Abstract
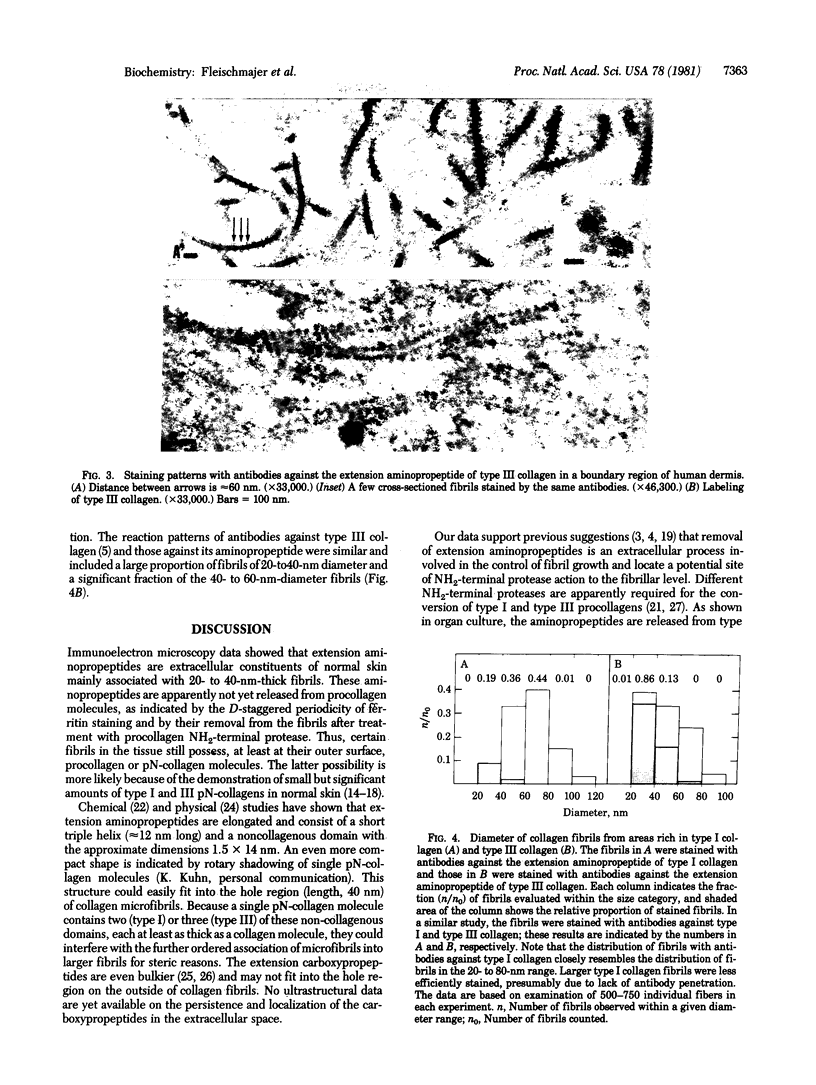
Human skin was labeled with purified antibodies against type II and III collagens and against their extension aminopropeptides by using the ferritin technique. Both aminopropeptides were visualized mainly along thin collagenous fibrils (diameter, 20-40 nm) and rarely in nonfibrillar regions of the skin. The labeling showed a periodicity of 60-65 nm resembling the D (67 nm) stagger of collagen molecules. Blocking of antibodies with aminopropeptides and treatment of tissues with procollagen NH2-terminal protease abolished labeling. Antibodies against type I collagen uniformly labeled approximately equal to 80% of the fibrils (diameter, 20-80 nm), while reaction with antibodies against type III collagen was restricted to thin fibrils. It is currently thought that the aminopropeptides of procollagen molecules are cleaved after they are released from the cell and before fibril formation. Our data indicate that aminopropeptides are removed at the fibrillar level and that fibril growth can be regulated by extracellular procollagen processing.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker U., Timpl R. NH2-terminal extensions on skin collagen from sheep with a genetic defect in conversion of procollagen into collagen. Biochemistry. 1976 Jun 29;15(13):2853–2862. doi: 10.1021/bi00658a024. [DOI] [PubMed] [Google Scholar]
- Bruns R. R., Hulmes D. J., Therrien S. F., Gross J. Procollagen segment-long-spacing crystallites: their role in collagen fibrillogenesis. Proc Natl Acad Sci U S A. 1979 Jan;76(1):313–317. doi: 10.1073/pnas.76.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers P. H., McKenney K. H., Lichtenstein J. R., Martin G. R. Preparation of type III procollagen and collagen from rat skin. Biochemistry. 1974 Dec 3;13(25):5243–5248. doi: 10.1021/bi00722a030. [DOI] [PubMed] [Google Scholar]
- Cassidy K., Eikenberry E. F., Olsen B., Brodsky B. X-ray diffraction investigations of collagen fibril structure in dermatosparactic lamb tissues. Lab Invest. 1980 Dec;43(6):542–546. [PubMed] [Google Scholar]
- Davidson J. M., McEneany L. S., Bornstein P. Intermediates in the limited proteolytic conversion of procollagen to collagen. Biochemistry. 1975 Nov 18;14(23):5188–5194. doi: 10.1021/bi00694a026. [DOI] [PubMed] [Google Scholar]
- Engel J., Bruckner P., Becker U., Timpl R., Rutschmann B. Physical properties of the amino-terminal precursor-specific portion of type I procollagen. Biochemistry. 1977 Sep 6;16(18):4026–4033. doi: 10.1021/bi00637a014. [DOI] [PubMed] [Google Scholar]
- Fessler J. H., Fessler L. I. Biosynthesis of procollagen. Annu Rev Biochem. 1978;47:129–162. doi: 10.1146/annurev.bi.47.070178.001021. [DOI] [PubMed] [Google Scholar]
- Fessler L. I., Fessler J. H. Characterization of type III procollagen from chick embryo blood vessels. J Biol Chem. 1979 Jan 10;254(1):233–239. [PubMed] [Google Scholar]
- Fessler L. I., Timpl R., Fessler J. H. Assembly and processing of procollagen type III in chick embryo blood vessels. J Biol Chem. 1981 Mar 10;256(5):2531–2537. [PubMed] [Google Scholar]
- Fjolstad M., Helle O. A hereditary dysplasia of collagen tissues in sheep. J Pathol. 1974 Mar;112(3):183–188. doi: 10.1002/path.1711120309. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R., Damiano V., Nedwich A. Scleroderma and the subcutaneous tissue. Science. 1971 Mar 12;171(3975):1019–1021. doi: 10.1126/science.171.3975.1019. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R., Gay S., Perlish J. S., Cesarini J. P. Immunoelectron microscopy of type III collagen in normal and scleroderma skin. J Invest Dermatol. 1980 Aug;75(2):189–191. doi: 10.1111/1523-1747.ep12522644. [DOI] [PubMed] [Google Scholar]
- Hoffmann H. P., Olsen B. R., Chen H. T., Prockop D. J. Segment-long-spacing aggregates and isolation of COOH-terminal peptides from type I procollagen. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4304–4308. doi: 10.1073/pnas.73.12.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., Wilkes C. M., Martin G. R. Interaction of fibronectin with collagen fibrils. Biochemistry. 1981 Apr 14;20(8):2325–2330. doi: 10.1021/bi00511a039. [DOI] [PubMed] [Google Scholar]
- Lapiere C. M., Nusgens B. Polymerization of procollagen in vitro. Biochim Biophys Acta. 1974 Apr 11;342(2):237–246. doi: 10.1016/0005-2795(74)90078-6. [DOI] [PubMed] [Google Scholar]
- Lenaers A., Ansay M., Nusgens B. V., Lapière C. M. Collagen made of extended -chains, procollagen, in genetically-defective dermatosparaxic calves. Eur J Biochem. 1971 Dec 10;23(3):533–543. doi: 10.1111/j.1432-1033.1971.tb01651.x. [DOI] [PubMed] [Google Scholar]
- Lenaers A., Lapiere C. M. Type III procollagen and collagen in skin. Biochim Biophys Acta. 1975 Jul 21;400(1):121–131. doi: 10.1016/0005-2795(75)90132-4. [DOI] [PubMed] [Google Scholar]
- Limeback H. F., Sodek J. Procollagen synthesis and processing in periodontal ligament in vivo and in vitro. A comparative study using slab-gel fluorography. Eur J Biochem. 1979 Oct 15;100(2):541–550. doi: 10.1111/j.1432-1033.1979.tb04200.x. [DOI] [PubMed] [Google Scholar]
- Nowack H., Gay S., Wick G., Becker U., Timpl R. Preparation and use in immunohistology of antibodies specific for type I and type III collagen and procollagen. J Immunol Methods. 1976;12(1-2):117–124. doi: 10.1016/0022-1759(76)90101-0. [DOI] [PubMed] [Google Scholar]
- Nusgens B. V., Goebels Y., Shinkai H., Lapière C. M. Procollagen type III N-terminal endopeptidase in fibroblast culture. Biochem J. 1980 Dec 1;191(3):699–706. doi: 10.1042/bj1910699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B. R., Guzman N. A., Engel J., Condit C., Aase S. Purification and characterization of a peptide from the carboxy-terminal region of chick tendon procollagen type I. Biochemistry. 1977 Jun 28;16(13):3030–3036. doi: 10.1021/bi00632a034. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Orford C. R. Dermatan sulphate-rich proteoglycan associates with rat tail-tendon collagen at the d band in the gap region. Biochem J. 1981 Jul 1;197(1):213–216. doi: 10.1042/bj1970213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Orford C. R., Hughes E. W. Proteoglycan-collagen arrangements in developing rat tail tendon. An electron microscopical and biochemical investigation. Biochem J. 1981 Jun 1;195(3):573–581. doi: 10.1042/bj1950573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. D., McKenney K. H., Lustberg T. J. Characterization of collagen precursors found in rat skin and rat bone. Biochemistry. 1977 Jun 28;16(13):2980–2985. doi: 10.1021/bi00632a027. [DOI] [PubMed] [Google Scholar]
- Timpl R., Glanville R. W., Nowack H., Wiedemann H., Fietzek P. P., Kühn K. Isolation, chemical and electron microscopical characterization of neutral-salt-soluble type III collagen and procollagen from fetal bovine skin. Hoppe Seylers Z Physiol Chem. 1975 Nov;356(11):1783–1792. doi: 10.1515/bchm2.1975.356.2.1783. [DOI] [PubMed] [Google Scholar]
- Timpl R., Wick G., Gay S. Antibodies to distinct types of collagens and procollagens and their application in immunohistology. J Immunol Methods. 1977;18(1-2):165–182. doi: 10.1016/0022-1759(77)90168-5. [DOI] [PubMed] [Google Scholar]
- Tuderman L., Kivirikko K. I., Prockop D. J. Partial purification and characterization of a neutral protease which cleaves the N-terminal propeptides from procollagen. Biochemistry. 1978 Jul 25;17(15):2948–2954. doi: 10.1021/bi00608a002. [DOI] [PubMed] [Google Scholar]
- Veis A., Anesey J., Yuan L., Levy S. J. Evidence for an amino-terminal extension in high-molecular-weight collagens from mature bovine skin. Proc Natl Acad Sci U S A. 1973 May;70(5):1464–1467. doi: 10.1073/pnas.70.5.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick G., Olsen B. R., Timpl R. Immunohistologic analysis of fetal and dermatosparactic calf and sheep skin with antisera to procollagen and collagen type I. Lab Invest. 1978 Aug;39(2):151–156. [PubMed] [Google Scholar]
- Wiedemann H., Chung E., Fujii T., Miller E. J., Kühn K. Comparative electron-microscope studies on type-III and type-I collagens. Eur J Biochem. 1975 Feb 21;51(2):363–368. doi: 10.1111/j.1432-1033.1975.tb03936.x. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Yamada S. S. Development of a new primary fixative for electron microscopic immunocytochemical localization of intracellular antigens in cultured cells. J Histochem Cytochem. 1979 May;27(5):947–960. doi: 10.1177/27.5.90071. [DOI] [PubMed] [Google Scholar]











