Abstract
Background
After resecting tumors confined to one femoral condyle, a unicondylar osteoarticular allograft can be used for reconstruction without sacrificing the uninvolved condyle. However, unicondylar osteoarticular allografts have been associated with a high rate of joint degeneration. We describe a unicondylar osteoallograft prosthesis composite reconstruction replacing only one side of the joint to reduce compartment degeneration and avoid contamination of the tibia, but the survival, function, and complications of a unicondylar osteoallograft prosthesis composite are unclear.
Description of Technique
We located a bone resection plane intraoperatively as planned before surgery using a computer-assisted navigation system. The tumor then was removed en bloc and the unicondylar defect filled with a size-matched allogeneic unicondyle. The allograft cartilage was removed. Thereafter, the condyle of the femoral component was resurfaced with a unicompartmental knee prosthesis to form a unicondylar osteoallograft prosthesis composite, however the tibia was left undisturbed. Navigation allowed precise apposition between the unicondylar osteoallograft prosthesis composite and host bone to ensure mechanical alignment and congruency of the joint surface before fixation with a plate.
Methods
We retrospectively reviewed 12 patients who underwent unicondylar osteoallograft prosthesis composite reconstructions after unicondylar resection for tumors. One patient died from tumor-related causes without unicondylar osteoallograft prosthesis composite failure after 18 months. We observed the survival rate of unicondylar osteoallograft prosthesis composite reconstruction and related complications. Function and radiographs also were documented according to the Musculoskeletal Tumor Society (MSTS) functional scoring system and the International Society of Limb Salvage radiographic scoring system. The minimum followup was 8 months (median, 37 months; range, 8–65 months).
Results
At last followup, 10 of the 12 unicondylar osteoallograft prosthesis composite reconstructions were still in place. Three reconstructions failed owing to two local recurrences (both treated with amputation) and there was one infection (treated with revision and maintenance of the implant at last followup). The average MSTS functional score at last followup was 27 points and the radiographic score 91%.
Conclusions
Our observations suggest unicondylar osteoallograft prosthesis composite reconstruction might be a reliable technique with relatively few major complications and at least short-term maintenance of the tibial cartilage.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
It is challenging for orthopaedic surgeons to reconstruct massive unicondylar osteoarticular defects in the distal femur after resection of aggressive or recurrent benign bone tumors and malignant bone sarcomas [2, 6, 12]. Owing to requirements for stability, motion, and function, reconstructing this joint has been demanding. Available options have included endoprostheses [3, 4, 10], bicondylar osteoarticular allografts [12, 23], and unicondylar osteoarticular allografts [2, 17, 18]. Endoprostheses can provide immediate fixation and rapid return to weightbearing, but they are associated with major complications including aseptic loosening (6%–84% at 5 to 10 years), infection (7%–41%), and periprosthetic fracture (5%–15%) [13, 14]. Compared with endoprosthetic replacement, bicondylar osteoarticular allografts have provided a more biologic reconstruction and could restore the articular surface. Moreover, they provide a site to reattach the surrounding ligaments and soft tissues [19, 20]. However, these two reconstructions require sacrificing the uninvolved condyle and have been associated with major complications rates including infection (10%–23%), allograft fracture (17%–31%), nonunion (17%–20%), and cartilage degeneration (25%–31%) [21–23].
As an alternative, a unicondylar osteoarticular allograft might be a reasonable option for tumors confined to one condyle. Such a reconstruction might reduce the risk of complications associated with endoprosthetic and bicondylar osteoarticular allograft reconstruction while preserving bone stock, which presumably would allow for easier salvage procedures with conventional total knee prostheses in cases of allograft subchondral collapse. In a study of 40 unicondylar osteoarticular allograft reconstructions, Musculo et al. reported an overall survival rate at 5 and 10 years was 85% when the bicondylar osteoarticular allograft was used to reconstruct either the distal femoral or proximal tibial condyle [17]. The mean radiographic score for the 33 surviving unicondylar osteoarticular allografts was 89%, with an average functional score of 27 points. Nevertheless, 39% (13) of the remaining unicondylar osteoarticular allografts had some articular deterioration, 18% (six) had joint narrowing of 2 mm, 9% (three) had joint narrowing of 4 mm, and 12% (four) had some form of subchondral bone collapse [17]. Bianchi et al. [2] reported two of 10 unicondylar osteoarticular allografts had excellent function, five had good, and three had fair function with a minimum followup of 4 years (average, 11 years) according to the Musculoskeletal Tumor Society score (MSTS) described by Enneking et al. [8]. Radiographically, five patients had mild and five had severe degenerative changes [2]. The dead chondrocytes in a frozen unicondylar osteoarticular allograft were likely related to degeneration. Moreover, the size of the unicondylar osteoarticular allograft could not be matched exactly, which caused malalignment and increased the speed of the degenerative process. Other factors such as processing (cytoprotective agents and technique for rate of slow freezing) and stability of the ligamentous repair could lead to cartilage degeneration. To reduce the degenerative changes of the graft compartment, the unicondylar osteoallograft prosthesis composite was implanted in this study. Computer-assisted navigation surgery also was used for precise tumor resection and planned reconstruction.
We then determined (1) the survival rate of the unicondylar osteoallograft prosthesis composite and limb salvage, (2) complications associated with the procedure, and (3) functional outcomes and radiographic scores of patients treated with a unicondylar osteoallograft prosthesis composite.
Surgical Technique
We performed unicondylar osteoallograft prosthesis composite reconstruction based on aggressive benign tumors or malignant tumors confined to one femoral condyle, as determined by imaging modalities. The specific indications for unicondylar osteoallograft prosthesis composite reconstruction were: (1) unicondylar osteoarticular defects after tumor resection, and (2) young active patients with a long life expectancy. The contraindications were: (1) a major neurovascular bundle was involved in the tumor, (2) a tumor affected both femoral condyles, and (3) inability to obtain a host-soft tissue reconstruction for adequate stability. For giant cell tumors, the resection margins were at least marginal, whereas wide margins were achieved for all malignant tumors. After an appropriate resection with a safe margin, the unaffected condyle remained intact. We did not use curettage for giant cell tumors because the involved condyle had a pathologic fracture or collapse of the articular surface after the intralesional curettage.
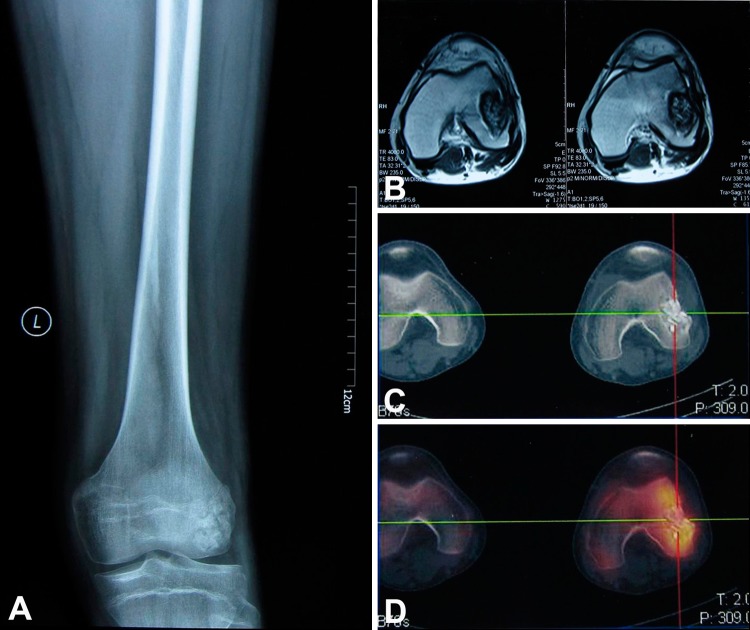
Preoperative radiographs, CT scans, bone scans, and MRI of each patient were obtained (Fig. 1).The DICOM images from the CT, bone scan, and MRI were integrated in the navigation system. As different image datasets shared identical spatial coordinates after image fusion, a three-dimensional (3-D) bone tumor model was generated as reported by Wong et al. [24]. All reconstructed two-dimensional and 3-D images were used for preoperative surgical planning. The plane of the tumor resection was defined and marked using multiple virtual screws placed along the margin of the planned resection. A margin that was 2 cm or more from the reactive zone of the lesion was defined as a safe margin. If the safe marginal area was confined to the unicondyle, a unicondylectomy was indicated.
Fig. 1A–D.
The images illustrate the case of a 24-year-old man with a diagnosis of recurrent Ewing’s sarcoma who received intralesional curettage and autogenous cancellous bone graft in his hometown hospital because of misdiagnosis. (A) A preoperative AP radiograph shows the extension of the tumor that compromised the lateral femoral condyle. (B) The MRI scans determined tumor extension. (C) The CT scan showed the area of bone destruction. (D) The bone scan showed increased uptake in the area of tumor invasion. These images were used to design the levels of tumor resection with the computer-assisted navigation system.
The bone bank of our institution provided nonirradiated allografts. The allografts were harvested under sterile conditions and stored at −80°C according to previously reported techniques [15]. No cryopreservation reagent was used. The cruciate and collateral ligaments of the allograft were preserved to augment those of the host tissue after the allograft was implanted. We made no attempt to preserve the viability of the chondrocytes. Bacteriologic and viral tests were performed in accordance with the recommendations of the Chinese Association of Tissue Banks [16]. The surgeon selected allografts by comparing the age, sex, height, weight, and radiographs (AP and lateral views) of the patient with corresponding data from the donor to achieve the closest anatomic match. Three major considerations were width of the distal femur on the AP view, the thickness of the distal femur on the lateral view, and the width of the femoral shaft [16]. In the operating room, the frozen allograft was thawed in a warm antibiotic-impregnated saline solution for preparation of implantation.
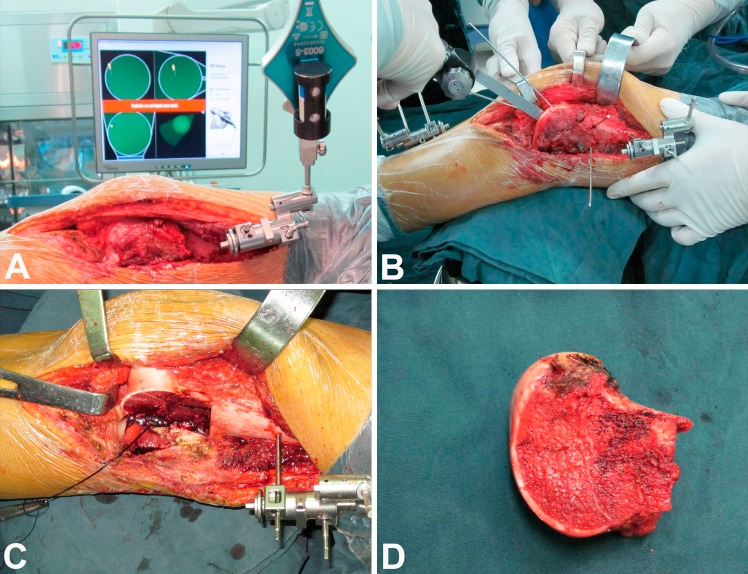
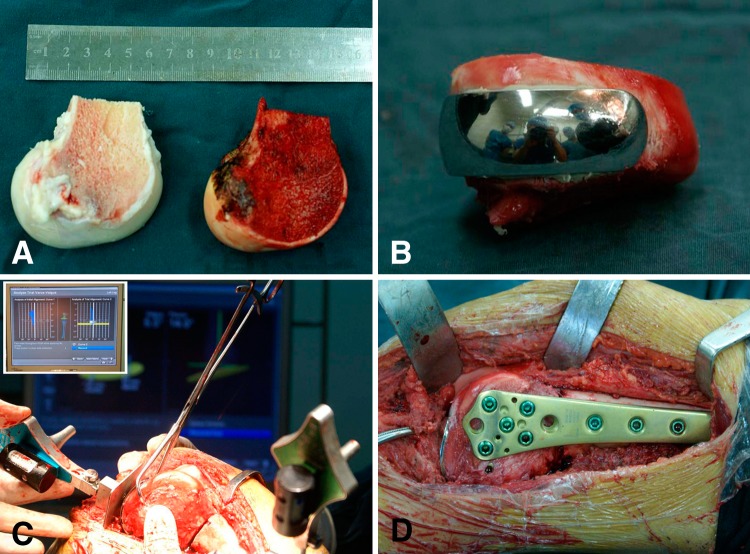
We used a computer-assisted navigation system (Stryker Pacific Ltd, Hong Kong, China) for precise resection and accurate reconstruction. The surgical approach was determined by the tumor site (medial or lateral condylar lesion) and performed according to reported protocol [18]. Through an extended anteromedial or anterolateral approach, we exposed the knee and distal femur, enhancing exposure by everting the patella or sliding the patella with the knee flexed. We then sectioned the cruciate and collateral ligaments of the affected condyle close to their femoral attachments to ensure enough length for reattachment. The navigation-guided surgical resection was performed as previously reported [24]. After appropriate registration and calibration, the surgeons located and marked the anatomic position of intended bone resection plane with a K-wire by using navigation tools. The tumor then was removed en bloc with an oscillating saw (Fig. 2). The surgically removed tissue was grossly observed by an experienced pathology technician. Eight samples from different sides of removed tissue (proximal, distal, lateral, medial, up, and down) then were taken, sectioned, and stained. A margin that was 2 cm or more from the reactive zone of the lesion was defined as a safe margin. After thawing, the surgeons cut the allograft to the proper size to match the bone defect, and then fixed the cemented femoral component of the unicompartmental knee prosthesis (Waldemar Link Co, Hamburg, Germany) to the allograft to form a unicondylar osteoallograft prosthesis composite. The opposing tibial plateau was not replaced by a metallic prosthesis to prevent possible contamination of tumor cells to the tibia and to retain the cartilage and bone stock. The surgeon then implanted the unicondylar osteoallograft prosthesis composite to fill the osteoarticular defect. With the help of the navigation system, precise apposition of the unicondylar osteoallograft prosthesis composite and the host bone was achieved. In addition, the degrees of varus or valgus of the tibia and femur were measured to confirm the mechanical alignment to avoid deformity. Care was taken to restore the anatomic congruency of joint surface. The allograft and host bone junctions were tailored with a high-speed burr to achieve maximal cortical contact before fixation with a locking plate (Fig. 3). The surgeon then attached the soft tissues from the allograft to the corresponding host tissues to obtain the greatest possible stability. After lavage, the joint capsule, subcutaneous tissue, and skin were closed in layers.
Fig. 2A–D.
The photographs show how the tumor resection was performed using computer-assisted navigation. (A) After appropriate exposure, a tracker was fixed to the femur to perform registration and calibration. This enabled the surgeons to match precisely the operative anatomy to the virtual image generated by the navigation system. (B) The anatomic position of the intended bone resection plane was located and marked with a K-wire by using navigated tools. (C) The lateral femoral condyle was removed using an oscillating saw. (D) The en bloc tumor resection had a wide margin.
Fig. 3A–D.
The photographs show the unicondylar osteoallograft prosthesis composite reconstruction that was performed using computer-assisted navigation. (A) After thawed, the osteoarticular allograft was cut into the proper size to match the bone defect The allograft is on the left and the resected femoral condyle is on the right. (B) The cemented femoral component was fixed to the allograft to form a unicondylar osteoallograft prosthesis composite. (C) The navigation system enabled the surgeons to precisely implant the unicondylar osteoallograft prosthesis composite. Congruency of the articular surface was meticulously adjusted. The insert in the upper left corner shows mechanical alignment was checked to avoid a varus or valgus deformity. (D) The unicondylar osteoallograft prosthesis composite was fixed to the host bone with a locking plate.
Prophylactic antibiotics were given intravenously until drains were removed on the third postoperative day, followed by 1 month of oral antibiotic therapy. Anticoagulation therapy was routinely used. Postoperatively, the knee was immobilized in a hinged brace for 3 months, allowing for flexion and extension. Patients were permitted only partial weightbearing at 12 weeks postoperatively. Full weightbearing was permitted after achieving radiographic union between the unicondylar osteoallograft prosthesis composite and host bone.
Patients and Methods
We retrospectively reviewed demographic, clinical, and surgical details of 12 patients with bone tumors in the distal femur who underwent unicondylar osteoallograft prosthesis composite reconstructions at our hospital from 2005 to 2011. The preoperative diagnoses were giant cell tumor in five patients (42%), osteosarcoma in three (25%), Ewing’s sarcoma in three (25%), and chondrosarcoma in one (8%). The average age of the patients was 29 years (range, 17–44 years) at the time of reconstruction. Eight (67%) patients were males and four (33%) were females. Eleven patients received primary unicondylar osteoallograft prosthesis composite implantation and one received secondary procedures after infection. The affected sites were the right medial condyles in three patients (25%), right lateral condyles in two (17%), left medial condyles in three (25%), and left lateral condyles in four (33%). One patient with osteosarcoma died from his primary disease at 18 months postoperatively. No patients were lost to followup. The minimum followup was 8 months (median, 37 months; range, 8–65 months). The study was approved by the institutional review board of Xi-Jing Hospital, the Fourth Military Medical University.
Staging studies were performed in all patients, including radiographs, CT scans, bone scan, and MRI of the affected limb. Each patient also underwent a chest CT. According to the MSTS staging system for primary malignant bone tumors [8], the seven malignant tumors were classified as Stage IIB in the current study. Another five aggressive benign lesions were Stage 3 lesions based on the system of Enneking et al. for staging benign soft tissue masses [8]. The giant cell tumors were all Grade 3 tumors according to Campanacci et al. [5]. Six patients with osteosarcomas and Ewing’s sarcomas received preoperative and postoperative chemotherapy. We recommended postoperative radiotherapy to three patients with Ewing’s sarcomas but owing to financial difficulties none of the patients received the therapy. None of the other nine patients (with giant cell tumors and osteosarcomas) received radiotherapy (Table 1).
Table 1.
Demographic and clinical data
| Patient | Sex | Age (years) | Diagnosis | Location | Chemotherapy (preoperative/postoperative) | Stage | Followup (months) | UOPC status | Limb status | Recurrence/metastasis | Patient status |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 17 | ES | LLC | Yes/yes | IIB | 8 | Removed | Amputation | Yes/no | ANED |
| 2 | Male | 24 | RES | LLC | Yes/yes | IIB | 26 | Preserved | Preserved | No/no | ANED |
| 3 | Male | 27 | ES | RLC | Yes/yes | IIB | 36 | Revised | Preserved | No/no | ANED |
| 4 | Male | 37 | CS | RMC | No/no | IIB | 40 | Preserved | Preserved | No/no | ANED |
| 5 | Male | 18 | OS | RMC | Yes/yes | IIB | 18 | Preserved | Preserved | No/yes | DOD |
| 6 | Male | 23 | OS | LMC | Yes/yes | IIB | 37 | Preserved | Preserved | No/no | ANED |
| 7 | Female | 28 | OS | LMC | Yes/yes | IIB | 12 | Removed | Amputation | Yes/no | ANED |
| 8 | Male | 25 | RGCT | LLC | No/no | III | 58 | Preserved | Preserved | No/no | ANED |
| 9 | Male | 29 | GCT | RLC | No/no | III | 62 | Preserved | Preserved | No/no | ANED |
| 10 | Male | 44 | GCT | RMC | No/no | III | 65 | Preserved | Preserved | No/no | ANED |
| 11 | Female | 36 | GCT | LMC | No/no | III | 60 | Preserved | Preserved | No/no | ANED |
| 12 | Female | 34 | GCT | LLC | No/no | III | 27 | Preserved | Preserved | No/no | ANED |
UOPC = unicondylar osteoarticular prosthesis composite; RES = recurrent Ewing’s sarcoma; ES = Ewing’s sarcoma; CS = chondrosarcoma; OS = osteosarcoma; GCT = giant cell tumor; RGCT = recurrent giant cell tumor; LLC = left lateral condyle; LMC = left medial condyle; RLC = right lateral condyle; RMC = right medial condyle; ANED = alive with no evidence of disease; DOD = dead of disease.
Patients were seen postoperatively at 2 weeks, 1 month, and 3 months after surgery; every 3 months thereafter until 2 years; and then annually. Plain radiographs and physical examination were performed at each followup. Chest CT was performed every 3 months until 2 years after surgery and then every 6 months to evaluate for metastatic disease. Function was assessed according to a classification system proposed by the MSTS [8] with a maximum of 30 possible points. In brief, this system assigned numerical values (0–5) for each of the six categories: pain, function, emotional acceptance, support, walking, and gait. Major complications including infection, allograft fracture, nonunion, and articular collapse were recorded [21–23].
Three of us (HF, ZG, ZW) independently evaluated the plain radiographs of patients according to the grading system established by International Society of Limb Salvage (ISOLS) [11], which has eight categories, including healing of osteotomies, contour of the graft, fixation of the graft, density of the graft, stability of the joint, diameter of the graft, and changes of the joint. We calculated the score by adding the value for each criterion and dividing the maximum attainable score. The score was expressed as a percentage with a maximum score of 100%. We considered the unicondylar osteoarticular prosthesis composite reconstruction a failure when it was removed during a revision procedure or amputation.
Results
One patient died from tumor-related causes without unicondylar osteoarticular prosthesis composite reconstruction failure by the 18-month radiographic followup. Among the remaining 11 unicondylar osteoarticular prosthesis composite reconstructions, three primary reconstructions failed: one from infection and two from local recurrence. At the time of the last evaluation, 10 of the original 12 unicondylar osteoarticular prosthesis composite reconstructions were still in place; three of these 10 unicondylar osteoarticular prosthesis composite reconstructions were in patients who were followed up more than 5 years.
Complications included infection (one case) and local recurrence (two cases). For the patient with infection, the unicondylar osteoarticular prosthesis composite was removed and an antibiotic-impregnated polymethylmethacrylate spacer was implanted. According to the infecting microorganisms, appropriate antibiotics were administered for 1 to 3 months. After achieving infection control, the patients underwent a second unicondylar osteoarticular prosthesis composite reconstruction for limb salvage. Two patients with local recurrence had above-knee amputations owing to neurovascular bundle involvement.
The 10 patients who retained the unicondylar osteoarticular prosthesis composite at their last followup had an average functional score of 27 points (range, 20–29 points). Six patients had no pain (5 points) in the involved knee, two had intermediate pain (4 points), and two had modest pain (3 points). Six patients had no functional restrictions (5 points) and four had intermediate restrictions (4 points). Seven patients were enthused about the surgery (5 points) and three were intermediately satisfied (4 points). Five patients walked without the use of supports (5 points), four occasionally used a brace (4 points), and one mostly used a brace (3 points). Six patients could walk an unlimited distance (5 points), three had mildly limited distance (4 points), and one had limited distance (3 points). Five patients had normal gait (5 points), four had intermediate gait (4 points), and one had minor cosmetic gait (3 points) (Fig. 4). The mean radiographic score of the 10 unicondylar osteoarticular prosthesis composite reconstructions was 91% (range, 76%–94%), which represented an excellent radiographic result (Fig. 5). According to the radiographic evaluation [1], all joint spaces were unchanged or had mild degeneration. None of the condyles of the proximal tibia or distal femur had severe osteoarthritis develop.
Fig. 4A–C.
The clinical functional outcome of unicondylar osteoallograft prosthesis composite reconstruction in a 25-year-old man with a diagnosis of recurrent giant cell tumor is shown. The photographs show (A) the weightbearing position, (B) extension, and (C) flexion of the knee.
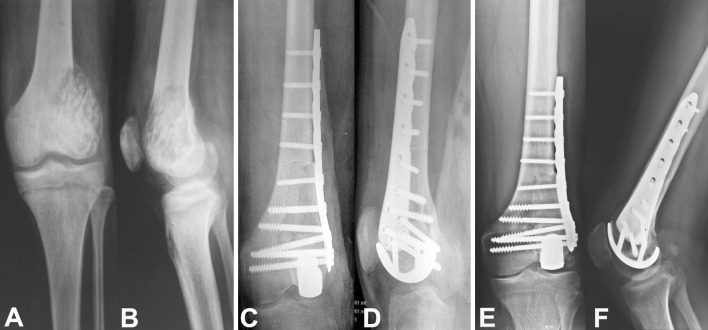
Fig. 5A–F.
A 25-year-old man with a diagnosis of recurrent giant cell tumor received a unicondylar osteoallograft prosthesis composite reconstruction after resection of the tumor The preoperative (A) AP and (B) lateral radiographs show the extension of the tumor that compromised the lateral femoral condyle. The intraoperative (C) AP and (D) lateral radiographs show successful unicondylar osteoallograft prosthesis composite reconstruction. The (E) AP and (F) lateral radiographs show solid union of the osteotomy without obvious joint deterioration after 58 months of followup.
Discussion
For aggressive benign and malignant bone tumors confined to one femoral condyle, it might be a more acceptable option only to remove the affected condyle [6, 9, 18]. Preservation of the uninvolved condyle may substantially improve the biomechanics of knee. One of the most challenging procedures has been to reconstruct the femoral condyle owing to the demands of stability and function of the knee. Implantation of a unicondylar osteoarticular allograft has been performed and the global rate of allograft survival at 5 and 10 years was 85%, with a mean followup of 148 months. The mean radiographic score was 89%, with an average functional score of 27 points [17, 18]. However, progressive degenerative changes in the graft compartment were seen in most patients, especially in relatively young and active ones. The collapse of the osteoarticular allograft ultimately required secondary resurfacing procedures. In this study, we used the unicondylar osteoarticular prosthesis composite for reconstruction and tried to reduce the incidence of degeneration.
Our study had several limitations. First, we had a small number of patients. The indications for this procedure are uncommon and it would be difficult to obtain a large number of patients in one institution. The limited study size did not allow sufficient power to explore the difference between the patients with different tumors. Second, the heterogeneous nature of the tumors made standardized treatment and comparisons difficult. Third, this was a retrospective clinical study with potentially uncontrolled variables, such as different locations (medial and lateral) and variable required bone and soft tissue resections. Finally, because several surgeons were involved during the period of treatment, the surgeon’s experience, ability to interpret MR images, and ability to measure tumor response to neoadjuvant treatment also introduced heterogeneity in treatment. The study has inadequate power to evaluate the unicondylar osteoarticular prosthesis composite reconstruction thoroughly. However, the unicondylar osteoarticular prosthesis composite is still an accessible alternative to reduce joint degeneration after unicondylar allograft transplantation.
Prosthetic reconstruction has its own advantages, such as maintenance of motion and immediate functional restoration. However, this reconstruction requires sacrificing the uninvolved condyle and the contralateral side of the joint, which leads to excessive bone loss. Moreover, possible multiple revision surgeries with limited bone stock is another difficult problem. Although high survival rates have been reported with this type of reconstruction [4, 14], complication and failure rates also have been high in other series [3, 10]. Henderson et al. [13] reviewed 534 endoprosthetic failures from five institutions. Soft tissue (Type 1) failures accounted for 12% of all failures. The highest rates of Type I failures were observed in reconstructions of the shoulder and hip, with most attributable to instability. Aseptic loosening (Type 2) accounted for 19% of all failures. Aseptic loosening in the distal part of the femur accounted for 6.8% of the failures and was the greatest of all locations. Structural failures (Type 3) accounted for 17% of all failures and were greatest with distal humeral and distal femoral replacements. Infection (Type 4) was the most common mode of failure overall and was the most common cause of failure at all locations except the proximal part of the femur. The relative incidence of endoprosthetic failures attributable to tumor progression (Type 5) was greatest with the distal humeral and proximal femoral replacements and least with total femoral and combined distal femoroproximal tibial replacements.
As diagnostic and therapeutic techniques improve, patients who have a musculoskeletal sarcoma can expect increased survival, fewer complications, and a better quality of life. Therefore, increasing emphasis has been placed on biologic reconstructive alternatives. Campanacci et al. [6] first reconstructed the distal femur with a patellar autograft after a unicondylar resection. They reported graft fusion and good stability in all 19 patients with 2 to 9 years followup. Numerous authors have reported the surgical techniques, survival, and failure of unicondylar and bicondylar osteoarticular allograft reconstructions [2, 17, 19, 20, 23], with the 5-year survival rates of bicondylar and unicondylar osteoarticular allografts ranging from 65% to 85%. The survival rate of the unicondylar osteoarticular allograft was a little higher (Table 2).
Table 2.
Survival and function of different types of knee reconstruction
| Study | Number of grafts | Type of reconstruction | Mean followup (months) | 5-year survival | 10-year survival | Failure of reconstruction | Functional score (MSTS) |
|---|---|---|---|---|---|---|---|
| Muscolo et al. [19] | 75 | BOA | 82 | 71% | 71% | 14 (19%) | 26 |
| Toy et al. [23] | 26 | BOA | 156 | 69% | 63% | 15 (58%) | – |
| Muscolo et al. [20] | 52 | BOA | 123 | 65% | 65% | 20 (38%) | 26 |
| Muscolo et al. [17] | 40 | UOA | 132 | 85% | 85% | 6 (15%) | 27 |
| Bianchi et al. [2] | 10 | UOA | 120 | – | – | 4 (40%) | Excellent (2); Good (5); Fair (3) |
| Current study | 12 | UOPC | 37 | 71% | – | 3 (25%) | 27 |
BOA = bicondylar osteoarticular allograft; UOA = unicondylar osteoarticular allograft; UOPC = unicondylar osteoallograft prosthesis composite; MSTS = Musculoskeletal Tumor Society.
The unicondylar osteoarticular prosthesis composite reconstruction is an original procedure designed by us and no other tumor center has reported on this type of reconstruction before. There are no other published data for reference. To prevent possible contamination of the tumor to the tibial plateau and maximally maintain cartilage and bone stock we did not replace the tibial plateau. Theoretically, a unicompartmental replacement of both sides of the joint would delay development of osteoarthritis. However, the procedure requires sacrifice of the tibial plateau cartilage. Additional studies are needed to compare whether replacing only one or both sides of the joint will minimize degeneration without the risk of contaminating the tibia with tumor.
We reviewed the mid-term survival rate and functions of unicondylar osteoarticular prosthesis composite reconstructions after resection of distal femoral tumors. Because we do not have enough cases with osteoarticular allograft transplantation alone to use as controls, we compared our functional score and survival rate with those from other groups [18–20, 23]. Three of 12 unicondylar osteoarticular prosthesis composite reconstructions failed and the limb preservation was 83% at 5 years. Our findings were not as good as those reported by Muscolo et al. [17, 18] for unicondylar osteoarticular allografts (85% graft survival and 100% limb preservation at 5 years), but were similar to the survival rate in other reports [20, 23]. The survival rate reported by Musculo et al. might be attributed to many factors, including their well-known dedication to allograft surgery, extensive bone bank, and matching of allografts to patients using CT measurements. The average MSTS function score of our patients was 27, as high as other reported scores [17, 19, 20]. The failure rate of reconstruction was 25%, which was lower than that for bicondylar osteoarticular allograft reconstruction in the study by Toy et al. [23] and unicondylar osteoarticular allograft reconstruction in the study by Bianchi et al. [2]. Our study indicated that unicondylar osteoarticular prosthesis composite reconstruction might be a reliable technique.
The primary complications of osteoarticular allograft reconstruction were local recurrence, allograft fracture, nonunion, infection, allograft resorption, articular collapse, and joint degeneration (Table 3). The local recurrence rates for bicondylar osteoarticular allograft reconstruction ranged from 0% [23] to 8% [20]. For unicondylar osteoarticular allograft reconstruction, they varied from 0% [2] to 5% [17]. Therefore these procedures have similar recurrence rates. The local recurrence rate was 17% in the current study. The discrepancy might have been a result of the heterogeneous nature of the tumor cases, the surgeons’ techniques, and patient selection [17]. The recurrence of Ewing’s sarcoma was observed in one patient who did not receive radiotherapy owing to finical difficulties. Another patient with recurrence of osteosarcoma had a large soft tissue invasion. These factors might contribute to the high local recurrence rate. In a future study, we should optimize patient selection. Good chemotherapeutic response is critical in determining the suitability of a limited resection for high-grade sarcomas of bone. We believe greater emphasis should be placed on meticulous preoperative evaluation, including CT, MRI, and bone scans. These could help surgeons confirm whether lesions are confined to one condyle. Moreover, all previous biopsy sites and all potentially contaminated tissues, including any needle biopsy tracks, should be removed en bloc with the surgical specimen [18]. Infection has been a major complication in bulk allograft reconstructions after tumor resection. It occurred in 5% (2/40) of unicondylar osteoarticular allograft reconstructions [17] to 25% (13/52) of bicondylar osteoarticular allograft reconstructions [20]. The unicondylar osteoarticular prosthesis composite reconstruction had a similar infection rate of 8% (1/12) in the current study. The patient with the infection received a second unicondylar osteoarticular prosthesis composite reconstruction and achieved good function after infection control.
Table 3.
Complications of different types of distal knee reconstruction
| Study | Number of grafts | Type of reconstruction | Mean followup (months) | Local recurrence | Allograft fractures | Infection | Resorption | Nonunion | Articular collapse | Moderate and severe joint degeneration |
|---|---|---|---|---|---|---|---|---|---|---|
| Muscolo et al. [19] | 75 | BOA | 82 | 4 (6%) | 3 (5%) | 6 (10%) | 1 (2%) | 0 | 0 | 17/48 (35%) |
| Toy et al. [23] | 26 | BOA | 156 | 0 | 5 (19%) | 6 (23%) | 1 (4%) | 5 (19%) | 0 | 8/26 (31%) |
| Muscolo et al. [20] | 52 | BOA | 123 | 4 (8%) | 3 (6%) | 13 (25%) | 0 | 0 | 4 (8%) | 23/32 (72%) |
| Muscolo et al. [17] | 40 | UOA | 132 | 2 (5%) | 1 (2.5%) | 2 (5%) | 1 (2.5%) | 0 | 0 | 13/33 (39%) |
| Bianchi et al. [2] | 10 | UOA | 120 | 0 | 0 | 0 | 0 | 0 | 1 (10%) | 5/10 (50%) |
| Current study | 12 | UOPC | 37 | 2 (17%) | 0 | 1 (8%) | 0 | 0 | 0 | 0 |
BOA = bicondylar osteoarticular allograft; UOA = unicondylar osteoarticular allograft; UOPC = unicondylar osteoallograft prosthesis composite.
Because there were no viable chondrocytes in the frozen osteoarticular allograft, progressive degeneration of the articular surface was inevitable in all bicondylar and unicondylar osteoarticular allograft reconstructions. The rate of moderate and severe joint degeneration reportedly ranges from 31% to 72% (Table 3). To prevent degenerative changes, fresh allograft with superior chondrocyte viability (30% to 90%) has been used but limited availability of donor tissue has limited its application [12]. The unicondylar osteoarticular prosthesis composite reconstruction provided another alternative, and our patients showed no moderate or severe degenerative changes. The low friction between the femoral metal component and contralateral tibial plateau could maximally reduce the incidence of degeneration. Furthermore, to refine the construction, computer-assisted navigation was used to adjust the distal articular surface and ensure mechanical alignment. In the current study, the 3-D bone tumor model generated in the navigation system allowed easier understanding of the exact anatomic tumor location and relationship with the surrounding structures. Intraoperatively, image guidance with the help of fusion images provided precise visual orientation. Cartiaux et al. [7] investigated the surgical accuracy of an experienced surgeon in performing a pelvic tumor resection with a 1-cm surgical margin. They reported that the surgeon could achieve a 1-cm surgical margin (± 5 mm) with a probability of only 52%. On the contrary, surgeons using a navigation system have a greater chance of reproducing their surgical plans and enhancing the accuracy of bone tumor surgery [24].
If the reconstruction using a unicondylar osteoarticular prosthesis composite is well planned and technically well performed, fixation with small fragment screws may be superior to use of a locking plate with the possibility of stress shielding of the autogenous bone. However, we do not have an extensive bone bank to obtain precisely matched allografts and therefore used a locking compression plate to provide greater stability to the reconstruction. The fixation technique needs to be enhanced in future studies.
Unicondylar osteoarticular allograft reconstruction has been developed to treat defects after resection of aggressive benign bone tumors and malignant bone sarcomas. However, progressive degenerative changes in the graft compartment were seen in most patients. To reduce complications, we described a new surgical technique that used a unicondylar osteoarticular prosthesis composite to reconstruct the knee that presumably would minimize the risk of degeneration. The failure and limb salvage rates after unicondylar osteoarticular prosthesis composite reconstruction were similar to those for bicondylar and unicondylar osteoarticular allografts. No moderate or severe degenerative changes were observed in patients with more than 5 years followup. Unicondylar osteoarticular prosthesis composite reconstruction might be a reliable technique that allows restoration of knee anatomy and function with reduced degeneration.
Acknowledgments
We thank Jun Fu MD for technical support in image integration for the computer-assisted navigation surgery.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Aubin PP, Cheah HK, Davis AM, Gross AE. Long-term followup of fresh femoral osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res. 2001;391(suppl):S318–S327. doi: 10.1097/00003086-200110001-00029. [DOI] [PubMed] [Google Scholar]
- 2.Bianchi G, Staals EL, Donati D, Mercuri M. The use of unicondylar osteoarticular allografts in reconstructions around the knee. Knee. 2009;16:1–5. doi: 10.1016/j.knee.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Biau D, Faure F, Katsahian S, Jeanrot C, Tomeno B, Anract P. Survival of total knee replacement with a megaprosthesis after bone tumor resection. J Bone Joint Surg Am. 2006;88:1285–1293. doi: 10.2106/JBJS.E.00553. [DOI] [PubMed] [Google Scholar]
- 4.Bickels J, Wittig JC, Kollender Y, Henshaw RM, Kellar-Graney KL, Meller I, Malawer MM. Distal femur resection with endoprosthetic reconstruction: a long-term followup study. Clin Orthop Relat Res. 2002;400:225–235. doi: 10.1097/00003086-200207000-00028. [DOI] [PubMed] [Google Scholar]
- 5.Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69:106–114. [PubMed] [Google Scholar]
- 6.Campanacci M, Cervellati C, Donati U. Autogenous patella as replacement for a resected femoral or tibial condyle: a report on 19 cases. J Bone Joint Surg Br. 1985;67:557–563. doi: 10.1302/0301-620X.67B4.4030850. [DOI] [PubMed] [Google Scholar]
- 7.Cartiaux O, Docquier PL, Paul L, Francq BG, Cornu OH, Delloye C, Raucent B, Dehez B, Banse X. Surgical inaccuracy of tumor resection and reconstruction within the pelvis: an experimental study. Acta Orthop. 2008;79:695–702. doi: 10.1080/17453670810016731. [DOI] [PubMed] [Google Scholar]
- 8.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 9.Farooque M, Sharma RK. Patellar reconstruction of the condyles in giant cell tumours of the knee. Int Orthop. 1995;19:355–358. doi: 10.1007/BF00178348. [DOI] [PubMed] [Google Scholar]
- 10.Gerrand CH, Currie D, Grigoris P, Reid R, Hamblen DL. Prosthetic reconstruction of the femur for primary bone sarcoma. Int Orthop. 1999;23:286–290. doi: 10.1007/s002640050373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glasser D, Langlais F. The ISOLS radiological implant evaluation system. In: Langlais F, Tomeno B, editors. Limb Salvage: Major Reconstructions in Oncologic and Nontumoral Conditions. Heidelberg, Germany: Springer-Verlag; 1991. pp. 23–31. [Google Scholar]
- 12.Gross AE, Kim W, Las Heras F, Backstein D, Safir O, Pritzker KP. Fresh osteochondral allografts for posttraumatic knee defects: long-term followup. Clin Orthop Relat Res. 2008;466:1863–1870. doi: 10.1007/s11999-008-0282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson ER, Groundland JS, Pala E, Dennis JA, Wooten R, Cheong D, Windhager R, Kotz RI, Mercuri M, Funovics PT, Hornicek FJ, Temple HT, Ruggieri P, Letson GD. Failure mode classification for tumor endoprostheses: retrospective review of five institutions and a literature review. J Bone Joint Surg Am. 2011;93:418–429. doi: 10.2106/JBJS.J.00834. [DOI] [PubMed] [Google Scholar]
- 14.Kawai A, Muschler GF, Lane JM, Otis JC, Healey JH. Prosthetic knee replacement after resection of a malignant tumor of the distal part of the femur: medium to long-term results. J Bone Joint Surg Am. 1998;80:636–647. doi: 10.1302/0301-620X.80B4.8216. [DOI] [PubMed] [Google Scholar]
- 15.Li D, Bi L, Meng G, Wang J, Lv R, Liu M, Liu J, Hu Y. Mineral status and mechanical properties of cancellous bone exposed to hydrogen peroxide for various time periods. Cell Tissue Bank. 2011;12:51–58. doi: 10.1007/s10561-009-9161-0. [DOI] [PubMed] [Google Scholar]
- 16.Li D, Bi L, Meng GL, Liu M, Jin J, Liu Y, Wang Z, Liu J, Hu YY. Multi-variety bone bank in China. Cell Tissue Bank. 2010;11:233–240. doi: 10.1007/s10561-009-9151-2. [DOI] [PubMed] [Google Scholar]
- 17.Muscolo DL, Ayerza MA, Aponte-Tinao LA, Abalo E, Farfalli G. Unicondylar osteoarticular allografts of the knee. J Bone Joint Surg Am. 2007;89:2137–2142. doi: 10.2106/JBJS.F.01277. [DOI] [PubMed] [Google Scholar]
- 18.Muscolo DL, Ayerza MA, Aponte-Tinao LA, Abalo E, Farfalli G. Unicondylar osteoarticular allografts of the knee: surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2):206–217. doi: 10.2106/JBJS.H.00311. [DOI] [PubMed] [Google Scholar]
- 19.Muscolo DL, Ayerza MA, Aponte-Tinao LA, Ranalletta M. Use of distal femoral osteoarticular allografts in limb salvage surgery. J Bone Joint Surg Am. 2005;87:2449–2455. doi: 10.2106/JBJS.D.02170. [DOI] [PubMed] [Google Scholar]
- 20.Muscolo DL, Ayerza MA, Farfalli G, Aponte-Tinao LA. Proximal tibia osteoarticular allografts in tumor limb salvage surgery. Clin Orthop Relat Res. 2010;468:1396–1404. doi: 10.1007/s11999-009-1186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Power RA, Wood DJ, Tomford WW, Mankin HJ. Revision osteoarticular allograft transplantation in weight-bearing joints: a clinical review. J Bone Joint Surg Br. 1991;73:595–599. doi: 10.1302/0301-620X.73B4.2071641. [DOI] [PubMed] [Google Scholar]
- 22.Terek RM, Hulstyn MJ. Osteoarticular allograft reconstruction for tumors of the distal femur and proximal tibia. Oper Tech Orthop. 2004;14:236–242. doi: 10.1053/j.oto.2004.11.001. [DOI] [Google Scholar]
- 23.Toy PC, White JR, Scarborough MT, Enneking WF, Gibbs CP. Distal femoral osteoarticular allografts: long-term survival, but frequent complications. Clin Orthop Relat Res. 2010;468:2914–2923. doi: 10.1007/s11999-010-1470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong KC, Kumta SM, Chiu KH, Antonio GE, Unwin P, Leung KS. Precision tumour resection and reconstruction using image-guided computer navigation. J Bone Joint Surg Br. 2007;89:943–947. doi: 10.1302/0301-620X.89B7.19067. [DOI] [PubMed] [Google Scholar]