Abstract
Background
Perthes-like hip deformities encompass variable proximal femoral abnormalities and associated acetabular dysplasia that can be reconstructed with contemporary hip preservation procedures. Nevertheless, the necessity and indications for surgical correction of associated acetabular dysplasia have not been established.
Questions/Purposes
We determined whether patient-specific factors (sex, age, BMI, previous surgery, hip pain and function) and/or structural deformity characteristics (radiographic parameters of acetabular morphology) were associated with our indications for acetabular reorientation in surgical reconstruction of Perthes-like hip deformities.
Methods
We compared patient-specific characteristics and radiographic parameters of acetabular morphology in 94 patients (97 hips) with residual Perthes deformities who underwent joint preservation surgery without or with a periacetabular osteotomy (PAO) as part of the reconstruction.
Results
Patient sex, BMI, preoperative Harris hip score, and previous hip surgery were not associated with our indications for a combined femoral and PAO procedure. Radiographic parameters associated with the indication for a PAO included the lateral center-edge angle, anterior center-edge angle, acetabular inclination, and acetabulum-head index. No or mild secondary osteoarthritis and joint congruency were associated with the indication for a PAO as part of the reconstruction.
Conclusions
Contemporary hip preservation surgery for residual Perthes deformities covers a wide spectrum of procedures. We believe a PAO should be considered in the surgical treatment plan for symptomatic patients having radiographic parameters indicating acetabular dysplasia, no or mild secondary osteoarthritis, and adequate joint congruity.
Level of Evidence
Level III, prognostic study. See Instructions for Authors for a complete description of levels of evidence.
Introduction
Perthes-like hip deformities are the result of vascular insult to the proximal femur during skeletal development [6, 14, 21]. Such deformities can be associated with Legg-Calvé-Perthes disease, infection, trauma, and treatment of developmental dysplasia of the hip (DDH) complicated by femoral head vascular insufficiency. The residual Perthes-like skeletal deformity is variable [8, 21] and can encompass a large aspheric femoral head, a short and wide femoral neck, coxa vara, a high greater trochanter, variable femoral version, and osteochondral lesions of the femoral head. The acetabular-sided disease variably includes secondary acetabular dysplasia, acetabular version deformities, and abnormalities of the labrochondral complex. These structural malformations are associated with a pathomechanical environment that can feature femoroacetabular impingement (FAI), structural instability (acetabular dysplasia), or both [8, 21]. In many patients, residual Perthes deformities are associated with hip pain, functional limitations, and secondary osteoarthritis (OA) over time [2, 10, 17]. Therefore, joint preservation hip surgery can be considered for patients with symptomatic Perthes-like deformities without advanced secondary OA.
Skeletally mature adolescents and young adult patients who present with symptomatic Perthes-like deformities are challenging to treat, and there is considerable controversy regarding optimal treatment strategies [21]. Treatment options include nonsurgical measures (NSAIDs, activity modification, physiotherapy) or hip preservation surgical procedures. Surgical procedures that can be considered for this patient population encompass a wide spectrum, including proximal femoral osteotomy, femoral osteochondroplasty, surgical hip dislocation, trochanteric advancement, acetabular osteotomy, and hip arthroscopy. Given the complexity of residual Perthes deformities, the role for hip arthroscopy is limited [4, 15, 18, 23, 26]. Over the past several years, we have utilized various femoral procedures to manage residual Perthes deformities, and most recently, surgical dislocation of the hip has emerged as our primary technique [9]. Surgical dislocation provides wide exposure of the acetabular rim and proximal femur enabling comprehensive treatment of complex hip deformities. This approach also allows uninhibited, dynamic examination of the hip to assess pathomechanics under direct visualization. Secondary acetabular dysplasia can then be addressed with acetabular reorientation if deemed necessary.
Anderson et al. [1] and Shore et al. [25] recently reported on the early results of operations performed with a surgical hip dislocation in patients with Legg-Calvé-Perthes disease. Both studies reported low complication rates and clinical improvement in hip function scores when the surgical dislocation approach was used to address FAI deformities and associated intraarticular abnormalities. In the setting of residual Perthes deformities and associated acetabular dysplasia, a PAO (usually combined with femoral procedures) has also demonstrated improved clinical outcome scores, with a mean improvement of 22 to 24 points in the modified Harris hip score at 1 to 9 years’ followup [3, 8]. Despite these recent reports supporting the concepts of hip preservation surgery, the indications for performing a PAO in residual Perthes deformities remain unclear.
Residual Perthes hip deformities commonly have components of intraarticular and/or extraarticular impingement. Correction of the impingement dimensions of the disease is commonly performed, and most surgeons agree this is a fundamental component of surgical correction for the vast majority of patients. In contrast, the identification and treatment of associated structural instability (due to acetabular dysplasia) are more controversial and remain a topic of continued debate [21]. Unlike DDH, the acetabular dysplasia that is often noted in Perthes disease is a result of secondary acetabular remodeling in response to the large, aspherical femoral head [21, 24]. In DDH, the acetabulum is usually underdeveloped secondary to the lack of a seated femoral head influencing growth. Established parameters of classic acetabular dysplasia do not necessarily apply to residual Perthes hips because of the altered mechanics associated with the large aspheric femoral head. Therefore, it is currently unclear when acetabular reorientation is indicated for reconstructing Perthes-like hip deformities. This is an important clinical issue, as neglected structural instability (acetabular dysplasia) may result in continued symptoms and failure of the hip preservation procedure. On the other hand, acetabular reorientation adds to the magnitude of the surgical procedure and has the potential to produce secondary FAI [20]. Therefore, it is important to determine the factors associated with the indication for an acetabular reorientation in residual Perthes-like hip deformities. Such data could be used to develop guidelines for preoperative and intraoperative surgical decision making in these complex reconstructive procedures.
We therefore determined whether patient-specific factors (sex, age, BMI, previous surgery, hip pain and function) and/or structural deformity characteristics (radiographic parameters of acetabular morphology) were associated with our indications for acetabular reorientation in surgical reconstruction of residual Perthes-like hip deformities.
Patients and Methods
We queried the hip preservation database of one of the authors (JCC) to identify all patients treated surgically for symptomatic residual Perthes deformities of the hip and identified 99 patients (103 hips) treated between 1999 and 2011. All patients had persistent hip pain that was refractory to at least 3 months of nonoperative treatment consisting of activity modification, NSAIDs, and physical therapy. Preoperative radiographs for five patients (six hips) were not available and these patients were excluded from the study. The remaining 97 hips (94 patients) were then divided into two cohorts depending on whether a PAO was performed as part of the hip reconstruction (Table 1). The first study cohort (Group I) included patients treated with isolated femoral procedures (without a PAO) and was comprised of 52 hips in 50 patients. The indications for isolated femoral procedures were (1) intraarticular and/or extraarticular FAI in the absence of structural instability and (2) intraarticular disease. The contraindications were (1) advanced osteoarthritis, (2) severe incongruity, and (3) associated structural instability (acetabular dysplasia). The femoral procedures performed included 45 surgical dislocations, four isolated osteochondroplasties, and four proximal femoral osteotomies (including one combined surgical dislocation with proximal femoral osteotomy). Sixteen hips were in females (31%) and 36 in males (69%). The median age at the time of surgery was 18 years (range, 10–44 years). Twenty-six (50%) of these patients had prior hip surgery. The second cohort (Group II) included patients treated with a PAO as part of the reconstruction and was composed of 45 hips in 44 patients. The indications for PAO were (1) hip flexion of at least 90°, (2) radiographic evidence of acetabular dysplasia, (3) good or excellent congruity with functional views, and (4) dynamic instability when assessed intraoperatively. The contraindications for PAO were (1) hip flexion of less than 90°, (2) severe osteoarthritis, (3) severe incongruity with functional radiographs, and (4) stable joint with intraoperative dynamic examination. Femoral procedures performed in conjunction with the PAOs included 24 surgical dislocations, two isolated osteochondroplasties, and 12 proximal femoral osteotomies. Six hips in the PAO group had no femoral-sided treatment. Twenty-two hips were in females (49%) and 23 in males (51%). The median age at the time of surgery was 20 years (range, 13–45 years). Seventeen (38%) hips had undergone previous surgery, including six (13%) hips with previous pelvic osteotomy. Clinical and radiographic data were collected under a Washington University Institutional Review Board-approved protocol.
Table 1.
Demographics and characteristics for Group I and Group II patients
| Variable | Group I (no PAO) | Group II (PAO) | p value |
|---|---|---|---|
| Number of patients/hips | 50/52 | 44/45 | |
| Sex (number of hips in females) | 16 (30.8%) | 22 (48.9%) | 0.07 |
| Age (years)* | 18.4 (10.5–44.1) | 19.6 (12.6–44.8) | 0.03 |
| Side (number of right hips) | 23 (44.2%) | 21 (46.7%) | 0.81 |
| BMI* | 25.2 (14.8–41.9) | 24.8 (19.1–31.7) | 0.6 |
| Any previous surgery (number of hips) | 22 (42.3%) | 15 (33.3%) | 0.364 |
| Proximal femoral osteotomy | 10 (19.2%) | 8 (17.8%) | |
| Femoral osteochondroplasty | 0 | 3 (6.7%) | |
| Pelvic osteotomy | 8 (15.4%) | 4 (8.9%) | |
| Trochanteric advancement | 2 (1.9%) | 2 (4.4%) | |
| Relative neck lengthening | 0 | 3 (6.7%) | |
| Surgical dislocation | 0 | 4 (8.9%) | |
| Hip arthroscopy | 3 (5.8%) | 1 (2.2%) | |
| Femoral lengthening | 2 (3.8%) | 0 | |
| ORIF femoral neck | 1 (1.9%) | 0 | |
| SCFE pinning | 1 (1.9%) | 0 | |
| I&D | 1 (1.9%) | 0 | |
| Preoperative Harris hip score (points) (n = 43 hips/group) | |||
| Mean | 56.9 (23.1–79.2) | 59.0 (28.6–92.4) | 0.88 |
| Median | 58.3 | 59.4 | 0.88 |
* Values are expressed as median, with range in parentheses; PAO = periacetabular osteotomy; ORIF = open reduction and internal fixation; SCFE = slipped capital femoral epiphysis; I&D = irrigation and débridement.
The femoral procedures performed during the time course of this study varied and evolved with the introduction of the surgical dislocation approach to our practice (Fig. 1). The decision to perform an isolated femoral procedure or to a incorporate a PAO as part of the reconstruction was based on multiple factors, including the patient history (abductor fatigue pain associated with weightbearing), physical examination (hip flexion > 90° and a positive apprehension sign), and preoperative radiographic evaluation indicative of acetabular dysplasia. The decision was also based on the dynamic examination of the hip intraoperatively with subluxation or dislocation of the hip anteriorly with extension/external rotation or posterosuperiorly with flexion/internal rotation both within the functional ROM. Finally, intraoperative fluoroscopic evaluation with demonstration of good or excellent congruity of the hip in a functional position (flexion/abduction/external rotation), which mimicked the osteotomy, was also part of the decision-making process. All patients treated with a surgical dislocation procedure had a dynamic hip examination intraoperatively to assess stability and to assist in our final decision about performing a PAO (Fig. 2).
Fig. 1A–B.
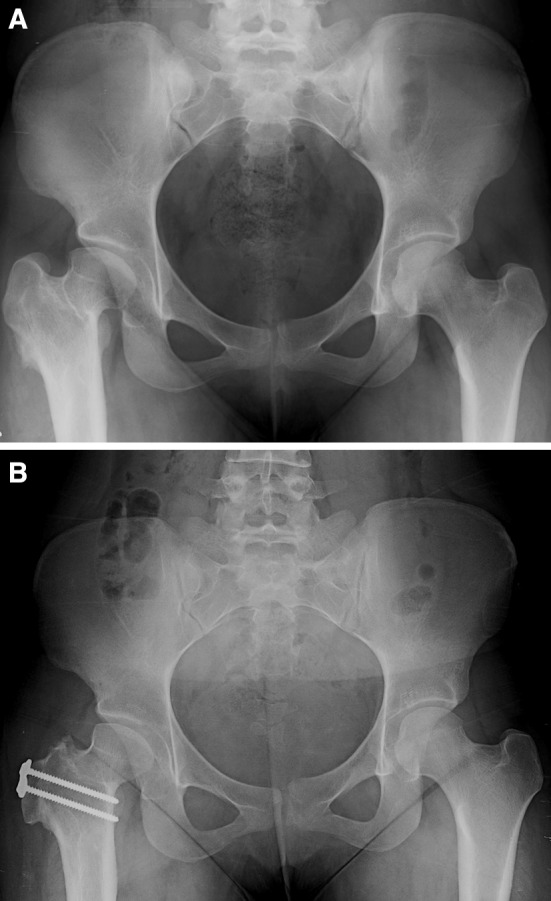
(A) A preoperative radiograph shows the hips of a 17-year-old girl with a history of right hip Perthes-like deformity secondary to childhood sepsis. The patient had a proximal femoral osteotomy at the age of 7 years. The patient was treated with a surgical dislocation, femoral neck lengthening, head-neck osteochondroplasty, and trochanteric advancement. Examination of the hip during the surgical dislocation procedure did not elicit instability, and therefore, a PAO was not performed. (B) The patient has markedly decreased pain and improved function 1 year after surgery.
Fig. 2A–B.
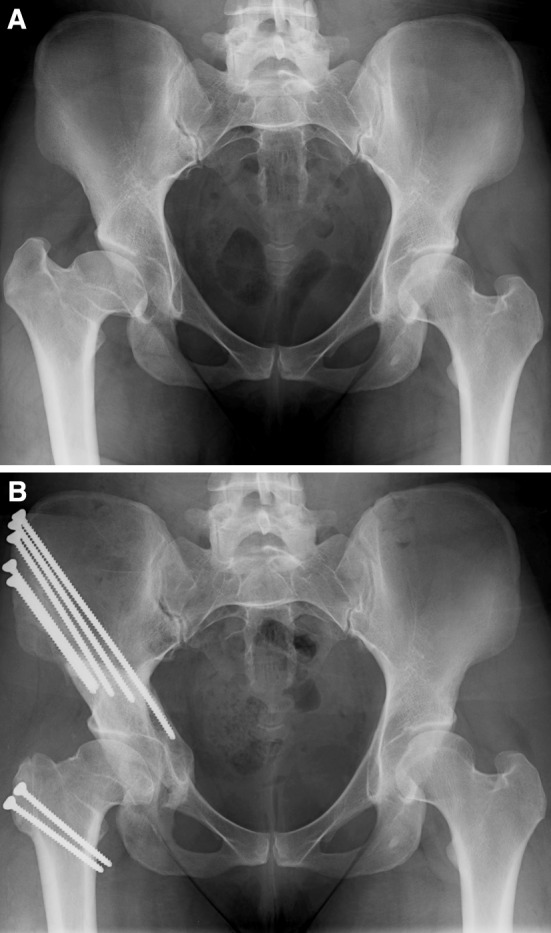
(A) A preoperative radiograph shows the hips of a 29-year-old woman with activity-related right hip pain. She was diagnosed with Legg-Calvé-Perthes disease at the age of 14 years. She was treated with surgical dislocation and osteochondroplasty. After this portion of the procedure, the hip was examined and was thought to be unstable. A PAO was performed during the same surgical setting. (B) Two years after surgery, the patient has excellent results and no restriction of activity.
Patient demographic information and clinical data were obtained from retrospective review of prospectively collected data in our hip preservation database. These data are obtained from self-administered patient questionnaires. Any data points requiring additional investigation (eg, type of previous surgery) were obtained by review of the medical records. These data were extracted and reviewed by three of the coauthors (JRR, JDN, JJN) who were not involved in the clinical and/or surgical care of the patients. Demographic data included sex, BMI, and history of previous surgery. Additionally, preoperative Harris hip scores were recorded as a marker of hip pain and function.
All patients had AP pelvic radiographs and 85% (82 of 97 hips) had false-profile radiographs available to assess acetabular morphology. These radiographs were obtained with standard techniques as previously described [7]. One of the authors (JRR) who was not involved in the clinical care of the patients performed radiographic evaluation. The lateral center-edge angle (LCEA) [3, 28], acetabular inclination (AI) [27], center-trochanteric distance (CTD) [22], acetabulum-head index (AHI) [12], Tönnis OA grade [27], modified Stulberg classification [11], acetabular retroversion (crossover sign) [13], and anterior center-edge angle (ACEA) [16] were recorded. The LCEA was determined by using the AP view according to the technique described by Wiberg [28], with values of less than 20° indicating dysplasia [5]. AI (or Tönnis angle) represents the horizontal orientation of the weightbearing zone (sourcil) of the acetabulum [27], with values of greater than 10° indicating dysplasia. This was measured on the AP pelvic radiograph. The CTD indicates whether the tip of the greater trochanter is above or below the center of the femoral head. It is the vertical distance from the center of the femoral head to the tip of the greater trochanter, along the femoral shaft axis [22]. Given the femoral head deformity in this group of patients, the center was defined as the intersection of the longest and shortest diameters of the femoral head. A negative value indicates the tip of the trochanter is proximal to the center of the femoral head. Values ranging from −1.1 cm to 1.0 cm are considered normal, whereas values of less than −1.7 cm are considered pathologic. Values between −1.2 cm and −1.7 cm are considered controversial [22]. AHI is a measurement used to assess the lateral subluxation of the hip. This was measured according to the technique described by Heyman and Herndon [12]. This is calculated as the ratio of the distance from the medial side of the femoral head to the edge of the acetabulum, over the largest diameter of the femoral head along a parallel line to the first measurement. An AHI of less than 0.80 is considered abnormal [19, 21]. The ACEA was obtained from the false-profile radiograph [16], with values of less than 20° indicating dysplasia. The degree of OA present in each hip was determined using the Tönnis classification system [27] on the AP pelvis radiograph. In this system, grades of OA range from 0 to 3 as follows: Grade 0, no signs of OA; Grade 1, increased sclerosis of the head and acetabulum, slight joint space narrowing, and slight lipping at the joint margins; Grade 2, small cysts in the head or acetabulum or moderate joint space narrowing; and Grade 3, large cysts in the head or acetabulum, joint space obliteration, or severe joint space narrowing, severe deformity of the femoral head, or evidence of necrosis. With strict application of this grading system, loss of femoral head sphericity would result in a Grade 2 or 3 classification for all hips. However, in this subgroup of hips, we excluded this criterion, given all cases by definition have femoral head asphericity. Finally, the modified Stulberg classification system as reported by Herring et al. [11] was used as follows: Stulberg Class I, a normal hip; Stulberg Class II, femoral head sphericity fitting within 2 mm of each other on the AP and frog leg lateral views; Stulberg Class III, aspherical femoral head by more than 2 mm on either view; Stulberg Class IV, at least 1 cm of flattening of the femoral head and acetabulum; and Stulberg Class V, at least 1 cm of flattening of the femoral head and a normal acetabulum. To assess the intraobserver reliability of these radiographic measurements, 10 radiographs were remeasured and showed intraclass correlation coefficients of 0.98 for AI, 0.99 for LCEA, 1.0 for ACEA, 0.99 for CTD, and 0.98 for AHA and kappa values of 1.0 for OA grade and Stulberg class.
Univariate statistical analyses were performed to identify demographic and radiographic parameters associated with the use of PAO. A power analysis showed, to detect a 10% difference in the presence of an abnormal LCEA (with an alpha of 0.05 and power 0.80), at least 50 hips were required. We determined differences in continuous demographic and radiographic parameters between Groups I and II using the Mann-Whitney U test. No assumptions of data normality were made. We determined differences in categorical demographic and radiographic parameters between Groups I and II using the chi-square test. Radiographic parameters were additionally analyzed according to threshold values as described above.
Results
Patient sex, BMI, previous surgery, and hip pain and function were similar in Groups I (no PAO) and II (PAO) (Table 1). Patients in Group II (average, 20 years) were slightly older (p = 0.03) than those treated in Group I (average, 18 years) (Table 1). Several radiographic features occurred more frequently in Group II (Table 2). Patients in Group II had more severe acetabular dysplasia, as evidenced by the differences in LCEA, ACEA, AI, and AHI. We observed no difference in CTD in hips in the two groups. Hips with a crossover sign were less likely to undergo a PAO, compared to hips without a crossover sign (p 0.02), with 62% of hips in Group I having a crossover sign compared to 38% in Group II (Table 3). Hips with an LCEA of less than 20°, an ACEA of less than 20°, an AI of greater than 10°, and/or an AHI of less than 0.8 were all associated with the decision to perform a PAO (Table 3). Combining three parameters of acetabular dysplasia (LCEA < 20°, ACEA < 20°, AI > 10°) for analysis, hips with zero or one finding had PAO in 12% (three of 26), hips with two findings had PAO in 47% (eight of 17), and hips with three findings had PAO in 67% (26 of 39). Of 13 hips with these three findings not undergoing PAO, eight of them had Tönnis Grade 2 or greater or Stulberg Class IV or V. Among all hips, if Tönnis Grade 2 or greater and Stulberg Class IV or V was present, only 21% had PAO (five of 24).
Table 2.
Radiographic parameters of Group I and Group II hips
| Parameter | Group I (no PAO) (n = 52) | Group II (PAO) (n = 45) | p value |
|---|---|---|---|
| LCEA (°) | 25.7 (−6 to 42) | 13.0 (−32 to −39) | < 0.001 |
| ACEA (°) | 22.4 (−28.3 to 53.5) | 7.8 (−46 to 47.5) | < 0.001 |
| AI (°) | 11.8 (−2.6 to 29.2) | 23.4 (−3 to 50) | < 0.001 |
| CTD (cm) | −1.8 (−4.7 to −0.1) | −2.2 (−5.5 to 0.4) | 0.2 |
| AHI | 0.75 (0.2 to 1.0) | 0.62 (0.3 to 0.9) | 0.001 |
Values are expressed as median, with range in parentheses; PAO = periacetabular osteotomy; LCEA = lateral center-edge angle; ACEA = anterior center-edge angle; AI = acetabular inclination; CTD = center-trochanteric distance; AHI = acetabulum-head index.
Table 3.
Radiographic parameter threshold values associated with indication for combined surgical dislocation and PAO
| Parameter | Number of hips | p value | |
|---|---|---|---|
| Group I (no PAO) (n = 52) | Group II (PAO) (n = 45) | ||
| Crossover sign | 32 (61.5%) | 17 (37.8%) | 0.02 |
| Stulberg class | |||
| II | 11 (21.2%) | 14 (31.1%) | |
| III | 14 (26.9%) | 20 (44.4%) | |
| IV–V | 27 (51.9%) | 11 (24.4%) | 0.006 |
| IV | 21 (40.4%) | 8 (17.6%) | |
| V | 6 (11.5%) | 3 (6.7%) | 0.5 |
| Tönnis OA grade | |||
| 0 | 7 (13.5%) | 10 (22.2%) | |
| 1 | 24 (46.2%) | 26 (57.8%) | |
| ≥ 2 | 21 (40.4%) | 9 (20.0%) | 0.03 |
| LCEA < 20° | 22 (42.3%) | 37 (82.2%) | < 0.001 |
| ACEA < 20° | 20 (44.4%) | 29 (78.4%) | 0.002 |
| AI > 10° | 30 (57.7%) | 42 (93.3%) | < 0.001 |
| CTD | |||
| > −1.2 cm | 17 (32.7%) | 10 (22.2%) | |
| −1.7 to −1.2 cm | 9 (17.3%) | 6 (13.3%) | |
| < −1.7 cm | 26 (50.0%) | 29 (64.4%) | 0.152 |
| AHI < 0.8 | 32 (61.5%) | 37 (82.2%) | 0.025 |
PAO = periacetabular osteotomy; OA = osteoarthritis; LCEA = lateral center-edge angle; ACEA = anterior center-edge angle; AI = acetabular inclination; CTD = center-trochanteric distance; AHI = acetabulum-head index.
Discussion
Symptomatic, residual Perthes deformities are complex and challenging to manage with hip preservation surgery. This is due, in large part, to the variability of deformities and the potential for a pathomechanical environment characterized by FAI, structural instability (acetabular dysplasia), or both. Accurate diagnosis of the pathomechanics of a given hip is dependent on multiple factors, including patient history, physical examination, radiographic evaluation, three-dimensional imaging, and intraoperative assessment. Surgical treatment is based on the pathomechanical diagnosis, underscoring the need for careful evaluation. The impingement features of these deformities are usually apparent, yet the clinical importance and need for surgical correction of associated acetabular dysplasia continue to be debated. Therefore, there is a need for information to guide surgeons regarding the importance of acetabular deformity correction. We therefore determined whether there were patient-specific factors (sex, age, BMI, previous surgery, hip pain and function) and/or structural deformity characteristics (radiographic parameters of acetabular morphology) associated with the indication for acetabular reorientation in surgical reconstruction of residual Perthes-like hip deformities.
This study has limitations. First, the decision to perform a PAO was based on several factors, including the preoperative and intraoperative assessment of the hip. This assessment did not follow a strict protocol and therefore could be criticized as subjective. We acknowledge this weakness yet suggest the parameters of hip morphology associated with the indication for a PAO identified in this study provide objective information that should be considered when formulating a plan in treating residual Perthes deformities. Secondly, we analyzed our data in reference to the indication for a PAO as determined by the treating surgeons. We currently do not have adequate clinical followup data on these patients to investigate the efficacy of our surgical procedures and decision-making process. Future studies will be performed to further analyze the clinical outcomes of these distinct patient cohorts. Thirdly, we realize Perthes-like hip deformities represent a wide spectrum of hip pathomorphologies that may vary due to demographic, geographic, and previous treatment variables. The deformity patterns we encounter may be different when compared to other institutions and/or surgeons. This should be considered when interpreting our results as they may not be generalizable to all patient cohorts with Perthes deformities. Additionally, we identified demographic and radiographic parameters associated with the use of PAO with univariate statistical analyses. Multivariate statistical analyses to identify confounding parameters were not performed.
Our current treatment for residual Perthes deformities most commonly includes a first-stage surgical hip dislocation. The hip is dynamically assessed to characterize the pathomechanics (FAI and/or instability), and the intraarticular disease is evaluated. This is performed with direct visualization within the functional ROM. This includes flexion with full arc of motion, as well as the hip in 10° of extension with 10° of external rotation, flexion to 45° with 10° of adduction and internal rotation, and finally flexion to 90° with 10° of internal rotation. We then use a variety of techniques to address the acetabular rim and proximal femur (Fig. 1). On the acetabular side, these include acetabular rim trimming, labral refixation/partial resection, and articular cartilage repair techniques. The femoral disease can be addressed with osteochondral lesion fixation or grafting, osteochondroplasty, relative neck lengthening, trochanteric osteotomy, and occasionally proximal femoral osteotomy. After these components of the procedure are completed, the hip stability is assessed again. If the radiographic parameters suggest instability (LCEA < 20°, ACEA < 20°, AI > 10°, AHI < 0.8), adequate ROM is present (at least 90° of flexion and 20° of abduction), and the hip is adequately congruent with fluoroscopic functional views, we proceed with a PAO at the same surgical setting (Fig. 2). We have found this sequence of evaluating and treating residual Perthes deformities to provide comprehensive surgical correction and improved hip mechanics. Future studies will focus on collecting and analyzing the clinical results of this surgical strategy and better defining the clinical outcomes of Perthes deformities treated with and without acetabular reorientation.
Acknowledgments
We thank Debbie Long for assistance with the preparation of this manuscript.
Footnotes
The institution of one or more of the authors (JJN) has received, during the study period, funding from Arthrex Hip Anatomical Study. The institution of one or more of the authors (JCC) has received, during the study period, research support from Zimmer, Inc (Warsaw, IN, USA) and the Curing Hip Disease Fund (St Louis, MO, USA). One of the authors (JCC) certifies that he, or a member of his immediate family, has received or may receive payments or benefits, during the study period, an amount less than $10,000, from Biomet Inc (Warsaw, IN, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Anderson LA, Erickson JA, Severson EP, Peters CL. Sequelae of Perthes disease: treatment with surgical hip dislocation and relative femoral neck lengthening. J Pediatr Orthop. 2010;30:758–766. doi: 10.1097/BPO.0b013e3181fcbaaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aronson J. Osteoarthritis of the young adult hip: etiology and treatment. Instr Course Lect. 1986;35:119–128. [PubMed] [Google Scholar]
- 3.Beck M, Mast JW. The periacetabular osteotomy in Legg-Perthes-like deformities. Semin Arthroplasty. 1997;8:102–107. [Google Scholar]
- 4.Bowen JR, Kumar VP, Joyce JJ, 3rd, Bowen JC. Osteochondritis dissecans following Perthes’ disease: arthroscopic-operative treatment. Clin Orthop Relat Res. 1986;209:49–56. [PubMed] [Google Scholar]
- 5.Byrd JW, Jones KS. Hip arthroscopy in the presence of dysplasia. Arthroscopy. 2003;19:1055–1060. doi: 10.1016/j.arthro.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Catterall A, Pringle J, Byers PD, Fulford GE, Kemp HB, Dolman CL, Bell HM, McKibbin B, Ralis Z, Jensen OM, Lauritzen J, Ponseti IV, Ogden J. A review of the morphology of Perthes’ disease. J Bone Joint Surg Br. 1982;64:269–275. doi: 10.1302/0301-620X.64B3.6807991. [DOI] [PubMed] [Google Scholar]
- 7.Clohisy JC, Carlisle JC, Beaule PE, Kim YJ, Trousdale RT, Sierra RJ, Leunig M, Schoenecker PL, Millis MB. A systematic approach to the plain radiographic evaluation of the young adult hip. J Bone Joint Surg Am. 2008;90(suppl 4):47–66. doi: 10.2106/JBJS.H.00756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clohisy JC, Nunley RM, Curry MC, Schoenecker PL. Periacetabular osteotomy for the treatment of acetabular dysplasia associated with major aspherical femoral head deformities. J Bone Joint Surg Am. 2007;89:1417–1423. doi: 10.2106/JBJS.F.00493. [DOI] [PubMed] [Google Scholar]
- 9.Ganz R, Gill TJ, Gautier E, Ganz K, Krugel N, Berlemann U. Surgical dislocation of the adult hip: a technique with full access to the femoral head and acetabulum without the risk of avascular necrosis. J Bone Joint Surg Br. 2001;83:1119–1124. doi: 10.1302/0301-620X.83B8.11964. [DOI] [PubMed] [Google Scholar]
- 10.Harris WH. Etiology of osteoarthritis of the hip. Clin Orthop Relat Res. 1986;213:20–33. [PubMed] [Google Scholar]
- 11.Herring JA, Kim HT, Browne R. Legg-Calvé-Perthes disease. Part I. Classification of radiographs with use of the modified lateral pillar and Stulberg classifications. J Bone Joint Surg Am. 2004;86:2103–2120. [PubMed] [Google Scholar]
- 12.Heyman CH, Herndon CH. Legg-Perthes disease; a method for the measurement of the roentgenographic result. J Bone Joint Surg Am. 1950;32:767–778. [PubMed] [Google Scholar]
- 13.Jamali AA, Mladenov K, Meyer DC, Martinez A, Beck M, Ganz R, Leunig M. Anteroposterior pelvic radiographs to assess acetabular retroversion: high validity of the “cross-over-sign”. J Orthop Res. 2007;25:758–765. doi: 10.1002/jor.20380. [DOI] [PubMed] [Google Scholar]
- 14.Jonsater S. Coxa plana; a histo-pathologic and arthrografic study. Acta Orthop Scand Suppl. 1953;12:5–98. [PubMed] [Google Scholar]
- 15.Kuklo TR, Mackenzie WG, Keeler KA. Hip arthroscopy in Legg-Calvé-Perthes disease. Arthroscopy. 1999;15:88–92. doi: 10.1053/ar.1999.v15.0150081. [DOI] [PubMed] [Google Scholar]
- 16.Lequesne M, Seze S. [False profile of the pelvis: a new radiographic incidence for the study of the hip. Its use in dysplasias and different coxopathies] [in French] Rev Rhum Mal Osteoartic. 1961;28:643–652. [PubMed] [Google Scholar]
- 17.McAndrew MP, Weinstein SL. A long-term follow-up of Legg-Calvé-Perthes disease. J Bone Joint Surg Am. 1984;66:860–869. doi: 10.2106/00004623-198466060-00006. [DOI] [PubMed] [Google Scholar]
- 18.Medlock V, Rathjen KE, Montgomery JB. Hip arthroscopy for the late sequelae of Perthes disease. Arthroscopy. 1999;15:552–553. [Google Scholar]
- 19.Mose K, Hjorth L, Ulfeldt M, Christensen ER, Jensen A. Legg Calvé Perthes disease: the late occurence of coxarthrosis. Acta Orthop Scand Suppl. 1977;169:1–39. [PubMed] [Google Scholar]
- 20.Myers SR, Eijer H, Ganz R. Anterior femoroacetabular impingement after periacetabular osteotomy. Clin Orthop Relat Res. 1999;363:93–99. doi: 10.1097/00003086-199906000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Novais EN, Clohisy J, Siebenrock K, Podeszwa D, Sucato D, Kim YJ. Treatment of the symptomatic healed Perthes hip. Orthop Clin North Am. 2011;42:401–417. doi: 10.1016/j.ocl.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Omeroglu H, Ucar DH, Tumer Y. [A new measurement method for the radiographic assessment of the proximal femur: the center-trochanter distance] [in Turkish] Acta Orthop Traumatol Turc. 2004;38:261–264. [PubMed] [Google Scholar]
- 23.Roy DR. Arthroscopic findings of the hip in new onset hip pain in adolescents with previous Legg-Calvé-Perthes disease. J Pediatr Orthop B. 2005;14:151–155. doi: 10.1097/01202412-200505000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Sankar WN, Flynn JM. The development of acetabular retroversion in children with Legg-Calvé-Perthes disease. J Pediatr Orthop. 2008;28:440–443. doi: 10.1097/BPO.0b013e318168d97e. [DOI] [PubMed] [Google Scholar]
- 25.Shore BJ, Novais EN, Millis MB, Kim YJ. Low Early Failure Rates Using a Surgical Dislocation Approach in Healed Legg-Calve-Perthes Disease. Clin Orthop Relat Res. 2011 November 29 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 26.Suzuki A, Kasahara Y, Seto Y, Futami T, Furukawa K, Nishino Y. Arthroscopy in 10 children with perthes disease: pathologic changes of the synovium and the joint surface. Acta Orthop Scand. 2004;66:581–584. doi: 10.3109/17453679408994608. [DOI] [PubMed] [Google Scholar]
- 27.Tönnis D. Congenital Dysplasia and Dislocation of the Hip in Children and Adults. Berlin, Germany: Springer; 1987. [Google Scholar]
- 28.Wiberg G. Studies on dysplastic acetabula and congenital subluxation of the hip joint: with specific reference to the complication of osteoarthritis. Acta Chir Scand. 1939;83(suppl 58):5–135. [Google Scholar]


