Abstract
A prospective multicenter observational study was performed to investigate the epidemiology and outcomes of community-acquired severe sepsis and septic shock. Subjects included 1,192 adult patients admitted to the 22 participating intensive care units (ICUs) of 12 university hospitals in the Korean Sepsis Registry System from April, 2005 through February, 2009. Male accounted for 656 (55%) patients. Mean age was 65.0 ± 14.2 yr. Septic shock developed in 740 (62.1%) patients. Bacteremia was present in 422 (35.4%) patients. The 28-day and in-hospital mortality rates were 23.0% and 28.0%, respectively. Men were more likely to have comorbid illnesses and acute organ dysfunctions, and had higher mortality and clinical severity compared to women. While respiratory sources of sepsis were common in men, urinary sources were predominant in women. In the multivariate logistic regression analysis, cancer (odds ratio 1.89; 95% confidence interval 1.13-3.17), urinary tract infection (0.25; 0.13-0.46), APACHE II score (1.05; 1.02-1.09), SOFA score on day 1 (1.13; 1.06-1.21) and metabolic dysfunction (2.24, 1.45-3.45) were independent clinical factors for gender-related in-hospital mortality. This study provided epidemiological and clinical characteristics of community-acquired severe sepsis and septic shock in ICUs in Korea, and demonstrated the impact of clinical factors on gender difference in mortality.
Keywords: Epidemiology, Severe Sepsis, Septic Shock, Mortality, Risk Factor, Gender
INTRODUCTION
Severe sepsis is a syndrome triggered by systemic inflammation, coagulopathy, and acute organ dysfunction in response to infection (1). It is an important reason for admission to intensive care units (ICUs) and the leading cause of death in non-coronary ICUs, resulting in an economic burden in intensive care (2). Despite advances in knowledge of pathogenesis, diagnosis, therapeutic care, and supportive care, the incidence and mortality of severe sepsis and septic shock has increased over the past few decades. The proportion of patients with suggestive severe sepsis increased from 19.1% in the first 11 yr of the study period between 1979 and 2000 to 30.2% in later years in the United States (2, 3). Angus et al. (4) estimated that 751,000 cases of severe sepsis occur annually in the USA, with a mortality rate of 28.6%, leading to an average cost per case of US$22,100.
Previous epidemiological studies have reported that men have higher incidence and mortality compared with women (2, 4, 5), however, a limited number of factors have been identified that could explain the gender disparity. Certain sources of infection and organisms are more likely to cause sepsis or the development of acute organ dysfunction (1, 5-7), and chronic comorbid medical conditions strongly influence outcomes of patients with sepsis (8).
There is a limited but growing amount of information concerning the epidemiology of sepsis in a variety of countries around the world. Accurate national data on sepsis may be useful for a variety of reasons, including the establishment of health care policy and the allocation of healthcare resources (2). However, there is a paucity of data regarding epidemiology and outcomes of patients managed in ICUs for community-acquired severe sepsis and septic shock in Korea (9, 10).
The purpose of the study was to investigate epidemiology and outcomes of community-acquired severe sepsis and septic shock in ICUs in Korea, focusing on the impact of gender on the clinical characteristics and outcomes.
MATERIALS AND METHODS
Study design and settings
This is a prospective, multi-center, observational study to investigate the epidemiology and outcomes of patients with community-acquired severe sepsis and septic shock who were enrolled from April, 2005 to February, 2009, through the Korean Sepsis Registry System (KSRS) endorsed by the Korean Society of Infectious Diseases. The subjects included adult patients (≥ 18 yr) who had severe sepsis or septic shock within 24 hr of entry to the emergency room or ICU, and excluded those with nosocomial infection as defined elsewhere (11). Patients with health care-associated risk factors who developed severe sepsis or septic shock in the community were also included (12). Only first ICU admission from one patient was included and followed-up until death or hospital discharge.
Eight university hospitals (8 medical and 6 surgical ICUs) located in Seoul were invited and voluntary for participation from the beginning of the study, followed by both medical and surgical ICUs of other 4 university hospitals one year later (Seoul [n = 1], Gyeonggi [n = 2] and Gangwon [n = 1] provinces). The study protocol for enrollment and assessment of the patients were developed by the steering committee of the KSRS that consisted of representative infectious disease specialists.
Definitions
Infection was defined as microbiologically documented, and classified according to the standard definitions of the Centers for Disease Control and Prevention (11) and/or clinically suspected infection, plus the administration of antibiotics. Primary infection site was assessed by the infectious diseases specialist in each participating hospital, based on the responsible microorganisms isolated and the organ(s) affected. Definitive microorganisms were defined by organisms isolated from a normally sterile site by routine culture, including blood, peritoneal or pleural fluid, cerebrospinal fluid, surgical specimen, or synovial fluid, or a bacterial pathogen isolated from cultures of respiratory specimens or a urine specimen with bacterial growth of ≥ 1 × 103 colony-forming units/mL. Sepsis and sepsis-related conditions were diagnosed in accordance with the criteria proposed by ACCP/SCCM (American College of Chest Physician/Society of Critical Care Medicine) (1). Definition of severe sepsis was the presence of sepsis plus at least one organ or system dysfunction. The criteria for acute organ or system dysfunction were modified from previous criteria (13): cardiovascular dysfunction (systolic blood pressure ≤ 90 mmHg or mean arterial pressure ≤ 70 mmHg that responds to administration of intravenous fluid), renal dysfunction (urine output < 0.5 mL/kg/hr for 1 hr despite adequate fluid resuscitation or serum creatinine ≥ 177 µM not attributable to chronic kidney failure), respiratory dysfunction (ratio of partial arterial oxygen tension to inspired fractional oxygen ≤ 200), hematologic dysfunction (platelet < 100 × 103/µL or platelet decreased to < 50% in the 2 days preceding admission or INR (International Normalized Ratio) > 1.5 in the absence of systemic anticoagulant agents), hepatic dysfunction (bilirubin > 43 µM or alanine transaminase > 50 IU/L), central nervous system dysfunction (documentation of deterioration in mental status within 24 hr before admission), gastrointestinal dysfunction (active gastrointestinal tract bleeding requiring transfusions within 24 hr of onset of sepsis), metabolic dysfunction (arterial pH ≤ 7.30 or the base deficit ≥ 5.0 mM in association with a plasma lactate > 2 times the upper limit of the normal value). Septic shock was defined as either systolic blood pressure ≤ 90 mmHg or mean arterial pressure ≤ 70 mmHg for at least 1 hr after adequate fluid resuscitation or use of vasopressors to maintain systolic blood pressure > 90 mmHg or mean arterial pressure > 70 mmHg.
Data collection and management
Data from each patient were collected on standardized web-based case report form by the physicians or research nurses in each hospital. They were provided detailed instructions explaining the aim of the study, instructions for data collection, and definitions for variables by operating manual before starting data collection and throughout the study period. The coordinating center at the Korea University Anam Hospital supported data registration at each center, and all data were checked to be within acceptable ranges.
Data collection on admission included demographic data, presence of underlying comorbidity, Charlson's index score (14). Acute Physiology and Chronic Health Evaluation (APACHE) II score (15), Sequential Organ Failure Assessment (SOFA) score (16), microbiology and clinical infections were collected. We recorded the admission date to ICU and the date of in hospital-death or hospital discharge. Sepsis-related mortality was judged by investigators in the participating hospitals.
Statistical analyses
Descriptive analysis was performed. Differences of categorical variables were compared using the chi-square test or Fisher exact test. Continuous variables were expressed as mean ± standard deviation (SD), and Student's two-tailed t test and two-way ANOVA were used for comparing means. To determine the effect of gender on 28-day and in-hospital mortality, multiple logistic regression analysis with forward variable selection method was done. The all variables significant in univariate analysis (P < 0.05) were included in the multivariate model. Odds ratio (OR) and 95% confidence interval (CI) were calculated according to standard methods after adjusting for confounding factors. All tests were two-tailed and a P value < 0.05 was considered significant. Data were analyzed using SPSS version 13.0 for Windows (SPSS, Chicago, IL, USA).
Ethics statement
The study protocol was approved by the institutional review board of the Korea University Anam Hospital (IRB No. ED 0513). Since this observational study required no deviation from routine medical practice, informed consent was waived by board.
RESULTS
Demographic and basal clinical characteristics
During the study period, a total of 1,192 patients met the enrollment criteria for the community-acquired severe sepsis or septic shock. The demographic and baseline clinical characteristics of patients by gender are shown in Table 1. Men accounted for 55% (656/1,192), and had lower mean age than in women (63.9 ± 14.0 yr vs 66.4 ± 14.8 yr; P = 0.003). The peak of age distribution was 60-69-yr-of-age.
Table 1.
Demographic and basal characteristics of the 1,192 patients with community-acquired severe sepsis and septic shock

*ulcer diseases, hemiplegia, etc. SD, standard deviation.
Any type of comorbidity was present in 847 (71.1%) patients. Men had higher rates of central nervous system diseases, cancer, liver diseases and lung diseases. However, cardiovascular diseases and connective tissue diseases were higher in women. The severity of comorbidity as measured by Charlson's index score was also greater in men.
The most common primary site of infection was the respiratory system, followed by urinary tract, abdomen, skin and soft tissue and primary bloodstream. Men were more likely to have respiratory (39.2% vs 19.2%, P < 0.001), abdominal (26.8% vs 20.9%, P = 0.017) and skin and soft tissue infections (11.0% vs 4.7%, P < 0.001), compared to women. Women showed higher rates of urinary tract infections (11.4% vs 44.8%, P < 0.001).
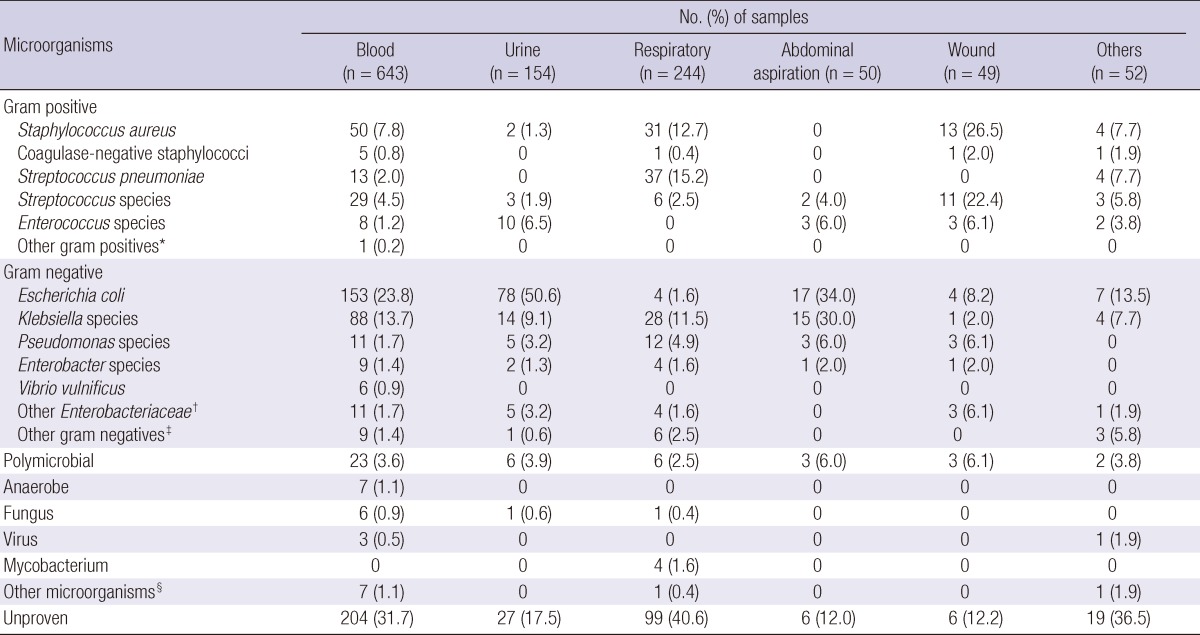
Clinical infections with identification of pathogens were documented in 831 (69.7%) patients. Distribution of microorganisms and sites of isolation were shown in Table 2. Gram negative bacteria were the predominant organisms of the isolates, accounting for 43.0% (n = 513), while gram positive bacteria accounted for 20.4% (n = 243). Polymicrobial infections accounted for 3.6%, followed by fungi (0.7%) and anaerobes (0.6%). Escherichia coli (22.1%) was the most common isolate, followed by Klebsiella species (12.6%) and Staphylococcus aureus (8.4%). Bacteremia developed in 35.4% (422/1,192) of patients, with the frequency of E. coli (37.7%; 159/422), Klebsiella species (22.0%; 93/422), and S. aureus (11.8%; 50/422). Based on clinical samples for the bacterial isolates, Streptococcus pneumoniae, S. aureus and Klebsiella species were more frequently isolated from respiratory samples. E. coli and Klebsiella species were the most frequent isolates from abdominal aspirates. Gram positive bacteria were more common in male patients than in female patients (25.3% vs 14.4%, P<0.001), whereas gram negative organisms were more common in female patients (36.6% vs 50.9%, P<0.001).
Table 2.
Frequencies of microorganisms isolated and the related clinical specimens in the 1,192 patients with community-acquired severe sepsis and septic shock
*Micrococcus species; †Proteus species, Morganella morganii, Serratia marcescens, Citrobacter species; ‡Hemophilus influenzae, Acinetobacter lwoffii, Raoultella ornithinolytica, Actinomyces species, Hafnia alvei, Legionella pneumophila, Aeromonas hydrophila, Salmonella species, Pantoea species, Vibrio cholera; §Orientia tsutsugamushi, Pneumocystis jiroveci, Plasmodium falciparum.
Clinical severity and outcomes
Clinical severity and outcomes of the 1,192 patients analyzed are shown in Table 3. The mean APACHE II score of patients on ICU admission was 18.8±7.3. The mean number of organ dysfunctions within 24 hr after admission was 2.6±1.6. The hematologic dysfunction was the most frequent, acute organ dysfunction, followed by hepatic, renal, respiratory, metabolic, cardiovascular, and gastrointestinal dysfunction. The mean SOFA score was 7.5 points on day 1, and decreased to 5.0 points on day 5. There were significant differences between men and women in the mean APACHE II score, the mean SOFA score, and in occurrence of acute organ dysfunction. Men were more likely to have renal, respiratory, hepatic, and metabolic organ dysfunction, compared to women (P<0.05).
Table 3.
Clinical severity and outcome of the 1,192 patients with community-acquired severe sepsis and septic shock
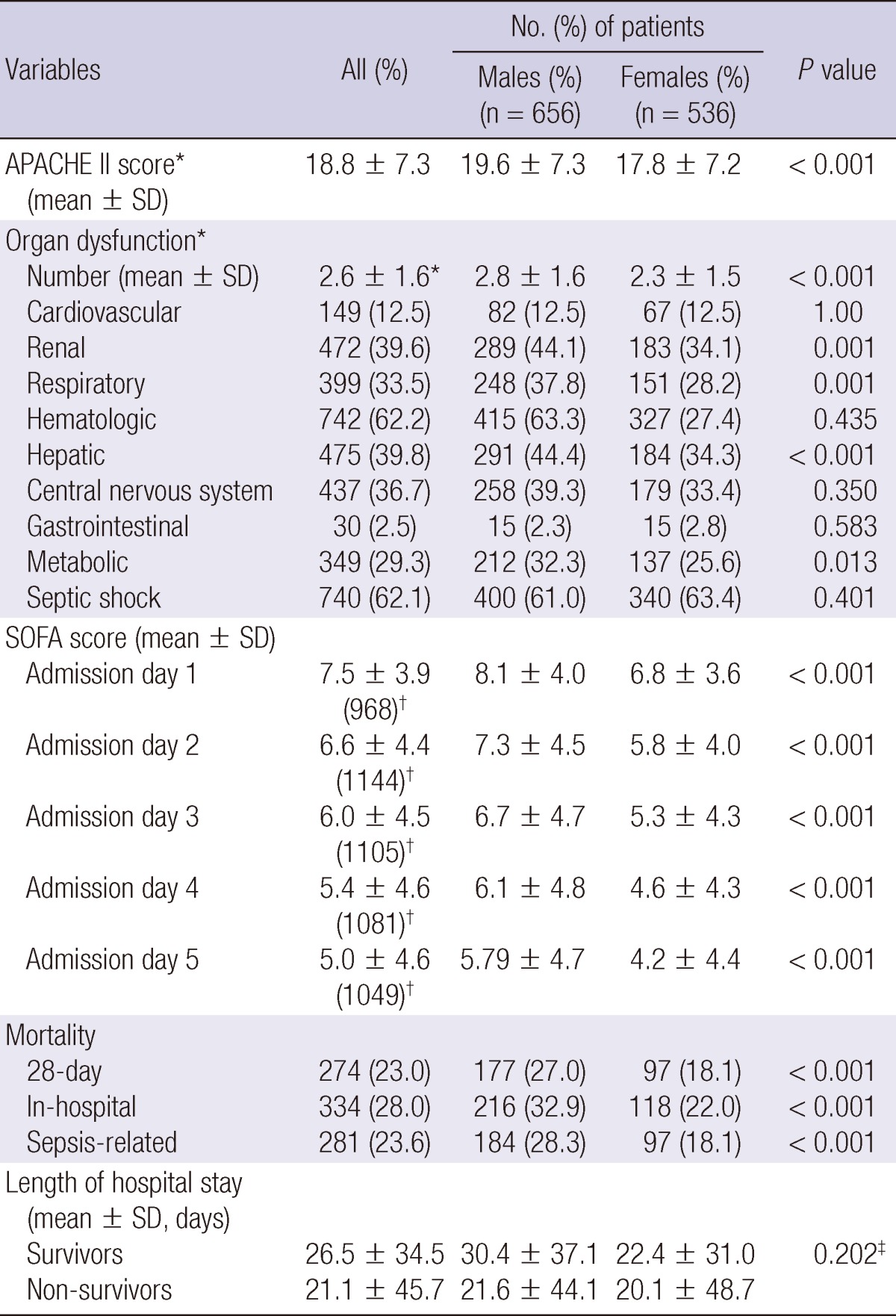
*within 24 hr of admission; †number of patient evaluated; ‡by two-way ANOVA. SD, standard deviation; APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sepsis-related Organ Failure Assessment.
Septic shock was present in 62.1% (740/1,192) of patients. There was no difference in the presence of septic shock between men and women. Development of septic shock was significantly associated with source of primary infection; abdomen (OR, 1.31; 95% CI, 0.96-1.77, P=0.088), bloodstream (2.24; 1.12-4.49, P=0.023), respiratory system (0.64; 0.48-0.84, P=0.001).
The overall 28-day, in-hospital, and sepsis-related mortality rates were 23.0% (n=274), 28.0% (n=334), and 23.6% (n=281), respectively (Table 3). The patients with septic shock had significantly higher mortality rates compared to those without septic shock: the 28-day mortality rate (26.1% vs 17.9%, P=0.001), in-hospital mortality rate (30.8% vs 23.5%, P=0.006), and sepsis-related mortality rate (27.2% vs 17.7%, P<0.001). There are also significant differences between men and women in 28-day, in-hospital, and sepsis-related mortality rates. Men had significantly higher mortality in all of the age groups compared to women (Fig. 1).
Fig. 1.
Gender difference of 28-day and in-hospital mortalities by sex.
The mean length of hospital stay for survivors and non-survivors was 26.5±34.5 days and 21.1±45.7 days, respectively. However, there was no significant gender difference in length of hospital stay for survivors or non-survivors.
Risk factors related to mortality difference by gender
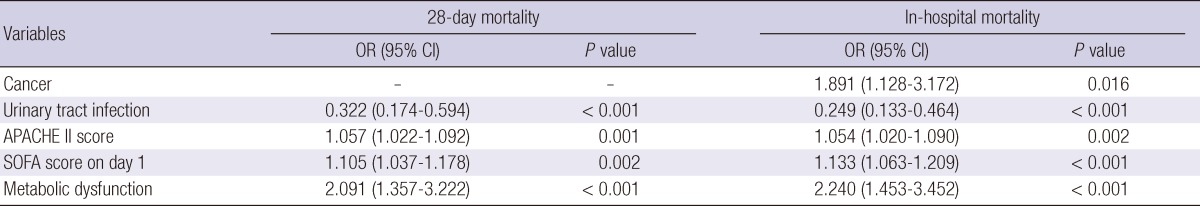
Multiple logistic regression analysis was performed to identify the risk factors which could explain the differences of the 28-day mortality and in-hospital mortality between men and women. Independent variables that were significantly different between men and women in univariate analysis were included in the models. The significant variables selected in the final model of the 28-day mortality were urinary tract infection (OR, 0.33; 95% CI, 0.17-0.59), APACHE II score (1.06; 1.02-1.09), SOFA score on day 1 (1.11; 1.04-1.18) and metabolic dysfunction (2.09; 1.36-3.22). In the model of in-hospital mortality, cancer (1.89; 1.13-3.17), urinary tract infection (0.25; 0.13-0.46), APACHE II score (1.05; 1.02-1.09), SOFA score on day 1 (1.13; 1.06-1.21) and metabolic dysfunction (2.24, 1.45-3.45) were significant variables selected (Table 4). However, both age and gender were not significant variables.
Table 4.
Multivariate logistic regression analysis of risk factors associated with gender difference in mortality
*The variables in the final model were selected by forward variable selection method. Age, sex, comorbid illness (cardiovascular, central nervous system, cancer, liver, lung, connective tissue disease, Charlson's score), sites of primary infection (abdomen, respiratory system, skin and soft tissue, urinary track), organ dysfunctions (renal, respiratory, hepatic, metabolic) and prognostic factors (APACHE II score and initial SOFA score) were included in initial model.
DISCUSSION
This prospective, multi-center, observational study has primarily investigated the epidemiological characteristics and clinical outcomes of patients with community-acquired severe sepsis and septic shock in the 22 ICUs of 12 university hospitals in Korea, although several analyses have been planned based on the database of the KSRS, including the recently published substudy on the prognostic factors for community-acquired bacteremic severe sepsis (10). The current study has disclosed the overall mortality, the most common site of primary infection, the predominant bacterial pathogens isolated from clinical specimens, clinical severity and occurrence of acute organ dysfunctions among adult patients with community-acquired severe sepsis. In addition, this study has shown gender-related differences in clinical characteristics and determined clinical factors related to gender-related mortality.
In this study, in-hospital mortality rate of 28.0% was within the ranges of the expected mortality according to a mean APACHE II score of 18.8 (i.e. 22%) and initial mean SOFA score of 7.5 (i.e. 33%) (15, 16), and was comparable to that of 28.6% from a review of hospital discharge data from seven U.S. states during 1995 (4) and that of 27.6% from sepsis and septic shock patients admitted through emergency departments in Australia and New Zealand (17). However, the current mortality rate is lower than those reported in other studies (18-20). The reasons for the differences in mortality rates may relate to differences in the definition used for diagnosis of severe sepsis and septic shock, community-acquired or hospital-acquired infection, severity of sepsis, and healthcare system issues.
In this study, we observed gender differences in mean age, chronic comorbidity, source of primary infection, occurrence of acute organ dysfunctions and mortality among the ICU patients with severe sepsis and septic shock. Men had significantly higher 28-day and in-hospital mortality rates in all age groups compared to women. However, both gender and age were not related to the higher mortality in men in our final multiple logistic regression models (Table 4). We also identified that clinical severity (APACHE II score and initial SOFA score), comorbidity (cancer), source of infection (urinary tract infection), and metabolic organ dysfunction were significantly independent risk factors for the gender-related mortality in our study population. Certain comorbid conditions such as diabetes mellitus, chronic liver diseases, HIV, and cancer have been suggested to increase the risk of developing sepsis (4, 21, 22). Sex disparity in the source of infection has also been examined in previous studies (23, 24). Urinary tract infection rate was lower in men than women, which can contribute to lower mortality rate in women in our study.
There has been an inconsistency regarding gender-mortality relationships in clinical sepsis studies. Women have better prognosis in some studies (25, 26), whereas other studies found no gender differences (2, 4). In contrast, in a large cohort study of ICU patients published recently, women with severe sepsis or septic shock have a higher risk of in-hospital mortality than men after multivariable adjustment. Although the study identified significant gender differences in some care delivery (deep venous thrombosis prophylaxis, invasive mechanical ventilation, hemodialysis catheter etc.) and in numerous other observed covariates (age, site of infection, functional status, and comorbid conditions), these factors cannot explain the observed gender difference (27). Considering these conflicting reports, the role of clinical severity, comorbidity and infection sites in gender-related mortality of severe sepsis in our study provide a catalyst for additional investigations.
In previous studies, infection with gram positive cocci (e.g., methicillin-resistant S. aureus) and the site of infection influenced the occurrence of acute organ dysfunction and subsequent clinical outcomes (6, 7). In this study, we observed that septic shock was present in 62.1% of the patients, and development of septic shock was significantly associated with source of primary infections such as abdomen, bloodstream and respiratory system. These findings might have clinical implications in management of septic shock after additional investigations.
This study has some limitations. First, this study did not include a random sample of ICUs, potentially leading to selection bias. Second, there could be an inclusion bias since all the septic patients may not have been entered into our database due to the long study period and voluntary participation. Third, this study analyzed the risk factors related to the gender difference in mortality. Further study needs determination of the risk factors for mortality, including variables of clinical severity and appropriateness of antimicrobial therapy.
In conclusion, to our knowledge, this study is the largest prospective multi-center, observational study to date providing the clinical characteristics and gender disparities in patients with community-acquired severe sepsis and septic shock in ICUs in Korea. In particular, this study concluded that men might be related with higher clinical severity and mortality, however, gender itself is not a significant risk factor for mortality of severe sepsis and septic shock.
Footnotes
This study was supported by grant from the Korean Society of Infectious Diseases and partly supported by grant from the Korean Health 21 R&D project, Ministry for Health, Welfare and Family Affairs, Korea (A102065).
References
- 1.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Lever A, Mackenzie I. Sepsis: definition, epidemiology, and diagnosis. BMJ. 2007;335:879–883. doi: 10.1136/bmj.39346.495880.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Brun-Buisson C, Doyon F, Carlet J, Dellamonica P, Gouin F, Lepoutre A, Mercier JC, Offenstadt G, Régnier B French ICU Group for Severe Sepsis. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. JAMA. 1995;274:968–974. [PubMed] [Google Scholar]
- 6.Alberti C, Brun-Buisson C, Chevret S, Antonelli M, Goodman SV, Martin C, Moreno R, Ochagavia AR, Palazzo M, Werdan K, et al. Systemic inflammatory response and progression to severe sepsis in critically ill infected patients. Am J Respir Crit Care Med. 2005;171:461–468. doi: 10.1164/rccm.200403-324OC. [DOI] [PubMed] [Google Scholar]
- 7.Guidet B, Aegerter P, Gauzit R, Meshaka P, Dreyfuss D. Incidence and impact of organ dysfunctions associated with sepsis. Chest. 2005;127:942–951. doi: 10.1378/chest.127.3.942. [DOI] [PubMed] [Google Scholar]
- 8.Pittet D, Thievent B, Wenzel RP, Li N, Gurman G, Suter PM. Importance of pre-existing co-morbidities for prognosis of septicemia in critically ill patients. Intensive Care Med. 1993;19:265–272. doi: 10.1007/BF01690546. [DOI] [PubMed] [Google Scholar]
- 9.Kim YM, Kwon YS, Kim JS, Kim SY, Jang GS, Lee SW, An Y, Park S, Hwang YI, Jang SH, Jung K, Kim D. Incidence and prognosis of disseminated intravascular coagulation in patients with severe sepsis or septic shock. Korean J Med. 2010;79:526–535. [Google Scholar]
- 10.Yoon YK, Kim MJ, Park DW, Kwon SS, Chun BC, Cheong HJ, Choi YH, Kim HY, Eom JS, Kim SI, et al. Prognostic factors of community-acquired bacteremic patients with severe sepsis: a prospective, observational study. Infect Chemother. 2012;44:168–174. [Google Scholar]
- 11.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 12.Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, Lamm W, Clark C, MacFarquhar J, Walton AL, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 13.Munford RS. Severe sepsis and septic shock. In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, editors. Harrison's Principles of Internal Medicine. 16th ed. New York McGraw-Hill companies, Inc.; 2005. pp. 1606–1612. [Google Scholar]
- 14.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 15.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 16.Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 17.Australasian resuscitation of sepsis evaluation; Australia and New Zealand Intensive Care Society Adult Patient Database Management Committee. The outcome of patients with sepsis and septic shock presenting to emergency departments in Australia and New Zealand. Crit Care Resusc. 2007;9:8–18. [PubMed] [Google Scholar]
- 18.Padkin A, Goldfrad C, Brady AR, Young D, Black N, Rowan K. Epidemiology of severe sepsis occurring in the first 24 hr in intensive care units in England, Wales, and Northern Ireland. Crit Care Med. 2003;31:2332–2338. doi: 10.1097/01.CCM.0000085141.75513.2B. [DOI] [PubMed] [Google Scholar]
- 19.Brun-Buisson C, Meshaka P, Pinton P, Vallet B. EPISEPSIS: a reappraisal of the epidemiology and outcome of severe sepsis in French intensive care units. Intensive Care Med. 2004;30:580–588. doi: 10.1007/s00134-003-2121-4. [DOI] [PubMed] [Google Scholar]
- 20.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 21.Sands KE, Bates DW, Lanken PN, Graman PS, Hibberd PL, Kahn KL, Parsonnet J, Panzer R, Orav EJ, Snydman DR, et al. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA. 1997;278:234–240. [PubMed] [Google Scholar]
- 22.Esper AM, Martin GS. The impact of comorbid [corrected] conditions on critical illness. Crit Care Med. 2011;39:2728–2735. doi: 10.1097/CCM.0b013e318236f27e. [DOI] [PubMed] [Google Scholar]
- 23.Esper AM, Moss M, Lewis CA, Nisbet R, Mannino DM, Martin GS. The role of infection and comorbidity: factors that influence disparities in sepsis. Crit Care Med. 2006;34:2576–2582. doi: 10.1097/01.CCM.0000239114.50519.0E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crabtree TD, Pelletier SJ, Gleason TG, Pruett TL, Sawyer RG. Gender-dependent differences in outcome after the treatment of infection in hospitalized patients. JAMA. 1999;282:2143–2148. doi: 10.1001/jama.282.22.2143. [DOI] [PubMed] [Google Scholar]
- 25.Melamed A, Sorvillo FJ. The burden of sepsis-associated mortality in the United States from 1999 to 2005: an analysis of multiple-cause-of-death data. Crit Care. 2009;13:R28. doi: 10.1186/cc7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Facing the challenge: decreasing case fatality rates in severe sepsis despite increasing hospitalizations. Crit Care Med. 2005;33:2555–2562. doi: 10.1097/01.ccm.0000186748.64438.7b. [DOI] [PubMed] [Google Scholar]
- 27.Pietropaoli AP, Glance LG, Oakes D, Fisher SG. Gender differences in mortality in patients with severe sepsis or septic shock. Gend Med. 2010;7:422–437. doi: 10.1016/j.genm.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]