog K, Sohrt J, Haist V, Hohling A, Baumgartner W, Rehage J. Diagnostic procedures and surgical treatment of craniodorsal coxofemoral luxation in calves. Vet Surg. 2007;36:99–106. doi: 10.1111/j.1532-950X.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 91.Stecher RM. Osteoarthritis of the hip in a gorilla; report of a third case. Clin Orthop Relat Res. 1958;12:307–314. [PubMed] [Google Scholar]
- 92.Stevenson DA, Mineau G, Kerber RA, Viskochil DH, Schaefer C, Roach JW. Familial predisposition to developmental dysplasia of the hip. J Pediatr Orthop. 2009;29:463–466. doi: 10.1097/BPO.0b013e3181aa586b. [DOI] [PubMed] [Google Scholar]
- 93.Takeyama A, Naito M, Shiramizu K, Kiyama T. Prevalence of femoroacetabular impingement in Asian patients with osteoarthritis of the hip. Int Orthop. 2009;33:1229–1232. doi: 10.1007/s00264-009-0742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tannast M, Goricki D, Beck M, Murphy SB, Siebenrock KA. Hip damage occurs at the zone of femoroacetabular impingement. Clin Orthop Relat Res. 2008;466:273–280. doi: 10.1007/s11999-007-0061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tardieu C. Short adolescence in early hominids: infantile and adolescent growth of the human femur. Am J Phys Anthropol. 1998;107:163–178. doi: 10.1002/(SICI)1096-8644(199810)107:2<163::AID-AJPA3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 96.Tayton E. Femoral anteversion: a necessary angle or an evolutionary vestige? J Bone Joint Surg Br. 2007;89:1283–1288. doi: 10.1302/0301-620X.89B10.19435. [DOI] [PubMed] [Google Scholar]
- 97.Teer JK, Mullikin JC. Exome sequencing: the sweet spot before whole genomes. Hum Mol Genet. 2010;19:R145–R151. doi: 10.1093/hmg/ddq333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tong SH, Eid MA, Chow W, To MK. Screening for developmental dysplasia of the hip in Hong Kong. J Orthop Surg (Hong Kong). 2011;19:200–203. doi: 10.1177/230949901101900214. [DOI] [PubMed] [Google Scholar]
- 99.Toogood PA, Skalak A, Cooperman DR. Proximal femoral anatomy in the normal human population. Clin Orthop Relat Res. 2009;467:876–885. doi: 10.1007/s11999-008-0473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Valdes AM, Evangelou E, Kerkhof HJ, Tamm A, Doherty SA, Kisand K, Tamm A, Kerna I, Uitterlinden A, Hofman A, Rivadeneira F, Cooper C, Dennison EM, Zhang W, Muir KR, Ioannidis JP, Wheeler M, Maciewicz RA, Meurs JB, Arden NK, Spector TD, Doherty M. The GDF5 rs143383 polymorphism is associated with osteoarthritis of the knee with genome-wide statistical significance. Ann Rheum Dis. 2011;70:873–875. doi: 10.1136/ard.2010.134155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Valdes AM, Spector TD. The genetic epidemiology of osteoarthritis. Curr Opin Rheumatol. 2010;22:139–143. doi: 10.1097/BOR.0b013e3283367a6e. [DOI] [PubMed] [Google Scholar]
- 102.Valdes AM, Spector TD, Tamm A, Kisand K, Doherty SA, Dennison EM, Mangino M, Tamm A, Kerna I, Hart DJ, Wheeler M, Cooper C, Lories RJ, Arden NK, Doherty M. Genetic variation in the SMAD3 gene is associated with hip and knee osteoarthritis. Arthritis Rheum. 2010;62:2347–2352. doi: 10.1002/art.27530. [DOI] [PubMed] [Google Scholar]
- 103.Waarsing JH, Kloppenburg M, Slagboom PE, Kroon HM, Houwing-Duistermaat JJ, Weinans H, Meulenbelt I. Osteoarthritis susceptibility genes influence the association between hip morphology and osteoarthritis. Arthritis Rheum. 2011;63:1349–1354. doi: 10.1002/art.30288. [DOI] [PubMed] [Google Scholar]
- 104.Walker JM. Histological study of the fetal development of the human acetabulum and labrum: significance in congenital hip disease. Yale J Biol Med. 1981;54:255–263. [PMC free article] [PubMed] [Google Scholar]
- 105.Walker JM, Goldsmith CH. Morphometric study of the fetal development of the human hip joint: significance for congenital hip disease. Yale J Biol Med. 1981;54:411–437. [PMC free article] [PubMed] [Google Scholar]
- 106.Weinstein SL. Natural history of congenital hip dislocation (CDH) and hip dysplasia. Clin Orthop Relat Res. 1987;225:62–76. [PubMed] [Google Scholar]
- 107.Wiberg G. The anatomy and roentgenographic appearance of a normal hip joint. Acta Chir Scand Suppl. 1939;83:7–38. [Google Scholar]
- 108.Wilkinson JA. Femoral anteversion in the rabbit. J Bone Joint Surg Br. 1962;44:386–397. doi: 10.1302/0301-620X.44B2.386. [DOI] [PubMed] [Google Scholar]
- 109.Wilkinson JA. Prime factors in the etiology of congenital dislocation of the hip. J Bone Joint Surg Br. 1963;45:268–283. [Google Scholar]
- 110.Wolpert L. Principles of Development. Oxford, UK: Oxford University Press; 2006. [Google Scholar]
- 111.Yiv BC, Saidin R, Cundy PJ, Tgetgel JD, Aguilar J, McCaul KA, Keane RJ, Chan A, Scott H. Developmental dysplasia of the hip in South Australia in 1991: prevalence and risk factors. J Paediatr Child Health. 1997;33:151–156. doi: 10.1111/j.1440-1754.1997.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 112.Yoshimura N, Campbell L, Hashimoto T, Kinoshita H, Okayasu T, Wilman C, Coggon D, Croft P, Cooper C. Acetabular dysplasia and hip osteoarthritis in Britain and Japan. Br J Rheumatol. 1998;37:1193–1197. doi: 10.1093/rheumatology/37.11.1193. [DOI] [PubMed] [Google Scholar]