Abstract
Background
Confirmation of early long-bone epiphyseal osteonecrosis in pediatric patients with leukemia allows for medical and surgical intervention before articular surface collapse. MRI detects early osteonecrosis, but multiple focused MR images are required to capture all lesions.
Questions/purposes
We determined whether whole-body MRI (WB-MRI) could (1) assist in diagnosing long-bone epiphyseal and other osteonecroses, (2) characterize articular surface involvement, and (3) detect preferential sites for osteonecrosis.
Patients and Methods
We retrospectively reviewed prospectively collected data on all 11 pediatric patients newly diagnosed with leukemia who had musculoskeletal pain develop that persisted 4 weeks or more during leukemia treatment. All were screened for osteonecrosis by WB-MRI, which consisted of a one-time scan of the entire body. Osteonecrosis was defined as circumscribed lesions with a distinct rim of low signal intensity in the normally high-intensity marrow on T1-weighted images and high signal intensity in the normally low-intensity marrow on short-tau inversion recovery images.
Results
WB-MRI confirmed osteonecrosis in nine of 11 patients. All patients had multisite lesions; eight had long-bone epiphyseal lesions, which comprised 66 of 129 (51%) of all lesions. Osteonecrosis involving greater than 50% of the epiphyseal surface was present in 57% of distal femoral and proximal tibial lesions. All humeral and femoral head lesions involved more than 1/3 of the medial surface volume but were asymptomatic. No articular collapse was present. All osteonecrotic lesions were more common in the lower extremities.
Conclusions
WB-MRI confirmed early epiphyseal osteonecrosis, with quantification of articular surface involvement. Lower limbs were preferentially affected, but asymptomatic humeral head osteonecrosis was present in five of nine patients.
Level of Evidence
Level IV, diagnostic study. See Instructions for Authors for a complete description of levels of evidence.
Introduction
During the past four decades, acute lymphoblastic leukemia (ALL) in children has become a curable disease, with 5-year disease free remission rates greater than 90% [22, 23]. With the clinical focus shifting toward minimizing morbidity of treatment while maintaining remission rates, osteonecrosis is progressively recognized as an important source of morbidity in survivors of ALL, with published prevalence rates between 1% and 65% [15, 16, 20]. Osteonecrosis is increasingly linked to systemic corticosteroids in chemotherapy protocols [7], and it has become clear many patients with ALL with osteonecrosis have multisite lesions, of which as much as 55% can be asymptomatic [12] . Large juxtaarticular lesions are at risk for an unfavorable outcome [10], and current literature suggests that articular surface collapse occurs in as many as 22% to 80% of patients who have osteonecrosis of the knee or hip area [11, 13]. From a practical point of view, early diagnosis is critical, as the many interventions designed to prevent joint deterioration or delay total joint arthroplasty [1, 26] are effective only if osteonecrosis is detected before substantial joint collapse occurs. Pharmacologic interventions (modification of corticosteroid type, intravenous bisphosphonates [1]) can take place during corticosteroid treatment, but joint-preserving surgery typically is performed after chemotherapy has been completed [26].
The presence of multisite lesions and the poor correlation between symptoms and osteonecrosis present challenges for early clinical diagnosis. Although histopathology can accurately confirm early and late lesions, this requires invasive procedure(s), especially if multiple lesions are present. Plain radiographs and bone scintigraphy provide limited or no utility in diagnosing early lesions [19]. MRI has emerged as the most sensitive and specific noninvasive assessment for early epiphyseal osteonecrosis, with excellent concordance with histopathology [8].
Some previous studies have used limited MRI dedicated to a specific region, typically to the lower limbs, and these localized MR images have been used to predict the ultimate outcome relating to articular surface collapse around the hips or knees after osteonecrosis [11, 13, 25]. However, from a practical point of view, multiple MR images would be required to capture multisite osteonecrosis. Whole-body MRI (WB-MRI) is an imaging technique that can analyze the entire skeleton with a single diagnostic test [6, 17] and has emerged as the preferred imaging modality for evaluation of patients with suspected osteonecrosis in various disorders [6, 9, 17]. In patients with juvenile systemic lupus erythematosus and juvenile dermatomyositis [4, 5], WB-MRI has confirmed multisite osteonecrosis, exclusively located in the lower limbs. In pediatric patients with hematologic malignancies there is one previous study using WB-MRI, which confirmed osteonecrosis in upper and lower extremities [1].
We therefore determined, in pediatric patients with leukemia who had persistent musculoskeletal pain develop, whether WB-MRI could (1) assist in diagnosis of long-bone epiphyseal and other osteonecroses, (2) quantify articular surface involvement, and (3) detect preferential sites for osteonecrosis, including potential upper limb involvement.
Patients and Methods
We retrospectively reviewed the imaging and medical records of 32 consecutive patients with a new diagnosis of childhood ALL from January 2006 to June 2008 who were assessed for presence of pain during routine visits while receiving therapy for ALL. All 11 of 32 patients (34.4%; six females, five males) who had persistent musculoskeletal pain develop during leukemia therapy who had undergone a routine WB-MRI to screen for potential osteonecrosis as a cause of pain were included in the study (Table 1). Persistent pain was defined as tenderness, aching, or other discomfort in the upper or lower extremities or spine area lasting greater than 4 weeks. Osteonecrotic lesion location and size were determined in a retrospective analysis of WB-MR images. Patients were excluded if they had transient or no pain and therefore did not have WB-MRI performed. Information was extracted from the charts and included leukemia subtype, chemotherapy protocol including type and cumulative corticosteroid dose, timing of osteonecrosis development, and location and severity of musculoskeletal pain. Written informed consent to publish the results was obtained from the patients and/or their parents in accordance with the local ethics committee standards.
Table 1.
Characteristics of patients with osteonecrosis
| Variable | Value |
|---|---|
| Demographic data | |
| Patients with ALL, musculoskeletal pain, and osteonecrosis | 9 |
| Male | 4 (44%) |
| Age at ALL diagnosis* (years) | 5.4 (4.5, 12) |
| Age < 10 years at ALL diagnosis | 5 (55%) |
| Timing of osteonecrosis confirmation | |
| Months from ALL diagnosis† | 12.8 (6.1) |
| Duration of musculoskeletal pain* (months) | 4.9 (3.8, 7.3) |
| Leukemia subtype | |
| Precursor B cell ALL | 7 (78%) |
| T cell ALL | 1 (11%) |
| Other ALL‡ | 1 (11%) |
| Glucocorticoid treatment before osteonecrosis diagnosis | |
| Dexamethasone only | 4 (45%) |
| Prednisone only | 0 |
| Dexamethasone and prednisone | 5 (55%) |
| Location of musculoskeletal pain | |
| Upper extremities | 3 (33%) |
| Lower extremities | 9 (100%) |
| Spine | 1 (11%) |
| Temporary refusal to walk | 3 (33%) |
* Values are expressed as median with 25th and 75th interquartile range in parentheses; †values are expressed as mean, with SD in parentheses; the remaining values are expressed as number of patients, with percentage in parentheses; ‡this patient had received corticosteroids before his leukemia diagnosis, and therefore his ALL was unclassified; ALL = acute lymphocytic leukemia.
We classified the ALL diagnoses according to the subtype (B cell or T cell) and further stratified them into standard-risk and high-risk subsets using the National Cancer Institute (NCI)/Rome criteria [3]. Standard risk was defined as an age of 1 to 9.99 years and a WBC count less than 50,000/μL; high risk was defined as an age of 10 years or older and/or WBC count of 50,000/μL or more. ALL therapy consisted of standard Children’s Oncology Group therapy regimens, including induction (the first month after leukemia diagnosis), consolidation, and maintenance phases. The induction phase includes the most intensive chemotherapy with high doses of corticosteroids (either dexamethasone or prednisone) in addition to other chemotherapeutic agents. The median age at ALL diagnosis for the 11 children was 5.4 years (range, 4.8–11.9 years). The diagnosis of osteonecrosis was made at median 4.9 months (range, 3.1–16.8 months) after leukemia diagnosis. Persistent pain developed most commonly during the maintenance phase of ALL treatment (eight patients), followed by induction (two patients) and consolidation phases (one patient) (Table 2). The median cumulative corticosteroid doses at osteonecrosis diagnosis were 483 mg/m2 dexamethasone (range, 228–718 mg/m2) in the dexamethasone only group and 695 mg/m2 dexamethasone and 486 mg/m2 prednisone in the group receiving both corticosteroids. The overall intensity of the ALL therapy was determined by the subtype of ALL and the NCI risk group assigned. No data were collected on other potential preleukemia risk factors for osteonecrosis. Preceding the WB-MRI, each patient underwent an initial radiograph of the symptomatic site(s).
Table 2.
Frequency and distribution of long-bone epiphyseal osteonecrosis*
| Variable | Value |
|---|---|
| Osteonecrosis affecting epiphyseal regions of the long bones | 8 |
| Number of epiphyseal lesions/patient† | |
| All age groups | 4.66 (2.74) |
| < 10 years | 4.6 (3.20) |
| ≥ 10 years | 4.75 (2.5) |
| Osteonecrosis affecting the lower extremities | |
| Proximal femur | 2 (100%) |
| Distal femur | 8 (100%) |
| Proximal tibia | 7 (86%) |
| Distal tibia | 7 (86%) |
| Osteonecrosis affecting the upper extremities | |
| Proximal humerus | 5 (60%) |
| Distal humerus | 6 (17%) |
* Only the first six calendar months of 2008 were included in the study; †values are expressed as mean, with SD in parentheses; the remaining values are expressed as number of patients, with percentage of bilateral lesions in parentheses.
In this pediatric ALL population, WB-MRI was chosen over multiple focused MR images because multisite osteonecrosis was suspected based on widespread pain as a presenting symptom and a report from the literature [1]. WB-MRI consisted of coronal fast-turbo spin echo T1-weighted images and axial and coronal short-tau inversion recovery (STIR) images of the entire body. General anesthesia was used in children younger than 6 years. The duration of a complete WB-MRI sequence was 45 minutes (including 10–15 minutes for STIR). Osteonecrosis was defined as a circumscribed lesion with a distinct rim of low signal intensity in the normally high-intensity marrow on T1-weighted images (the band sign) and high signal intensity in the normally low-intensity marrow on STIR images [21, 27]. The band sign corresponds to the classic double-line sign of osteonecrosis seen on T2-weighted images [27]. There was no attempt to differentiate between early and chronic osteonecrotic lesions based on signal intensity. Lesions that showed bone edema only without the typical rim for osteonecrosis (giving an appearance of decreased signal intensity with poorly defined margins on T1-weighted imaging) were excluded from the definition of osteonecrosis, as they can be seen with multiple other bone conditions, including bone marrow contusion, ischemia, and microfractures [14, 20, 21]. Rather, such lesions were called patchy according to a previously published definition [21] and were reported separately.
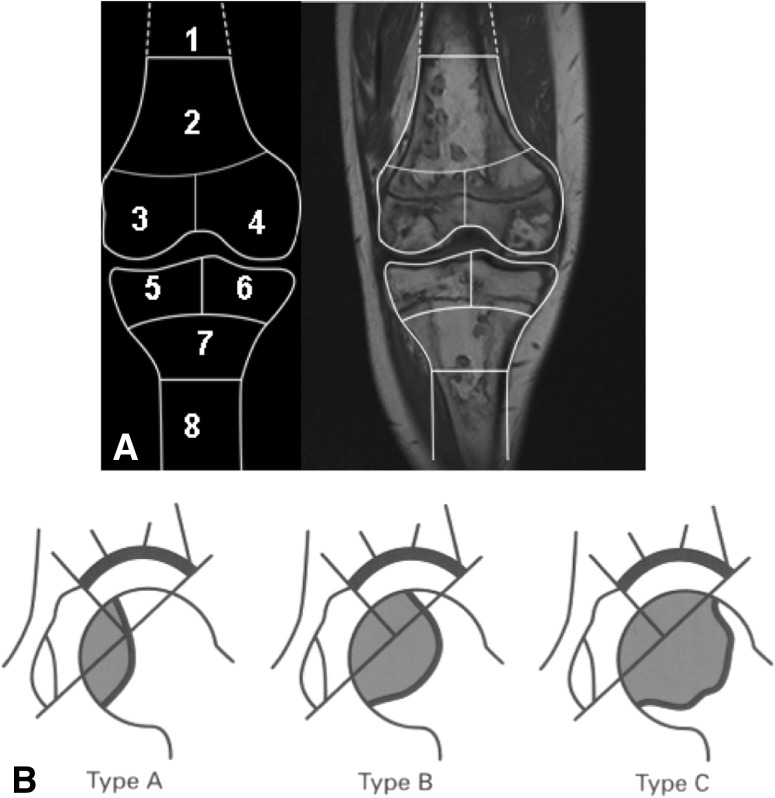
We developed a systematic WB-MRI protocol for assessing osteonecrotic lesions, dividing them into those affecting the epiphyses of the long bones (humerus, radius, ulna, femur, tibia, and fibula) versus those affecting other bone areas. Osteonecrotic lesions in the knee area were classified according to the method of Karimova et al. [12] with detailed analysis of epiphyseal, metaphyseal, and diaphyseal involvement of the distal femur and proximal tibia (Fig. 1A). These epiphyseal lesions were further characterized by (1) the presence or absence of articular surface collapse and (2) size (determined only for lesions extending to articular surface and coded as percentage of articular surface involvement in the osteonecrotic lesion). A similar method was used in the lower extremities to the distal tibia and proximal and distal fibula and in the upper extremities to the distal humerus, distal and proximal radius, and distal and proximal ulna. Osteonecrotic lesions affecting the proximal femur were categorized according to the method of Sugano et al. [25], which included analysis of the osteonecrotic lesion affecting the proportion of the weightbearing volume of the proximal femoral epiphysis (Fig. 1B). This method also was applied to the proximal humeral epiphysis. There was no attempt to measure the distance of osteonecrotic lesion to the articular surface for epiphyseal lesions that did not extend into the articular surface. Growth plates were classified as open versus closed for all affected epiphyseal areas.
Fig. 1A–B.
The classification schemes for osteonecrotic lesions for the knee area and the proximal femoral epiphysis are shown. (A) According to the MRI classification of osteonecrosis of the knee area using the method of Karimova et al. [10], the knee area is divided into eight zones and the presence or absence of an osteonecrotic lesion is recorded in each zone, including the distal femoral diaphysis (1), distal femoral metaphysis (2), medial and lateral distal femoral epiphyses (3 and 4), medial and lateral proximal tibial epiphyses (5 and 6), proximal tibial metaphysis (7), and proximal tibial diaphysis (8). Additional characteristics of lesions located in the epiphyseal zones included what percentage (≤ 25%, 25%–50%, or > 50%) of the articular surface was involved. (Published with permission from The American Journal of Roentgenology and adapted from Karimova EJ, Rai SN, Deng X, Ingle DJ, Ralph AC, Neel MD, Kaste SC. MRI of knee osteonecrosis in children with leukemia and lymphoma. Part 1. Observer agreement. AJR Am J Roentgenol. 2006;186:470–476.) (B) According to the MRI classification of osteonecrosis of the proximal femoral epiphysis using the method described by Sugano et al. [25], Type A lesions occupy the medial 1/3 or less of the weightbearing portion, Type B lesions occupy the medial 2/3 or less, and Type C lesions occupy more than 2/3 based on the central coronal section of the proximal femoral epiphyses on T1-weighted images. (Published with permission from Sugano N, Ohzono K, Masuhara K, Takaoka K, Ono K. Prognostication of osteonecrosis of the femoral head in patients with systemic lupus erythematosus by magnetic resonance imaging. Clin Orthop Relat Res. 1994;305:190–199.)
Descriptive statistics were generated for demographic and clinical characteristics. Numerical variables were summarized using mean and SD or median and interquartile range as appropriate. Categorical variables were summarized as proportions.
Results
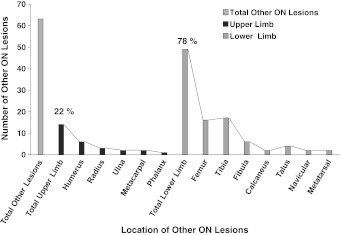
WB-MRI was performed in 11 patients and confirmed multisite osteonecrosis in nine (82%), whereas only one patient had evidence of osteonecrosis observed on radiographs. Long-bone epiphyseal lesions comprised the majority of the bony lesions, with 66 individual lesions at 42 epiphyses, most frequently located at the femur (Table 2). Five patients (56%) had humeral head involvement, of which 80% was bilateral. Although among each epiphyseal area some lesions extended to the articular surface, no articular surface collapse was present. All growth plates were open. WB-MRI confirmed 63 additional osteonecrotic lesions (Fig. 2), most commonly located in the diametaphyseal areas of the same bones that were affected by epiphyseal osteonecrosis. Unexpectedly, 13 of 129 (10%) lesions were in the small bones of the hands and feet (Fig. 2), all in patients who had additional lesions in upper and lower extremities. None of the osteonecrotic lesions in these bones were identified by focal radiographs but were readily identified by WB-MRI and explained the refusal to walk by three of our patients, all younger than 5 years. One patient was undergoing neurologic assessment for apparent unexplained regression of developmental milestones as she had resorted to crawling until WB-MRI revealed the correct diagnosis (Fig. 3). Multiple small patchy lesions observed on T1-weighted imaging were present in the remaining two of 11 patients.
Fig. 2.
A graph shows the frequency and distribution of osteonecrosis (ON) affecting other areas (nonlong-bone epiphyses) in the upper and lower extremities.
Fig. 3A–B.
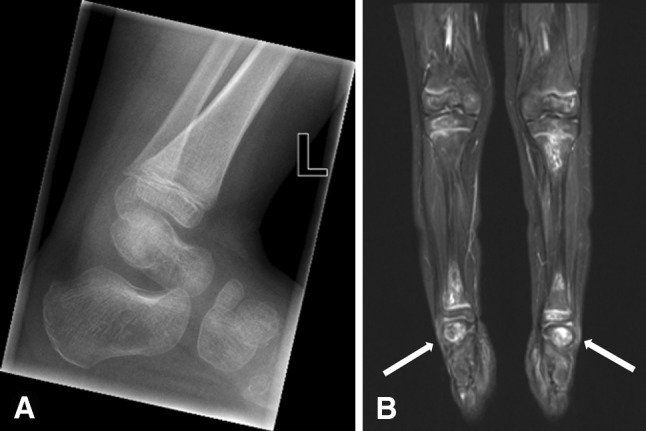
The feet and ankles of a 4-year-old girl with ALL, who transiently refused to walk because of severe foot pain, are shown in these images. (A) The lateral radiograph of her left ankle and hindfoot and midfoot does not show lesions suggestive of osteonecrosis. (B) WB-MRI (shown for below-the-knee lesions) performed on the same day shows multiple osteonecrotic lesions in the tibia and distinct osteonecrotic lesions in both calcanei (arrows).
Large juxtaarticular osteonecrotic lesions were present as follows: osteonecrosis affecting greater than 50% of the epiphyseal surface was present at 57% of distal femoral and proximal tibial lesions and at 46% of the distal tibial lesions. Osteonecrosis involving greater than 1/3 of the medial surface volume of the humeral or femoral head was present at all affected epiphyses (corresponding to Type B lesions [Fig. 1]), and involvement greater than 2/3 of the medial surface volume was present at 44% of the affected proximal humeral epiphyses and at 31% of the affected proximal femoral epiphyses (corresponding to Type C lesions [Fig. 1]).
Although osteonecrosis was present in both upper and lower extremities, all osteonecrotic lesions (epiphyseal and other) were more commonly located in the lower extremities. Epiphyseal osteonecrosis also was more frequently bilateral in the lower extremities than in the upper extremities (Table 2). It was preferentially present in the large bones (femur, tibia, and humerus) and was not detected in the ulna or fibula. No vertebral osteonecrosis was present. Pain complaints were general rather than well localized to specific part of the bone, and, all patients with lower limb osteonecrosis had nonspecific leg pain compared with generalized arm pain in 33% of patients with upper limb osteonecrosis. None of the patients with humeral or femoral head osteonecrosis had localized pain to these regions, despite large juxtaarticular lesions. No patient has required surgical intervention to date but pharmacologic intervention was required by eight (89%) patients, including change from dexamethasone to prednisone (five patients) and use of intravenous bisphosphonates (five patients).
Discussion
Osteonecrosis is a serious condition that can result in skeletal morbidity, even after leukemia is in remission. Its early diagnosis is crucial to allow for medical and surgical interventions designed to prevent or delay additional joint deterioration. In previous clinical studies, focused MR images of the lower limbs have been used, but multiple MR images are required to capture all lesions [11, 12, 20]. We therefore developed a diagnostic approach using WB-MRI to study the entire skeleton with one test. Our aims were to confirm whether WB-MRI could (1) detect epiphyseal and other osteonecrotic lesions, (2) quantify articular surface involvement, and (3) identify potentially preferential sites affected by osteonecrosis in pediatric patients who had persistent musculoskeletal pain develop during leukemia treatment.
We recognize limitations to our study. First, we used only WB-MRI to diagnose osteonecrosis as bone biopsies were believed to be invasive and/or impractical in this pediatric population. However, because we used an MRI definition of osteonecrosis that is characterized by the presence of the classic band sign and has been validated by histology correlation [8], we presume the osteonecrotic lesions in our patients are consistent with those reported in the literature. Similarly, after initiation of our study, other studies have emerged confirming the utility of WB-MRI in detecting osteonecrosis in various pediatric disorders, such as juvenile lupus [4], juvenile dermatomyositis [5], and hematologic malignancies [1]. Second, at the time of study onset, osteonecrosis was considered a rare complication of ALL therapy. Because we evaluated only patients with persistent pain, we may have underestimated the frequency of osteonecrosis in the entire cohort of 32 patients. The minimum frequency of osteonecrosis in the entire ALL cohort during our study period was 28% (nine of 32 patients) in keeping with previous reports [7, 11].
We found that osteonecrosis was widespread (82%) in the patients with leukemia who had persistent pain develop. Moreover, all nine patients had multifocal lesions. All patients had epiphyseal lesions affecting at least the hip, knee, or shoulder area, and interestingly, 10% of all lesions were detected in the small bones of the hands and feet. None of these lesions were seen on radiographs but were readily observed on WB-MRI and explained the refusal to walk in our young patients. Although our numbers are small, they are consistent with the findings by Beer et al., who by WB-MRI reported similar distribution of lesions in pediatric patients with malignancies who had musculoskeletal pain develop [1] (Table 3). However, a few key differences exist between our cohort and the majority of patients in studies reported in the literature (Table 3). The first is that older studies typically involve cohorts that had only focal imaging of symptomatic sites. The second is that as many studies have focused on lower limbs only, the prevalence of upper limb involvement has not been fully appreciated. The lower overall prevalences of osteonecrosis from 2% to 20 % have been reported in studies focusing on symptomatic patients only [2, 18, 24]. Higher prevalences between 24% to 65% have been reported in studies of consecutive patients evaluated by MRI [15, 20, 21]. However, Elmantaser et al. [7] postulated that rather than being simply a reflection of better imaging modalities, there may be a true increase in the prevalence of osteonecrosis secondary to higher dexamethasone use in the newer chemotherapy protocols. On review of the previous literature, the majority of the osteonecrosis lesions are diagnosed in the maintenance phase of chemotherapy, after the intensive induction phase, and an increase in prevalence of osteonecrosis is seen when comparing older with newer cohorts (Table 3).
Table 3.
Comparison of osteonecrosis data in pediatric patients with acute lymphocytic leukemia and hematologic malignancies reported in the literature
| Study | Number of patients | Period analyzed | Age range of patients at primary diagnosis (years) (median) | Symptomatic patients only | Imaging modality | Only lower limbs studied | % Diagnosed with ON in maintenance phase of chemotherapy | % With ON (number) | % With multisite ON (number) | Children older than 10 years predominantly affected |
|---|---|---|---|---|---|---|---|---|---|---|
| Strauss et al. [24] | 176 | 1987–1995 | NR | Yes | NR | No | 45 % | 7 (13) | 77 (10) | Yes |
| Mattano et al. [18] | 1409 | 1989–1995 | NR | Yes | MRI and other | No | 54 % | 8 (111) | 74 (82) | Yes |
| Ojala et al. [20] | 28 | 1992–1996 | 1.6–12.8 (8.3) | No | MRI | No | NR | 32 (9) | 89 (8) | No |
| Ojala et al. [21] | 24 | 1991–1996 | 2.1–15.0 (5.9) | No | MRI | Yes* | NR | 24 (9) | 78 (7) | No |
| Burger et al. [2] | 1951 | 1996–2000 | NR | Yes | NR | Yes | 35% | 2 (31) | 64 (20) | Yes |
| Karimova et al. [12] | 302 | 1994–2003 | 1.0–18.8 (10) | No | MRI | Yes | NR | 56 (168) | 80 (135) | Yes |
| Elmantaser et al. [7] | 186 | 1997–2007 | 1.7–13.6 (5.3) | Yes | MRI | No | NR | 10 (18) | 55 (10) | Yes |
| Kawedia et al. [15] | 365 | NR | NR | No | MRI | Yes | 55% | 65 (238) | NR | Yes |
| Beer et al. [1] | 5 | NR | NR | Yes | WB-MRI | No | NR | 80 (4) | 100% (4) | Yes |
| Current study | 11 | 2006–2008 | 4.8–11.9 (5.4) | Yes | WB-MRI | No | 89% | 82 (9) | 100% (9) | No |
ON = osteonecrosis; NR = not reported, WB-MRI = whole-body MRI; *four of 24 patients also had upper limb MRI, with no osteonecrosis of the humeral head detected.
Our analysis suggests that WB-MRI can confirm in sufficient detail osteonecrosis of epiphyseal and other areas to help predict the eventual skeletal outcome [13]. Nonepiphyseal lesions are expected to resolve in nearly all patients [21] and thus 63 of 129 (48.8%) lesions in our patients would be expected to have good outcomes. In contrast, epiphyseal osteonecrosis has been associated with worse outcomes owing to the risk of subsequent joint deterioration, although the pediatric data are limited to the hip and knee regions. In a retrospective analysis of proximal femoral epiphyseal osteonecrosis, Karimova et al. [11] suggested, if osteonecrosis affected greater than 30% of the femoral head volume, 80% of such hips will collapse within 2 years of diagnosis and 50% will require hip arthroplasty. In our series, all four affected proximal femoral epiphyses met these poor prognostic criteria. A long-term study of osteonecrosis affecting the knee area in 109 pediatric patients reported articular collapse occurred in 22% overall [13]. Risk factors for articular collapse included lesions extending to the articular surface of the distal femoral epiphysis, older age (> 11.5 years), and pain at osteonecrosis diagnosis. In our series, all eight patients with distal femoral lesions were clinically symptomatic, ½ of them were 10 years or older, and all distal femoral lesions extended to the articular surface.
We found that although osteonecrosis was present in upper and lower limbs, the lower limbs were most commonly affected, regardless of the type of osteonecrosis (epiphyseal versus other sites), had more symmetric epiphyseal involvement, and were more commonly painful than the upper limbs. High prevalence of lower limb involvement also was reported in previous studies of childhood leukemia [12, 21], although these findings are limited by the fact that the studies focused primarily on the lower limbs. WB-MRI has allowed a more comprehensive assessment of skeletal involvement and these studies suggest greater involvement of the knee area by osteonecrosis in patients with juvenile systemic lupus erythematosus (JSLE) compared with adult patients with lupus [4]. Castro et al. suggested that pediatric populations could be predisposed to knee trauma and exacerbation of osteonecrosis, because children and adolescents participate in physical activities that are more intense than those in which the adult population participates [4]. We found that humeral head lesions occurred in greater than 50% of our patients (all asymptomatic) in comparison to none in patients with JSLE and juvenile dermatomyositis who also had WB-MRI performed (Table 4). This difference may reflect the unique nature or treatment of pediatric leukemia, as asymptomatic humeral head lesions also were detected in 75% (three of four) of patients with hematologic malignancies using WB-MRI [1], suggesting that shoulder involvement may be more frequent than previously appreciated. Although we used a comprehensive MRI technique with T-1 weighted and STIR images, Castro et al. [5] (Table 4) reported that WB-STIR sequences alone with short imaging times of 10 to 15 minutes compare favorably with joint-specific MRI (15–20 minutes required to assess one joint). WB-STIR may become the ideal imaging modality of choice in the future, especially for younger patients who would benefit from shorter sedation times.
Table 4.
Studies of whole-body MRI and osteonecrosis in pediatric patients with nononcologic disease.
| Study | Disease | Number of patients | Age range of patients (years) (median) | Rationale for WB-MRI | % With ON (number) | % With asymptomatic ON (number) | % With multisite ON (number) | % With upper limb lesions (number) | Findings |
|---|---|---|---|---|---|---|---|---|---|
| Castro et al. [5] | Juvenile dermatomyositis | 1 | 13 | Comparison of whole-body STIR to joint-specific MRI (hips, ankles, knees) | 100 (1) | NR | 100 (1) | 0 (0) | Whole-body STIR detected all ON sites seen in joint-specific MRI and revealed diaphyseal sites that were missed by joint-specific MRI. |
| Castro et al. [4] | Juvenile systemic lupus erythematosus | 40 | 8.8–16.2 (12.6) | To study frequency of ON in consecutive patients | 18 (7) | 86 (6) | 86 (6) | 0 (0) | Knees were most frequently affected (six of seven patients). All patients with ON were older than 10 years. |
WB-MRI = whole-body MRI; ON = osteonecrosis; NR = not reported, STIR = short tau inversion recovery.
In our patients WB-MRI allowed, with one diagnostic test, a comprehensive diagnosis of osteonecrosis of epiphyseal and nonepiphyseal areas. Novel findings from our study include that lower limbs (1) were preferentially affected, (2) had more symmetrically distributed epiphyseal lesions, and (3) were more commonly symptomatic than upper limbs. WB-MRI also captured large humeral head lesions, which were clinically unsuspected and additionally detected clinically important lesions in the small bones of the feet. Our pilot data suggest that persistent pain for more than 4 weeks after starting chemotherapy (during the induction or consolidation period) or new or recurrent pain during the maintenance phase of chemotherapy should lead to screening for osteonecrosis. Moreover, because at least some epiphyseal lesions at risk for articular surface collapse were asymptomatic in our series and in those reported by others [12, 13], we suggest that WB-MRI be studied prospectively to better understand the natural history of osteonecrosis in pediatric patients with leukemia and to allow for early medical or surgical intervention.
Acknowledgments
We thank our colleagues in the Department of Oncology for referring patients to our study and to our colleagues in the Department of Radiology for performing the WB-MRI scans.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Beer M, Stenzel M, GIrschick H, Schlegel PG, Darge K. [Whole-body MR imaging in children with suspected osteonecrosis after intensive chemotherapy: preliminary results] [in German] Rofo. 2008;180:238–245. doi: 10.1055/s-2008-1027185. [DOI] [PubMed] [Google Scholar]
- 2.Burger B, Beier R, Zimmermann M, Beck JD, Reiter A, Schrappe M. Osteonecrosis: a treatment related toxicity in childhood acute lymphoblastic leukemia (ALL): experiences from trial ALL-BFM 95. Pediatr Blood Cancer. 2005;44:220–225. doi: 10.1002/pbc.20244. [DOI] [PubMed] [Google Scholar]
- 3.Carroll WL, Bhojwani D, Min DJ, Raetz E, Relling M, Davies S, Downing JR, Willman CL, Reed JC. Pediatric acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2003;2003:102–131. doi: 10.1182/asheducation-2003.1.102. [DOI] [PubMed] [Google Scholar]
- 4.Castro TC, Lederman H, Terreri MT, Caldana WI, Kaste SC, Hilario MO. The use of joint-specific and whole-body MRI in osteonecrosis: a study in patients with juvenile systemic lupus erythematosus. Br J Radiol. 2011;84:621–628. doi: 10.1259/bjr/34972239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castro TC, Lederman H, Terreri MT, Kaste SC, Hilario MO. Detection of multifocal osteonecrosis in an adolescent with dermatomyositis using whole-body MRI. Pediatr Radiol. 2010;40:1566–1568. doi: 10.1007/s00247-010-1636-4. [DOI] [PubMed] [Google Scholar]
- 6.Darge K, Jaramillo D, Siegel M. Whole-body MRI in children: current status and future applications. Eur J Radiol. 2008;68:289–298. doi: 10.1016/j.ejrad.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 7.Elmantaser M, Stewart G, Young D, Duncan R, Gibson B, Ahmed SF. Skeletal morbidity in children receiving chemotherapy for acute lymphoblastic leukaemia. Arch Dis Child. 2010;95:805–809. doi: 10.1136/adc.2009.172528. [DOI] [PubMed] [Google Scholar]
- 8.Glickstein MF, Burk DL, Jr, Schiebler ML, Cohen EK, Dalinka MK, Steinberg ME, Kressel HY. Avascular necrosis versus other diseases of the hip: sensitivity of MR imaging. Radiology. 1988;169:213–215. doi: 10.1148/radiology.169.1.3420260. [DOI] [PubMed] [Google Scholar]
- 9.Jaremko JL, Macmahon PJ, Torriani M, Merker VL, Mautner VF, Plotkin SR, Bredella MA. Whole-body MRI in neurofibromatosis: incidental findings and prevalence of scoliosis. Skeletal Radiol. 2012;41:917–923. doi: 10.1007/s00256-011-1333-x. [DOI] [PubMed] [Google Scholar]
- 10.Karimova EJ, Rai SN, Deng X, Ingle DJ, Ralph AC, Neel MD, Kaste SC. MRI of knee osteonecrosis in children with leukemia and lymphoma. Part 1. Observer agreement. AJR Am J Roentgenol. 2006;186:470–476. doi: 10.2214/AJR.04.1598. [DOI] [PubMed] [Google Scholar]
- 11.Karimova EJ, Rai SN, Howard SC, Neel M, Britton L, Pui CH, Kaste SC. Femoral head osteonecrosis in pediatric and young adult patients with leukemia or lymphoma. J Clin Oncol. 2007;25:1525–1531. doi: 10.1200/JCO.2006.07.9947. [DOI] [PubMed] [Google Scholar]
- 12.Karimova EJ, Rai SN, Ingle D, Ralph AC, Deng X, Neel MD, Howard SC, Pui CH, Kaste SC. MRI of knee osteonecrosis in children with leukemia and lymphoma. Part 2. Clinical and imaging patterns. AJR Am J Roentgenol. 2006;186:477–482. doi: 10.2214/AJR.04.1597. [DOI] [PubMed] [Google Scholar]
- 13.Karimova EJ, Wozniak A, Wu J, Neel MD, Kaste SC. How does osteonecrosis about the knee progress in young patients with leukemia? A 2- to 7-year study. Clin Orthop Relat Res. 2010;468:2454–2459. doi: 10.1007/s11999-010-1358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaste SC, Karimova EJ, Neel MD. Osteonecrosis in children after therapy for malignancy. AJR Am J Roentgenol. 2011;196:1011–1018. doi: 10.2214/AJR.10.6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawedia JD, Kaste SC, Pei D, Panetta JC, Cai X, Cheng C, Neale G, Howard SC, Evans WE, Pui CH, Relling MV. Pharmacokinetic, pharmacodynamic, and pharmacogenetic determinants of osteonecrosis in children with acute lymphoblastic leukemia. Blood. 2011;117:2340–2347; quiz 2556. [DOI] [PMC free article] [PubMed]
- 16.Korholz D, Bruder M, Engelbrecht V, Ruther W, Gobel U. Aseptic osteonecrosis in children with acute lymphoblastic leukemia. Pediatr Hematol Oncol. 1998;15:307–315. doi: 10.3109/08880019809014014. [DOI] [PubMed] [Google Scholar]
- 17.Ley S, Ley-Zaporozhan J, Schenk JP. Whole-body MRI in the pediatric patient. Eur J Radiol. 2009;70:442–451. doi: 10.1016/j.ejrad.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Mattano LA, Jr, Sather HN, Trigg ME, Nachman JB. Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: a report from the Children’s Cancer Group. J Clin Oncol. 2000;18:3262–3272. doi: 10.1200/JCO.2000.18.18.3262. [DOI] [PubMed] [Google Scholar]
- 19.Mont MA, Ulrich SD, Seyler TM, Smith JM, Marker DR, McGrath MS, Hungerford DS, Jones LC. Bone scanning of limited value for diagnosis of symptomatic oligofocal and multifocal osteonecrosis. J Rheumatol. 2008;5:1629–1634. [PubMed] [Google Scholar]
- 20.Ojala AE, Lanning FP, Paakko E, Lanning BM. Osteonecrosis in children treated for acute lymphoblastic leukemia: a magnetic resonance imaging study after treatment. Med Pediatr Oncol. 1997;29:260–265. doi: 10.1002/(SICI)1096-911X(199710)29:4<260::AID-MPO5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 21.Ojala AE, Paakko E, Lanning FP, Lanning M. Osteonecrosis during the treatment of childhood acute lymphoblastic leukemia: a prospective MRI study. Med Pediatr Oncol. 1999;32:11–17. doi: 10.1002/(SICI)1096-911X(199901)32:1<11::AID-MPO4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 22.Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, Ribeiro RC, Rubnitz JE, Raimondi SC, Onciu M, Coustan-Smith E, Kun LE, Jeha S, Cheng C, Howard SC, Simmons V, Bayles A, Metzger ML, Boyett JM, Leung W, Handgretinger R, Downing JR, Evans WE, Relling MV. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 24.Strauss AJ, Su JT, Dalton VM, Gelber RD, Sallan SE, Silverman LB. Bony morbidity in children treated for acute lymphoblastic leukemia. J Clin Oncol. 2001;19:3066–3072. doi: 10.1200/JCO.2001.19.12.3066. [DOI] [PubMed] [Google Scholar]
- 25.Sugano N, Ohzono K, Masuhara K, Takaoka K, Ono K. Prognostication of osteonecrosis of the femoral head in patients with systemic lupus erythematosus by magnetic resonance imaging. Clin Orthop Relat Res. 1994;305:190–199. doi: 10.1097/00003086-199408000-00023. [DOI] [PubMed] [Google Scholar]
- 26.Werner A, Jager M, Schmitz H, Krauspe R. Joint preserving surgery for osteonecrosis and osteochondral defects after chemotherapy in childhood. Klin Padiatr. 2003;215:332–337. doi: 10.1055/s-2003-45495. [DOI] [PubMed] [Google Scholar]
- 27.Zurlo JV. The double-line sign. Radiology. 1999;212:541–542. doi: 10.1148/radiology.212.2.r99au13541. [DOI] [PubMed] [Google Scholar]