Abstract
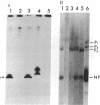
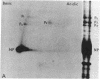
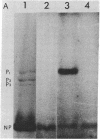
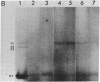
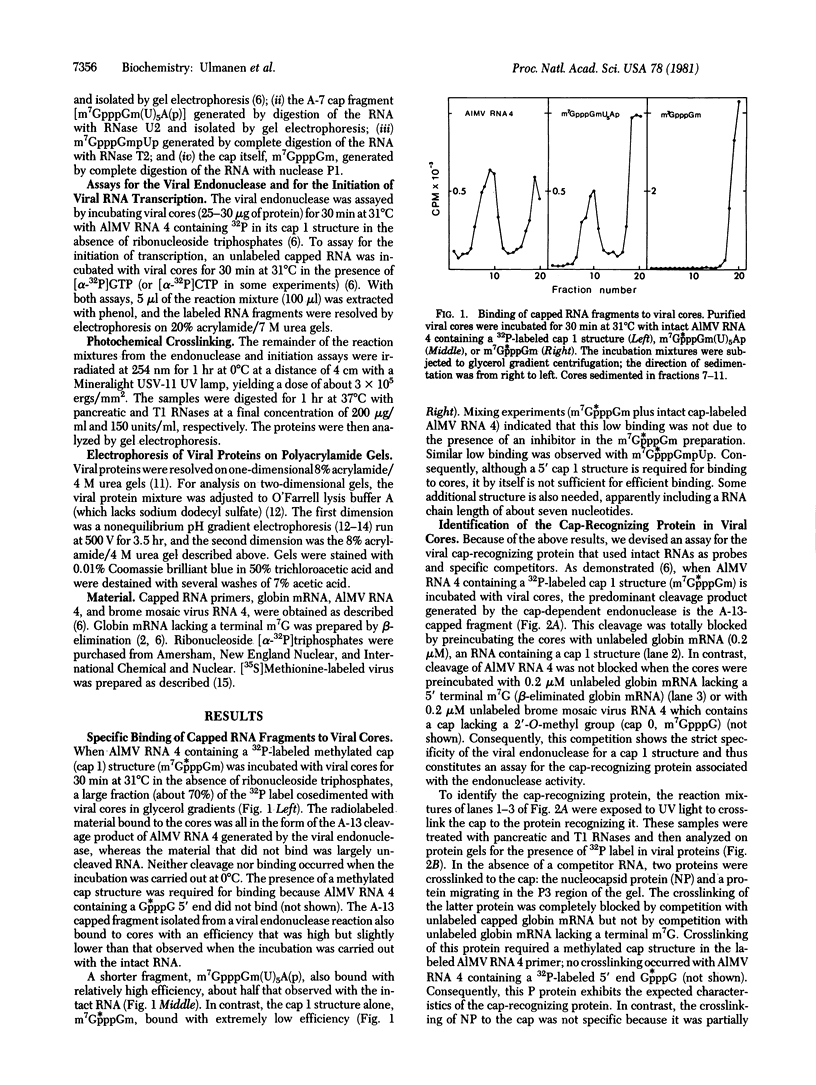
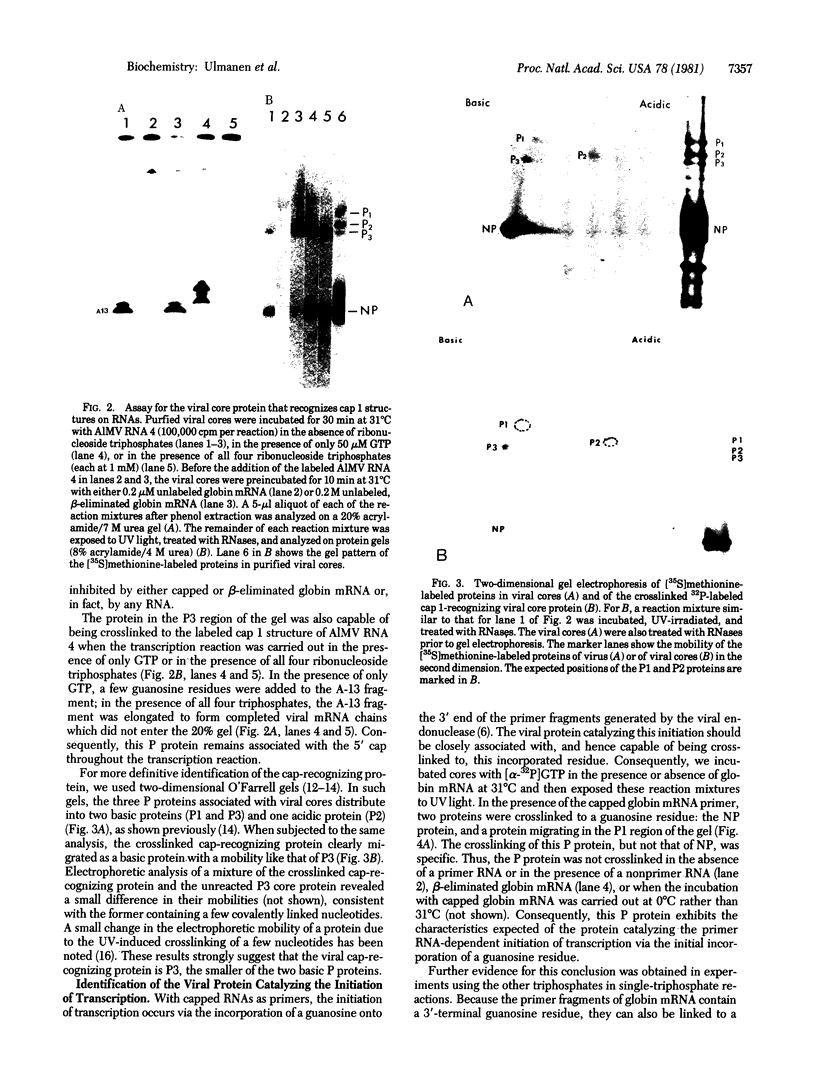
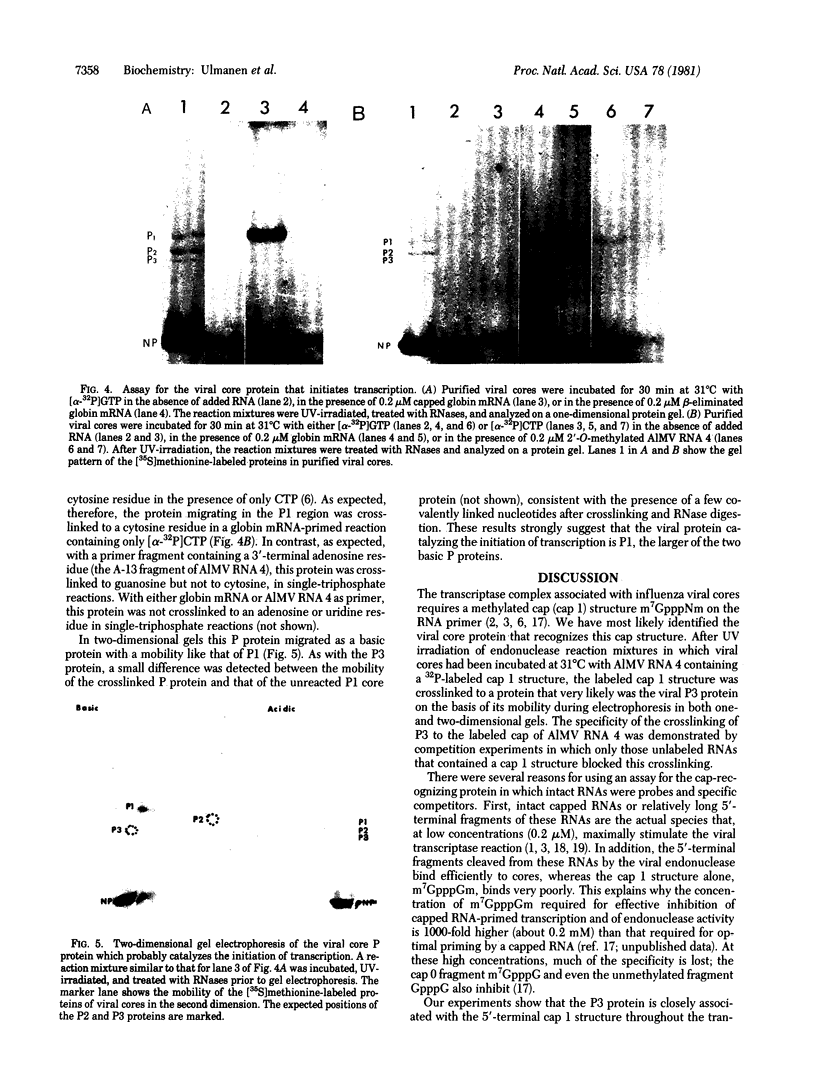
Purified influenza viral cores catalyze the entire process of viral RNA transcription, which includes the endonucleolytic cleavage of heterologous RNAs containing cap 1 (m7GpppNm) structures to generate capped primers 10-13 nucleotides long, the initiation of transcription via the incorporation of a guanosine residue onto the primers, and elongation of the viral mRNAs [Plotch, S. J., Bouloy, M., Ulmanen, L & Krug, R. M. (1980) Cell 23, 847-858]. To identify which viral core protein (nucleocapsid protein, P1, P2, or P3) recognizes the cap 1 structure on the RNA primer, we irradiated (UV) endonuclease reactions carried out by viral cores in the absence of ribonucleoside triphosphates, with a primer RNA labeled in its cap 1 structure with 32P. The labeled cap was crosslinked to a protein that had a mobility similar to that of the P3 protein, the smaller of the two basic P proteins, in both one- and two-dimensional gel electrophoresis. This strongly suggests that this crosslinked protein is the viral P3 protein. Competition experiments with unlabeled RNAs containing or lacking a cap 1 structure established that this protein recognizes the cap 1 structure on RNAs. This protein remained associated with the cap throughout the transcription reaction, even after the viral mRNA molecules were elongated. To identify the viral core protein that catalyzes the initiation of transcription via the incorporation of a guanosine residue onto primer fragments, we irradiated transcription reactions carried out by viral cores in the presence of [α-32P]GTP as the only ribonucleoside triphosphate with an unlabeled primer RNA. A labeled guanosine residue was crosslinked to a protein that had a mobility similar to that of the P1 protein, the larger of the two basic P proteins, in both one-and two-dimensional gel electrophoresis. The transcription reaction conditions required to bring this protein in close association with a labeled guanosine residue so that crosslinking could occur indicated that this association most likely occurred coincident with the guanosine residue's being incorporated onto the primer. These results suggest that the viral P1 protein catalyzes this incorporation and hence initiates transcription.
Keywords: UV-induced crosslinking, two-dimensional gel electrophoresis
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouloy M., Morgan M. A., Shatkin A. J., Krug R. M. Cap and internal nucleotides of reovirus mRNA primers are incorporated into influenza viral complementary RNA during transcription in vitro. J Virol. 1979 Dec;32(3):895–904. doi: 10.1128/jvi.32.3.895-904.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouloy M., Plotch S. J., Krug R. M. Both the 7-methyl and the 2'-O-methyl groups in the cap of mRNA strongly influence its ability to act as primer for influenza virus RNA transcription. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3952–3956. doi: 10.1073/pnas.77.7.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R., Chanock R. M., Lai C. J. Nonviral oligonucleotides at the 5' terminus of cytoplasmic influenza viral mRNA deduced from cloned complete genomic sequences. Cell. 1980 Sep;21(2):495–500. doi: 10.1016/0092-8674(80)90486-9. [DOI] [PubMed] [Google Scholar]
- Horisberger M. A. The large P proteins of influenza A viruses are composed of one acidic and two basic polypeptides. Virology. 1980 Nov;107(1):302–305. doi: 10.1016/0042-6822(80)90296-2. [DOI] [PubMed] [Google Scholar]
- Inglis S. C., Carroll A. R., Lamb R. A., Mahy B. W. Polypeptides specified by the influenza virus genome I. Evidence for eight distinct gene products specified by fowl plague virus. Virology. 1976 Oct 15;74(2):489–503. doi: 10.1016/0042-6822(76)90355-x. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Broni B. A., Bouloy M. Are the 5' ends of influenza viral mRNAs synthesized in vivo donated by host mRNAs? Cell. 1979 Oct;18(2):329–334. doi: 10.1016/0092-8674(79)90052-7. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Broni B. A., LaFiandra A. J., Morgan M. A., Shatkin A. J. Priming and inhibitory activities of RNAs for the influenza viral transcriptase do not require base pairing with the virion template RNA. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5874–5878. doi: 10.1073/pnas.77.10.5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug R. M., Etkind P. R. Cytoplasmic and nuclear virus-specific proteins in influenza virus-infected MDCK cells. Virology. 1973 Nov;56(1):334–348. doi: 10.1016/0042-6822(73)90310-3. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Ueda M., Palese P. Temperature-sensitive mutants of influenza WSN virus defective in virus-specific RNA synthesis. J Virol. 1975 Oct;16(4):790–796. doi: 10.1128/jvi.16.4.790-796.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Synthesis of influenza virus proteins in infected cells: translation of viral polypeptides, including three P polypeptides, from RNA produced by primary transcription. Virology. 1976 Oct 15;74(2):504–519. doi: 10.1016/0042-6822(76)90356-1. [DOI] [PubMed] [Google Scholar]
- Möller K., Brimacombe R. Specific cross-linking of proteins S7 and L4 to ribosomal RNA, by UV irradiation of Escherichia coli ribosomal subunits. Mol Gen Genet. 1975 Dec 9;141(4):343–355. doi: 10.1007/BF00331455. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Palese P., Ritchey M. B., Schulman J. L. P1 and P3 proteins of influenza virus are required for complementary RNA synthesis. J Virol. 1977 Mar;21(3):1187–1195. doi: 10.1128/jvi.21.3.1187-1195.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotch S. J., Bouloy M., Krug R. M. Transfer of 5'-terminal cap of globin mRNA to influenza viral complementary RNA during transcription in vitro. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1618–1622. doi: 10.1073/pnas.76.4.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotch S. J., Bouloy M., Ulmanen I., Krug R. M. A unique cap(m7GpppXm)-dependent influenza virion endonuclease cleaves capped RNAs to generate the primers that initiate viral RNA transcription. Cell. 1981 Mar;23(3):847–858. doi: 10.1016/0092-8674(81)90449-9. [DOI] [PubMed] [Google Scholar]
- Rochovansky O. M. RNA synthesis by ribonucleoprotein-polymerase complexes isolated from influenza virus. Virology. 1976 Sep;73(2):327–338. doi: 10.1016/0042-6822(76)90394-9. [DOI] [PubMed] [Google Scholar]