Abstract
Background
Complicated tibial fractures with severe soft tissue trauma are challenging to treat. Frequently associated acute compartment syndrome can result in scarring of muscles with impaired function. Several studies have shown a relationship between angiogenesis and more effective muscle regeneration. Vascular endothelial growth factor (VEGF) is associated with angiogenesis but it is not clear whether it would restore muscle force, reduce scarring, and aid in muscle regeneration after acute musculoskeletal trauma.
Questions/purposes
Therefore, we asked whether local application of VEGF (1) restores muscle force, (2) reduces scar tissue formation, and (3) regenerates muscle tissue.
Methods
We generated acute soft tissue trauma with increased compartment pressure in 22 rabbits and shortened the limbs to simulate fracture débridement. In the test group (n = 11), a VEGF-coated collagen matrix was applied locally around the osteotomy site. After 10 days of limb shortening, gradual distraction of 0.5 mm per 12 hours was performed to restore the original length. Muscle force was measured before trauma and on every fifth day after trauma. Forty days after shortening we euthanized the animals and histologically determined the percentage of connective and muscle tissue.
Results
Recovery of preinjury muscle strength was greater in the VEGF group (2.4 N; 73%) when compared with the control (1.8 N; 53%) with less connective and more muscle tissue in the VEGF group. The recovery of force was related to the percentage of connective tissue versus muscle fibers.
Conclusions
Local application of VEGF may improve restoration of muscle force by reducing connective tissue and increasing the relative amount of muscle fibers.
Clinical Relevance
VEGF may be useful to improve skeletal muscle repair by modulating muscle tissue regeneration and fibrosis reduction after acute trauma.
Introduction
Appropriate fracture healing requires intact soft tissue coverage. Closed fractures, however, can cause an acute compartment syndrome (ACS), which can be limb-threatening and requires immediate treatment, most often with fasciotomy [20] or by acute limb shortening [24].
ACS may lead to widespread muscle necrosis and intramuscular scar tissue formation. During regeneration, various cellular responses are synchronously activated. On injury, damaged tissue is infiltrated by fibroblasts, neutrophils, and monocytes and macrophages [30]. There is a close relationship between maintaining blood supply and muscle regeneration, indicating revascularization plays an important role in the success of muscle regeneration [6]. In models of revascularization, endothelial cells and capillary pericytes undergo degeneration and subsequently new capillaries begin to develop along the existing capillary basement membrane. The new capillaries sprout out from peripheral surviving capillaries toward the center of the injured area. Taken together, these observations suggest the newly developed capillaries would help to provide the injured area with oxygen and substrates and therefore aid in the regenerative process. Angiogenesis is highly regulated by factors such as vascular endothelial growth factor (VEGF). As a mitogenic factor, it acts on endothelial cells and plays a crucial role in vasculo- and angiogenesis [7]. Several studies suggest increased VEGF-A expression after acute exercise [5, 11, 14] or electrical stimulation [13, 31].
Soft tissue trauma not only leads to reduced muscle force attributable to scarring of the muscle fibers, but also to insufficient callus [22]. We recently found VEGF can enhance deficient callus resulting from acute soft tissue trauma of the lower extremity leading to an ACS [25]. Average callus diameter and torsional strength were substantially higher in the VEGF-treated group than in the control group without VEGF. Blood vessel formation in damaged bone increased with a substantially higher number of vessels in the VEGF-treated group as compared with controls. Because ischemia leads to necrosis of muscle tissue, treatment with proangiogenic factors might be a novel approach in the treatment of damaged muscle after trauma.
Therefore, we asked whether local application of VEGF (1) restores muscle force, (2) reduces scar tissue formation, and (3) regenerates muscle tissue.
Materials and Methods
We divided 22 male New Zealand White rabbits into two equal groups. Both groups received standardized musculoskeletal trauma to one lower leg resulting in a critical elevated intracompartmental pressure of the tibialis anterior muscle [21]. One group (test) was treated locally with VEGF whereas in the other group no VEGF was used (control). To compare the effect of the treatment on muscle force restoration, muscle force was measured before trauma and every fifth day after trauma until euthanasia. To study the effect of VEGF on scar tissue formation and regeneration of muscle tissue the specimens were examined histologically and histomorphometrically. Animals were administered general anesthesia for all procedures. The average weight of the animals was 3.8 kg. Data from these same animals were reported to explore the effects on callus [17]. To highlight the effect of VEGF on two different tissues (muscle and bone) and to emphasize different potential therapeutic strategies, we thought it was necessary to publish these results separately. The investigation was approved by the local University Animal Ethics Committee.
A tourniquet was placed on the upper thigh to stop arterial blood flow for 90 minutes. During the first 30 minutes of ischemia a contusion clamp (10-mm aluminum base, compression load of 100 kilo Pascal = 10 Newtons/cm2) was placed directly on the tibialis anterior muscle belly 15 mm below its origin. Arterial blood pressure was documented continuously after cannulating the medial auricular artery. The compartment pressure was gauged using a piezoelectric transducer (KODIAG; Braun-Dexon GmbH, Spangenberg, Germany). Bilateral pressure measurements confirmed the onset of a critically elevated intracompartmental pressure (defined as pressure greater than 30 mmHg) on the traumatized side. A unilateral external fixator (Orthofix® M-103; Orthofix SRL, Verona, Italy) was applied to the anteromedial aspect of the tibia. Both groups underwent osteotomy of the tibia with a 10-mm diaphyseal block being removed. Thus, the traumatized leg was shortened. In the test group (n = 11), the osteotomy was sheathed with a 1 × 0.5 × 0.5-mm collagen matrix coated with 12 μg VEGF surrounded by the traumatized tibialis anterior muscle. Hereby, the collagen matrix was contiguous to muscle (outer site) and bone (inner site). In the control group (n = 11), no collagen matrix was applied. Distraction started 10 days after surgery and was performed with 0.5 mm per 12 hours for 10 days until the original length was restored.
A power analysis was performed to determine the sample size required to detect a difference in the absolute muscle force of 25% (mean value of 1.8 N in the control and 2.4 N in the VEGF (+) group; common SD, 0.5) at Day 30 after trauma with 76% power and a two-sided alpha of 0.05. We assumed a 25% difference would be clinically important. The expected effect size greater than 20% for calculation was estimated from data on the use of long-term electrical stimulation to improve muscle force and muscle atrophy [2]. The calculation revealed that 11 animals would be required per group. Although muscle force restoration data of this type of model were not available at the time, one study showed a difference of 23% to be beneficial in the treatment of denervation atrophy [2].
Before surgery, we measured the baseline dorsiflexion force for each limb of each animal. These data were used as reference values for normalization. To measure the dorsiflexion force, a bipolar electrode for transcutaneous stimulation (amplitudes of 5.1 mA for durations of 2.56 ms) of the peroneal nerve was used. Each measurement consisted of 20 single fast signals at 50-ms intervals. Measurements were made at an interval of 5 days. On every testing day they were repeated four times on each side (traumatized and nontraumatized legs in each animal), resulting in 80 values per animal per side, which were presented as an overall average value. The contralateral side (nontraumatized) served as the paired control. Bilateral force measurements were obtained at Days 0 (before trauma), 5, 10, 15, 25, and 30 after trauma [21].
Animals were euthanized (intracardiac injection of T61®; Hoechst GmbH, Munich, Germany) on Day 40. We completely excised the anterior tibialis muscle. Five muscle sections were taken from each specimen 15 mm from the proximal insertion of the anterior tibial muscle. After processing the samples, we embedded the specimens in paraffin. Slices were prepared at a predefined level in a cross section from the same area of the earlier contusion and were stained histochemically (Heidenhain azan stain). Light microscopic analysis was used to quantify morphologic changes in muscle and connective tissue in one to five sections from each animal [21]. For determination of the ratio of muscle and connective tissue, single muscle fibers and the area of connective tissue were outlined semiautomatically and counted at 100-fold magnification. We measured digitized images of each muscle (treated and nontreated) using image-editing software (Image-Pro Plus, Version 7.0; Media Cybernetics, Inc, Bethesda, MD, USA). The ratio between muscle and connective tissue was calculated. Quantitative assessment included morphometric analysis of muscle and connective tissue postmortem. Each image was calibrated with the standard scale of 10 mm. Accuracy of the linear measurement of the system was 0.01 mm [21].
Muscle specimens were stained with α-smooth muscle actin antibody (α-SMA/monoclonal mouse antihuman smooth muscle actin, 1:400). The second antibody was antimouse IgG (Vector Laboratories, Burlingame, CA, USA) followed by avidin-biotin-complex detection (Vectastain® ABC Kit; Vector Laboratories) and counterstained with Vector® methyl-green (Carl Roth, Karlsruhe, Germany). For analysis, 10 areas were chosen and inspected with 100-fold magnification.
The variables of interest were restoration of muscle force, scar tissue formation, and regeneration of muscle tissue. We calculated mean values and SDs for each group and each variable at different times. Data were approximately normally distributed by normal probability plots. Differences in the variables between control and test groups were performed using the t-test for unpaired data. Paired t-tests were used to examine the differences for one group at different times and when comparing traumatized and nontraumatized extremities. For statistical analysis we used SPSS 19.0 (SPSS Inc, Chicago, IL, USA) and R 2.13.1 (R Development Core Team [2011]; R: A language and environment for statistical computing; R Foundation for Statistical Computing, Vienna, Austria).
Results
The VEGF-treated group regained 22% more muscle force output (back to 75% of original muscle force) than the control group (53% of original muscle force) (Table 1). There was no difference in force (p = 0.53) between the treated and the control limbs before trauma. After trauma and surgery, a difference (p < 0.001) was evident with the force of all traumatized extremities being less than that of the nontraumatized extremities. Muscle contractions were negligible between 0 and 5 days after surgery. The greatest increase in force was noted between 10 and 15 days after surgery with values almost doubling during this period. In general, muscle strength continued to improve until the 25th day after surgery (Fig. 1). In both groups, force was measured 30 days after surgery and compared with values before trauma. The difference between traumatized and nontraumatized limbs remained even after completion of the distraction in both groups (control group, p = 0.001; VEGF group, p = 0.008) (Table 2). By the final day of testing, more than 50% of the animals in the control group had a force less than 60% of the presurgery baseline, four animals had a measured force greater than 60% of the baseline measure, and just one animal had regained 88% of original muscle strength. In contrast, nine animals of the VEGF group regained more than half of their muscle force at Day 30 after surgery. Five of these nine animals regained more than 90%, a value none of the animals in the control group accomplished (Table 1). Compartment pressures of all animals normalized after acute shortening of the limb.
Table 1.
Muscle force and distribution of connective tissue 30 days after trauma*
| Specimen number | Muscle force | Connective tissue | Specimen (VEGF+) | Muscle force (VEGF+) | Connective tissue (VEGF+) |
|---|---|---|---|---|---|
| 1 | 3.8 | 3.7 | 1 | 44.9 | 2.6 |
| 2 | 66.6 | 6.4 | 2 | 100 | 0.6 |
| 3 | 85.0 | 14.5 | 3 | 61.8 | 2.8 |
| 4 | 32.0 | 14.7 | 4 | 42.7 | 2.0 |
| 5 | 40.0 | 11.3 | 5 | 100 | 2.5 |
| 6 | 51.5 | 17.4 | 6 | 48.3 | 2.3 |
| 7 | 23.0 | 15.8 | 7 | 100 | 1.7 |
| 8 | 60.0 | 6 | 8 | 100 | 2.0 |
| 9 | 78.1 | 6.5 | 9 | 81.4 | 5.2 |
| 10 | 87.5 | 9.0 | 10 | 95.9 | 3.1 |
| 11 | 57.6 | 3.3 | 11 | 52.5 | 2.6 |
| Average | 53.2 | 9.9 | Average | 75.3 | 2.5 |
* In percent; the pretrauma values are set as baseline (100%); VEGF = vascular endothelial growth factor.
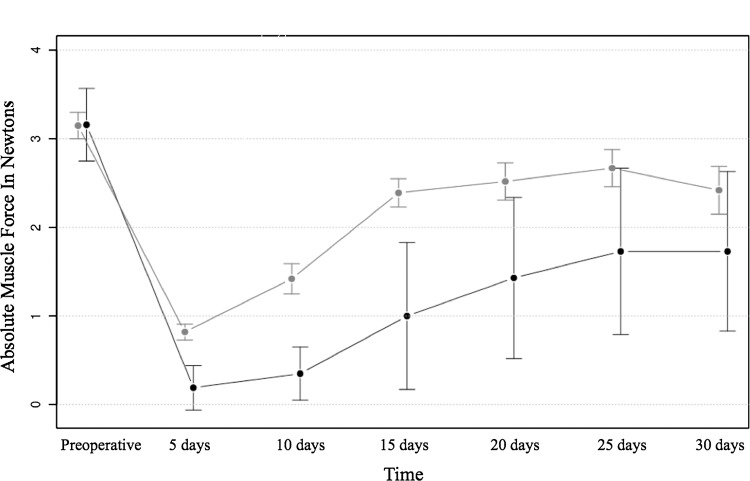
Fig. 1.
Progression of muscle force during the 30 days after trauma is shown. The VEGF group reached with a mean muscle force of 2.42 N at 30 days after surgery, 75% of their mean force preoperatively. In comparison, the control group only reached 53%. The greatest increase in force was noted between 10 and 15 days after surgery with values almost doubling during this period. In general, muscle strength continued to improve until the 25th day after surgery.
Table 2.
Absolute values in Newtons (N) of the measured force produced by dorsiflexion (control and VEGF(+) side)
| Days* | Mean value (N) | SD | Mean value (N) | SD |
|---|---|---|---|---|
| 0 | 3.16 | 0.41 | 3.15 | 0.15 |
| 5 | 0.19 | 0.25 | 0.82 | 0.09 |
| 10 | 0.35 | 0.30 | 1.42 | 0.17 |
| 15 | 1.00 | 0.83 | 2.39 | 0.16 |
| 20 | 1.43 | 0.91 | 2.52 | 0.21 |
| 25 | 1.73 | 0.94 | 2.67 | 0.21 |
| 30 | 1.73 | 0.90 | 2.42 | 0.27 |
VEGF = vascular endothelial growth factor; * Day 0 was before trauma, all other days were after trauma.
The control group showed four times the amount of connective tissue in the traumatized side in comparison to the nontraumatized side (Fig. 2). The amount was 1.7-fold higher in the VEGF group (traumatized versus nontraumatized) (Fig. 2). None of the animals in the VEGF group generated more than 10% connective tissue (control group, five animals). In contrast, the proportion of connective tissue was less than 5%. Compared with the nontraumatized leg, the quantity of connective tissue was 1% greater on the traumatized VEGF side (2.5% ± 0.9% versus 1.5% ± 0.5%). The average connective tissue composition of the traumatized side of the VEGF group (2.5% ± 0.9%) compared with the control group (9.9% ± 1.9%) was lower. On the sides without surgery, connective tissue percentages were similar in the control and treatment groups (1.5% ± 0.5% for VEGF + compared with 2.5% ± 0.9% for control).
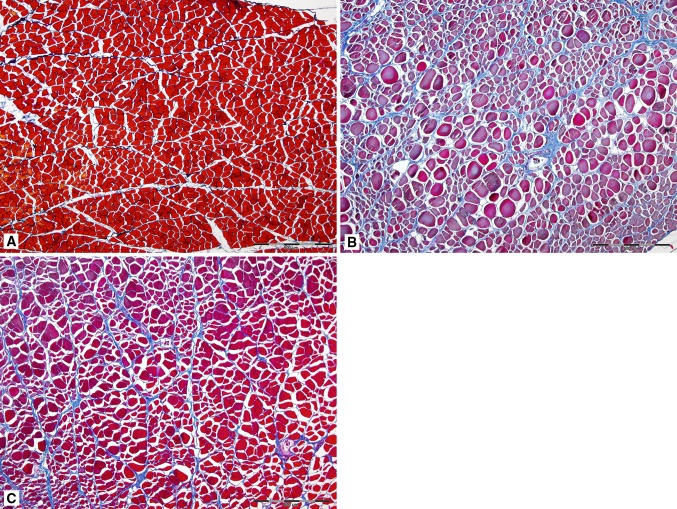
Fig. 2A–C.
Morphologic features of muscle and collagen expression in (A) normal uninjured muscle, (B) control traumatized tibialis anterior muscle, and (C) traumatized muscle regenerated in the presence of VEGF are shown (Stain, Heidenhain azan; original magnification, ×4). The muscle fibers are stained red and the connective tissue blue. There is less connective tissue in the traumatized muscle, which was treated with VEGF.
Histomorphometric evaluation of the muscle revealed the recovery of force was related to distribution of connective tissue and muscle fibers in the muscle. On average the VEGF-treated animals had more than 95% muscle tissue compared with 90% in the control group. Animals treated with locally applied VEGF regenerated more muscle tissue but generated less connective tissue. Immunohistochemical staining revealed no difference (p = 0.05) in the distribution and amount of vessels positively stained for α-SMA.
Discussion
Complicated tibia fractures with severe soft tissue trauma remain a clinical challenge because outcomes are often poor. Ischemic damage of the muscles after trauma provokes humoral responses and liberation of vasoactive substances with early onset of an ACS, which then again results in necrotic muscles. The generation of intramuscular connective tissue in the form of scar tissue with associated reduction of muscle fibers reduces overall muscle force production and therefore impairs the function of the injured limb, increasing the patient’s long-term suffering from an already traumatic injury. Hypoxia and necrosis of the soft tissues are risk factors for poor fracture healing. Therefore, we asked whether local application of VEGF (1) restores muscle force, (2) reduces scar tissue formation, and (3) regenerates muscle tissue.
We recognize limitations to our study. First, experimental studies like ours generally must be transferred to human clinical practice with caution. There are no clinical data on the use of VEGF in human musculoskeletal trauma. Second, the short half-life and high costs of VEGF might be a limiting factor in clinical use. Third, other delivery techniques rather than a collagen matrix need to be tested in terms of prolonged release. The VEGF-soaked collagen I matrix we used in our experiment is similar to the one used for clinical BMP application. The linear declining gradient with time in release kinetics of the collagen I matrix with a peak release in the first hours [15], seemed to be ideal for our experiment. Next to it, the collagen matrix is biodegradable and uncomplicated to apply to the injury side. However, there are different methods to deliver VEGF or its gene to the injury site. For example, implantable biodegradable scaffolds can act as slow release [18] or subcutaneous microosmotic pumps [12] can be used but did not seem to be appropriate in our experimental setup. Fourth, we did not wait to produce a full ACS with a contraction, but a critically elevated intracompartmental pressure as in our experiment might lead to an ACS if not treated. Each animal might tolerate different critical pressure thresholds. Fifth, we observed a positive effect of VEGF in rabbit limbs but these tissues are not identical to those of humans. Sixth, to determine whether VEGF had some biologic effects on the muscle no lengthening of the limb would be required. As we simulated fracture débridement, the limb was shortened by 10%. Consequently, length was restored to regain the physiologic prestress of the muscle which is needed for measurement of muscle force.
We found VEGF enhanced muscle force restoration and reduced the amount of connective tissue after traumatic injury and critically high intracompartmental pressure. The peak of this effect occurred between Days 15 and 20 after injury. A reduction of muscle force was seen when the average portion of connective tissue was greater than 10%, a feature noted particularly in the control group. The positive effect of VEGF on muscle force restoration and reduction of scar tissue presumably relies on induced chemotaxis on inflammatory cells and myogenic precursor cells [10]. Furthermore, VEGF seems to have an antiapoptotic effect and direct myogenic effect [1]. In mice with limb ischemia, local administration of a plasmid vector with a hypoxia-responsive element sequence allowing long-term VEGF expression, promoted gastrocnemius mass and force recovery and ameliorated limb necrosis better in comparison to the group treated without [33] as seen in our experiments. Another study showed that intramuscular administration of a plasmid engineered to induce VEGF expression enhanced preserved grip strength in a rat model of amyotrophic lateral sclerosis which suggests that VEGF administration might be neuroprotective and a practical approach for treating motor disorders [16]. Although data on muscle force restoration after musculoskeletal injury for this type of model were not available at the time we conducted our study, recently published studies [2, 4] confirm the restoration of muscle force we observed.
Quantitative assessment of the histologic specimens showed that the amount of fibrosis was reduced. Similar to our results, a reduction of fibrosis was observed after in vivo transplantation of VEGF-expressing cells in a muscle-derived stem cell (MDSC) transplantation-based skeletal muscle regeneration model [7]. The synergistic effect of VEGF and granulocyte-colony stimulating factor (G-CSF) was tested in a mouse limb ischemia model. There was a distinct reduction of the level of necrosis in the group treated with VEGF and G-CSF compared with the untreated ischemic group [29], which underlines the antifibrotic effect. Another study [3] showed that local and sustained release of VEGF from macroporous scaffolds used to transplant and disperse cultured myogenic cells limited fibrosis and accelerated the regenerative process. Furthermore, limb ischemia not only led to impaired angiogenesis, but also caused abnormal tissue fibrosis, as in our study. Conversely, the fibrotic area in the ischemic region was markedly reduced by injection of human mesenchymal stem cells transfected VEGF nanoparticles [32]. When VEGF was inhibited via its soluble antagonist (sFlt1), the level of angiogenesis was inhibited while an increase of fibrosis was observed [7]. Not only contractile properties but also passive mechanical properties such as muscle stiffness differ by the fibrotic alteration of the muscle as seen after trauma. We could show that force recreation decreases if the compliance of the muscle is reduced. We observed that VEGF reduces muscle scarring which coincides with findings in the literature.
We found local application of VEGF to the injury site improved the muscle tissue regeneration. VEGF reportedly not only enhances neovascularization [19] but also muscle regeneration after ischemia [5]. The expression of VEGF was examined in ischemic skeletal muscle and its regeneration in eight patients and 20 New Zealand White rabbits [28]. In that study acute ischemia led to diffuse generation and distribution of VEGF and VEGF receptor-2 of muscle cells and macrophages. Further, these cells had a high level of hypoxia inducible factor-1α, which leads to upregulation of VEGF and VEGF receptor-1 [28]. These findings underline that endogenous VEGF is released naturally in ischemic tissues as a factor needed for regeneration. After experimental muscle damage, VEGF and its receptors are expressed in regenerating muscle fibers, suggesting there is an autocrine pathway that may promote survival and regeneration of myocytes [23]. Levenberg et al. [17] reported a direct effect of VEGF-engineered myoblasts transplanted subcutaneously into nude mice. The transplantation led to substantially more muscle mass and higher neovascularization. VEGF can promote myogenesis and vascularization in numerous muscle injuries including cardiac injuries [26]. After ischemic injury to normal mouse skeletal tissue, VEGF expression increased and direct injection of adenovirus VEGF at the site of ischemic injury led to reduced apoptosis as compared with controls that did not receive VEGF [10]. In contrast to the observation of higher neovascularization, we did not observe a higher rate of blood vessels in the VEGF group. Because high local concentrations of VEGF might be detrimental for the regeneration process by generating excessively disorganized vascular growth, at lower VEGF dosages, this negative effect was not observed and muscle regenerations appeared to be predominant [23]. After experimental muscle damage with ischemia the delivery of adeno-associated virus-VEGF markedly promoted muscle fiber regeneration with a dose-dependent effect. This proregenerative effect was more evident when VEGF was delivered 5 days after muscle damage, suggesting a direct effect on myogenesis in addition to the well-established proangiogenic activity [9]. Moreover, vascular endothelial growth factor receptor-2 (VEGFR-2) is reportedly the main mediator of the effect of VEGF on myogenic cells [1]. Again, this suggests the potential importance of using VEGF to improve myogenesis. We also suspect VEGF might have had more a direct effect on stimulating myogenesis, as we saw better muscle regeneration rather than contributing to repair by aiding neovascularization as the number of vessels did not increase. In addition, this might be explained by the mechanism that VEGF stimulates satellite cell activation and proliferation, cells are protected from apoptosis, the inflammatory response is muted, and highly functional muscle tissue is formed [4]. Furthermore, muscle regeneration appears to be mediated by MDSCs [7] which are superior to myoblasts in terms of proliferating ability, multipotent differentiation, and strong self-renewal [27]. The main effects seem to be proregenerative and proangiogenic [23].
The therapeutic use of VEGF has been limited to date. There are no data regarding the treatment of posttraumatic muscle malfunctions with VEGF or the appropriate dose to be applied at the injury site. Comparison studies for dose effects do not exist but are required. Treatment of musculoskeletal trauma like the one described here and its complications are primarily surgical. However, administration of proangiogenic factors might provide a new perspective in the treatment of subsequent posttraumatic malfunctions. Additional work is needed to better define the potential therapeutic range of VEGF in this setup.
VEGF is a potent angiogenic factor with a unique role in induction of endothelial cell proliferation. It not only elicits migration of progenitor cells and promotion of capillary sprouting, but also improves muscle regeneration and reduces fibrosis. Our data highlight one part of the multifaceted capabilities of VEGF in skeletal muscle healing after acute soft tissue trauma. We found the application of VEGF improves muscle force. Nearly ½ of the animals regained original muscle strength. The amount of scar tissue was reduced. Thus, the observations suggest local application of VEGF may improve skeletal muscle repair by modulating angiogenesis, muscle fiber regeneration, and fibrosis reduction after acute trauma and highlight possible therapeutic uses for VEGF in the management of musculoskeletal injury.
Acknowledgments
We thank Karim Gorschlueter MD, former medical student at the University of Muenster, for assistance with the muscle force measurements and histologic and histomorphometric analyses, Torsten Blunk PhD for his support discussing the results, James Ridgley MD for critically reviewing the manuscript as a native speaker, and Daniela Keller Dipl. Math.-statistics for her excellent support.
Footnotes
One of the authors certifies that he (SPF) has or may receive payments or benefits, during the study period, an amount less than $10,000, from IZKF Wuerzburg (Interdisziplinäres Zentrum für Klinische Forschung), University of Wuerzburg, Wuerzburg, Germany.
One of the authors (SO) has or may receive payments or benefits, during the study period, an amount less than $10,000, from IMF (Innovative Medizinische Forschung), University of Muenster, Muenster, Germany.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the University of Wuerzburg, Wuerzburg, Germany, and the University of Muenster, Muenster, Germany, in equal parts.
References
- 1.Arsic N, Zacchigna S, Zentilin L, Ramirez-Correa G, Pattarini L, Salvi A, Sinagra G, Giacca M. Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol Ther. 2004;10:844–854. doi: 10.1016/j.ymthe.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Ashley Z, Salmons S, Boncompagni S, Protasi F, Russold M, Lanmuller H, Mayr W, Sutherland H, Jarvis JC. Effects of chronic electrical stimulation on long-term denervated muscles of the rabbit hind limb. J Muscle Res Cell Motil. 2007;28:203–217. doi: 10.1007/s10974-007-9119-4. [DOI] [PubMed] [Google Scholar]
- 3.Borselli C, Cezar CA, Shvartsman D, Vandenburgh HH, Mooney DJ. The role of multifunctional delivery scaffold in the ability of cultured myoblasts to promote muscle regeneration. Biomaterials. 2011;32:8905–8914. doi: 10.1016/j.biomaterials.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borselli C, Storrie H, Benesch-Lee F, Shvartsman D, Cezar C, Lichtman JW, Vandenburgh HH, Mooney DJ. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci USA. 2010;107:3287–3292. doi: 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breen EC, Johnson EC, Wagner H, Tseng HM, Sung LA, Wagner PD. Angiogenic growth factor mRNA responses in muscle to a single bout of exercise. J Appl Physiol. 1996;81:355–361. doi: 10.1152/jappl.1996.81.1.355. [DOI] [PubMed] [Google Scholar]
- 6.Brunelli S, Rovere-Querini P. The immune system and the repair of skeletal muscle. Pharmacol Res. 2008;58:117–121. doi: 10.1016/j.phrs.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Deasy BM, Feduska JM, Payne TR, Li Y, Ambrosio F, Huard J. Effect of VEGF on the regenerative capacity of muscle stem cells in dystrophic skeletal muscle. Mol Ther. 2009;17:1788–1798. doi: 10.1038/mt.2009.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol. 2002;29(6 suppl 16):10–14. doi: 10.1053/sonc.2002.37264. [DOI] [PubMed] [Google Scholar]
- 9.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 10.Germani A, Carlo A, Mangoni A, Straino S, Giacinti C, Turrini P, Biglioli P, Capogrossi MC. Vascular endothelial growth factor modulates skeletal myoblast function. Am J Pathol. 2003;163:1417–1428. doi: 10.1016/S0002-9440(10)63499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gustafsson T, Puntschart A, Kaijser L, Jansson E, Sundberg CJ. Exercise-induced expression of angiogenesis-related transcription and growth factors in human skeletal muscle. Am J Physiol. 1999;276:H679–H685. doi: 10.1152/ajpheart.1999.276.2.H679. [DOI] [PubMed] [Google Scholar]
- 12.Hill A, Geissler S, Weigandt M, Mader K. Controlled delivery of nanosuspensions from osmotic pumps: zero order and non-zero order kinetics. J Control Release. 2012;158:403–412. doi: 10.1016/j.jconrel.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Hudlicka O, Milkiewicz M, Cotter MA, Brown MD. Hypoxia and expression of VEGF-A protein in relation to capillary growth in electrically stimulated rat and rabbit skeletal muscles. Exp Physiol. 2002;87:373–381. doi: 10.1113/eph8702285. [DOI] [PubMed] [Google Scholar]
- 14.Kivelä R, Silvennoinen M, Lehti M, Jalava S, Vihko V, Kainulainen H. Exercise-induced expression of angiogenic growth factors in skeletal muscle and in capillaries of healthy and diabetic mice. Cardiovasc Diabetol. 2008;7:13. doi: 10.1186/1475-2840-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleinheinz J, Jung S, Wermker K, Fischer C, Joos U. Release kinetics of VEGF165 from a collagen matrix and structural matrix changes in a circulation model. Head Face Med. 2010;6:17. doi: 10.1186/1746-160X-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kliem MA, Heeke BL, Franz CK, Radovitskiy I, Raore B, Barrow E, Snyder BR, Federici T. Kaye Spratt S, Boulis NM. Intramuscular administration of a VEGF zinc finger transcription factor activator (VEGF-ZFP-TF) improves functional outcomes in SOD1 rats. Amyotroph Lateral Scler. 2011;12:331–339. doi: 10.3109/17482968.2011.574142. [DOI] [PubMed] [Google Scholar]
- 17.Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, Marini R, Blitterswijk CA, Mulligan RC, D’Amore PA, Langer R. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879–884. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 18.Lim TY, Poh CK, Wang W. Poly (lactic-co-glycolic acid) as a controlled release delivery device. J Mater Sci Mater Med. 2009;20:1669–1675. doi: 10.1007/s10856-009-3727-z. [DOI] [PubMed] [Google Scholar]
- 19.Lokmic Z, Darby IA, Thompson EW, Mitchell GM. Time course analysis of hypoxia, granulation tissue and blood vessel growth, and remodeling in healing rat cutaneous incisional primary intention wounds. Wound Repair Regen. 2006;14:277–288. doi: 10.1111/j.1743-6109.2006.00122.x. [DOI] [PubMed] [Google Scholar]
- 20.McQueen MM, Christie J, Court-Brown CM. Acute compartment syndrome in tibial diaphyseal fractures. J Bone Joint Surg Br. 1996;78:95–98. [PubMed] [Google Scholar]
- 21.Meffert RH, Frey SP, Jansen H, Ochman S, Raschke MJ, Langer M. Muscle strength quantification in small animals: a new transcutaneous technique in rabbits. J Orthop Res. 2008;26:1526–1532. doi: 10.1002/jor.20645. [DOI] [PubMed] [Google Scholar]
- 22.Meffert RH, Jansen H, Frey SP, Raschke MJ, Langer M. The influence of soft tissue trauma on bone regeneration after acute limb shortening. Clin Orthop Relat Res. 2007;460:202–209. doi: 10.1097/BLO.0b013e31804a5e12. [DOI] [PubMed] [Google Scholar]
- 23.Messina S, Mazzeo A, Bitto A, Aguennouz M, Migliorato A, Pasquale MG, Minutoli L, Altavilla D, Zentilin L, Giacca M, Squadrito F, Vita G. VEGF overexpression via adeno-associated virus gene transfer promotes skeletal muscle regeneration and enhances muscle function in mdx mice. FASEB J. 2007;21:3737–3746. doi: 10.1096/fj.07-8459com. [DOI] [PubMed] [Google Scholar]
- 24.Mollenhoff G, Josten C, Muhr G. Callotaxis: osteogenesis by stretching–a conservative possibility for restoring leg length after post-traumatic primary tibial shortening?][in German. Zentralbl Chir. 1997;122:970–973. [PubMed] [Google Scholar]
- 25.Ochman S, Frey S, Raschke MJ, Deventer JN, Meffert RH. Local application of VEGF compensates callus deficiency after acute soft tissue trauma: results using a limb-shortening distraction procedure in rabbit tibia. J Orthop Res. 2011;29:1093–1098. doi: 10.1002/jor.21340. [DOI] [PubMed] [Google Scholar]
- 26.Oshima H, Payne TR, Urish KL, Sakai T, Ling Y, Gharaibeh B, Tobita K, Keller BB, Cummins JH, Huard J. Differential myocardial infarct repair with muscle stem cells compared to myoblasts. Mol Ther. 2005;12:1130–1141. doi: 10.1016/j.ymthe.2005.07.686. [DOI] [PubMed] [Google Scholar]
- 27.Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, Mytinger J, Cao B, Gates C, Wernig A, Huard J. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rissanen TT, Vajanto I, Hiltunen MO, Rutanen J, Kettunen MI, Niemi M, Leppanen P, Turunen MP, Markkanen JE, Arve K, Alhava E, Kauppinen RA, Yla-Herttuala S. Expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 (KDR/Flk-1) in ischemic skeletal muscle and its regeneration. Am J Pathol. 2002;160:1393–1403. doi: 10.1016/S0002-9440(10)62566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sacramento CB, Silva FH, Nardi NB, Yasumura EG, Baptista-Silva JC, Beutel A, Campos RR, Moraes JZ, Junior HS, Samoto VY, Borojevic R, Han SW. Synergistic effect of vascular endothelial growth factor and granulocyte colony-stimulating factor double gene therapy in mouse limb ischemia. J Gene Med. 2010;12:310–319. doi: 10.1002/jgm.1434. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt-Bleek K, Schell H, Kolar P, Pfaff M, Perka C, Buttgereit F, Duda G, Lienau J. Cellular composition of the initial fracture hematoma compared to a muscle hematoma: a study in sheep. J Orthop Res. 2009;27:1147–1151. doi: 10.1002/jor.20901. [DOI] [PubMed] [Google Scholar]
- 31.Tang K, Breen EC, Wagner H, Brutsaert TD, Gassmann M, Wagner PD. HIF and VEGF relationships in response to hypoxia and sciatic nerve stimulation in rat gastrocnemius. Respir Physiol Neurobiol. 2004;144:71–80. doi: 10.1016/j.resp.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Yang F, Cho SW, Son SM, Bogatyrev SR, Singh D, Green JJ, Mei Y, Park S, Bhang SH, Kim BS, Langer R, Anderson DG. Genetic engineering of human stem cells for enhanced angiogenesis using biodegradable polymeric nanoparticles. Proc Natl Acad Sci USA. 2010;107:3317–3322. doi: 10.1073/pnas.0905432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yasumura EG, Stilhano RS, Samoto VY, Matsumoto PK, Carvalho LP. Valero Lapchik VB, Han SW. Treatment of mouse limb ischemia with an integrative hypoxia-responsive vector expressing the vascular endothelial growth factor gene. PLoS One. 2012;7:e33944. doi: 10.1371/journal.pone.0033944. [DOI] [PMC free article] [PubMed] [Google Scholar]