Abstract
There are several antigenic variants of Orientia tsutsugamushi. The 56-kDa type-specific antigen (TSA) is responsible for the antigenic variation. Nucleotide sequences of the 56-kDa TSA obtained from 44 eschar samples of Korean scrub typhus patients and from 40 representative strains retrieved from the GenBank database were analyzed phylogenetically. Clinical patient data were assessed based on the genotyping results. Of the 44 nucleotide sequences, 32 (72.7%) clustered with the Boryong genotype, which is the major genotype in Korea. Eleven nucleotide sequences (25%) clustered with the Kawasaki genotype, not identified in Korea until 2010. One nucleotide sequence was consistent with the Karp genotype. The clinical course of the patients infected with each genotype showed no differences. Diagnostic performance of the immunofluorescence assay (IFA) using the 56-kDa TSA from Gilliam, Karp and Boryong as test antigens were not different for the Boryong and Kawasaki genotypes. Although Boryong is still the predominant genotype, the results suggest that Kawasaki genotype is quite prevalent in Chungbuk province of Korea.
Keywords: Orientia tsutsugamushi, 56-kDa Type-Specific Antigen, Boryong Genotype, Kawasaki Genotype
INTRODUCTION
Tsutsugamushi disease (scrub typhus) occurs throughout Korea on a yearly basis and the number of the reported cases has increased recently. It is transmitted to humans by the bite of chigger mites (Leptotrombidium pallidum and L. scutellare) infected with Orientia tsutsugamushi; the former mite being distributed predominantly in central Korea and the latter in the southern Korea (1).
O. tsutsugamushi has several serotypes. The 56-kDa protein antigen of the outer membrane is the primary antigen, which conveys the type-specific antigenicity of O. tsutsugamushi. The three major prototypes are Gilliam, Karp and Kato (2-4). In addition to these prototype strains, many new isolates, which are antigenically different, have been identified in some Asian countries including Korea (2, 5-9). Patients infected with different strains of O. tsutsugamushi showed some differences in clinical features and antibiotic responses (10). However, there were no significant differences in disease severity and mortality (10). In another study, different strains of O. tsutsugamushi showed different fatality rates and pathogenicity in mice (11). Gilliam, Karp and Boryong are the main serotypes of O. tsutsugamushi in Korea. Karp and Gilliam type are found mainly in central Korea, whereas Boryong is predominant in the south (9). However, the geographic distribution and pathogenicity of each serotype has not been fully investigated in Korea, and previous studies include relatively small sample numbers from Chungbuk province (central part of Korea) (12). Therefore, the aim of this study was to investigate the genotypes of O. tsutsugamushi collected from patients with scrub typhus in central Korea and to perform phylogenetic analysis of DNA sequences of the 56-kDa typespecific protein genes of them. We also compared the clinical course of the patients infected with each genotype, and analyzed whether the difference of genotypes gave different results in a diagnostic serological test (immunofluorescence assay [IFA] using the 56-kDa TSA from Gilliam, Karp and Boryong as test antigens) which is commonly used in Korea to diagnose scrub typhus.
MATERIALS AND METHODS
Patients
Adult patients (n = 45) older than 18 yr of age who were diagnosed with scrub typhus at Chungbuk National University Hospital between September 1, 2009 and December 31, 2010 were enrolled in this study. Chungbuk National University Hospital is a tertiary university hospital located in Chungbuk province, in central Korea. A diagnosis of scrub typhus was made based on the presence of an eschar or maculopapular skin rash with at least two of the following symptoms: fever, myalgia, nausea, cough, headache, generalized weakness and abdominal discomfort. After gaining informed consent from the patients, eschar was obtained and stored at -70℃. Patient data, including addresses, the areas where the chigger bites were thought to have occurred, the time to defervescence (defined as the interval between the time at which the first dose of antibiotics [doxycycline] was administered and the time at which the body temperature decreased to < 37.3℃ and was maintained for more than 48 hr without antipyretics), and the presence or absence of hypotension and major organ involvement such as pneumonia, renal failure, myositis or meningoencephalitis, were recorded. Baseline and follow-up blood samples were also collected from each patient. Paired sera were used for the serological IFA test, which was based upon antigens from the Gilliam, Karp and Boryong strains. A positive result was defined as a ≥ 4-fold change in the titer in paired sera, or a titer ≥ 1:80 in a single serum sample. The cut-off value of ≥ 1:80 was defined as a positive result by the manufacturer (GreenCross, Yongin, Korea).
DNA preparation and PCR
DNA from the eschar samples was purified using a DNeasy Blood & Tissue kit (Qiagen, Hilden, Germany). To obtain the entire open reading frame (ORF) sequences of the 56-kDa type-specific protein of O. tsutsugamushi, the ORF was divided into two areas (front and back) and PCR primers were designed for each area. For the front part, primer pairs targeted the upstream sequence of the 5' end and the middle portion of the ORF. For the back part, primer pairs targeted the middle portion and the downstream sequence of the 3' end of the ORF. The PCR primers are shown in Fig. 1 and Table 1.
Fig. 1.
Positions of the primers designed to obtain the entire open reading frame (ORF) sequences of the 56-kDa type-specific protein. The ORF is represented by the heavy line.
Table 1.
Primers and PCR conditions used to obtain the ORF sequences of the 56-kDa type-specific protein
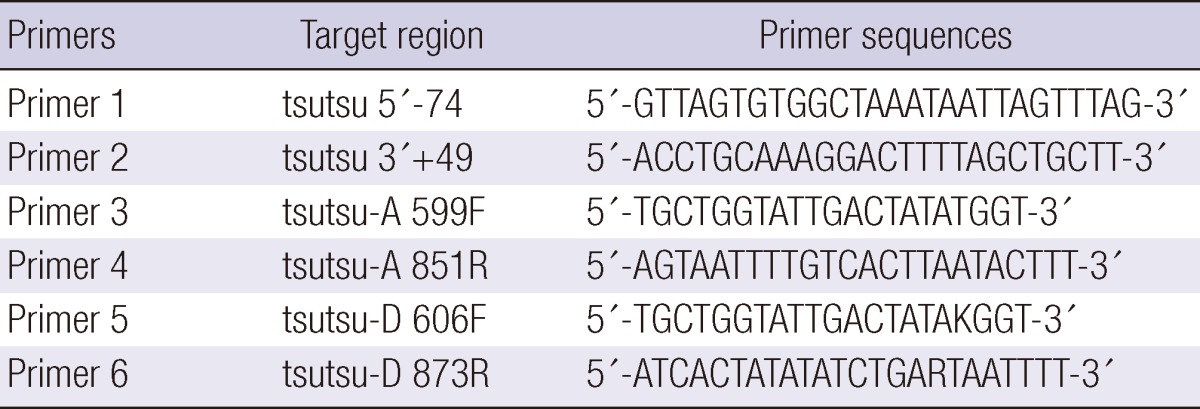
Primer pairs for the front portion: primer 1 (forward) and primer 4 or primer 6 (reverse).
Primer pairs for the back portion: primer 2 (forward) and primer 3 or primer 5 (reverse).
Each reaction mixture (50 µL) contained 5 µL of template DNA, 1 µL of primers (10 pM of each), 5 U of Taq DNA polymerase (Enzynomics, Daejeon, Korea), 2.5 mM of each dNTP, 10 mM Tris-HCl (pH 9.0), 40 mM KCl and 1.5 mM MgCl2. The PCR conditions were: 35 cycles of denaturation at 94℃ for 30 sec, annealing at 52℃ for 1 min, and extension at 72℃ for 30 sec. The amplified products were analyzed on 0.8% agarose gel electrophoresis.
DNA sequencing and phylogenetic analysis
The PCR products were purified using the QIAquick kit (Qiagen, Hilden, Germany). DNA sequencing was performed at Cosmogenetech (Seoul, Korea) using an ABI 373 XL DNA sequencer (Applied Biosystems, Foster City, CA, USA). DNA sequences were verified and aligned using Lasergene sequence analysis software (DNASTAR 5.0, Madison, WI, USA). Sequences were also aligned using the CLUSTAL_V program (13). A phylogenetic tree was constructed and analyzed using the sequences obtained in this study and those obtained from the GenBank database. Phylogenic trees were constructed using the neighbor-joining algorithms and viewed using NJ Plot (14). Branch lengths were proportional to sequence divergence and can be measured relatively to the scale bar included in the figure (Fig. 2).
Fig. 2.
Phylogenetic tree showing the nucleotide sequences of the 56-kDa type-specific protein genes of Orientia tsutsugamushi isolates from this study compared with nucleotide sequences from selected strains available in GenBank. Samples from the present study are identified by their strain name (e.g., CBNU-1). Reference strains from GenBank database are identified by their accession number. The nucleotide sequences were aligned using CLUSTAL V (13) and phonogram was generated by the neighborjoining method using the tree drawing program embedded within the Lasergene sequence analysis software package (DNASTAR 5.0, Madison, WI, USA) and viewed using NJPlot (14). The scale bars represent the number of substitutions per nucleotide. Branch labels record the stability of the branches over 1000 bootstrap replicates. Only bootstrap values > 600% are shown in the tree. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test is shown next to the branches.
Statistical analyses
Statistical analyses were performed using SPSS for Windows, version 11.0 (SPSS Inc., Chicago, IL, USA). Pearson's chi-square test was used for the categorical variables. A P value ≤ 0.05 was considered significant.
Ethical statement
The study protocol was approved by the institutional review board of Chungbuk National University Hospital (IRB No. 2012-02-012). Informed consent was obtained from all patients or their guardians.
RESULTS
A total of 45 patients was enrolled in the study and all 45 patient specimens were PCR-positive. From these PCR products, 44 samples yielded sequences corresponding to the 56-kDa type-specific antigen of O. tsutsugamushi.
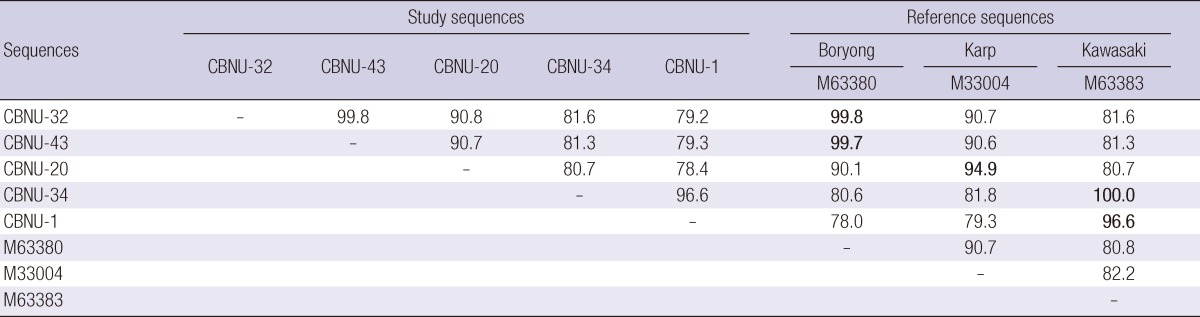
Several ORF sequences for 56-kDa type-specific protein of O. tsutsugamushi were obtained from the GenBank database and 40 representative sequences were selected from them. The 44 DNA sequences obtained in the present study and the 40 representative sequences from the GenBank database were analyzed phylogenetically (Fig. 2). The majority of the sequences obtained in this study showed the Boryong genotype (32/44, 72.7%). Eleven PCR products (25%) were of the Kawasaki cluster genotype, which was not identified in Korea until 2010 (15, 16). One PCR product showed the Karp cluster genotype (CBNU-20). Those ORF sequences were registered in GenBank (accession number JQ898348 - JQ898391). The sequence identity and similarity information of some representative study sequences and reference sequences are summarized in Table 2.
Table 2.
Percent sequence similarity between some study sequences and reference strains
Only representative sequences from Fig. 2 were used for comparison.
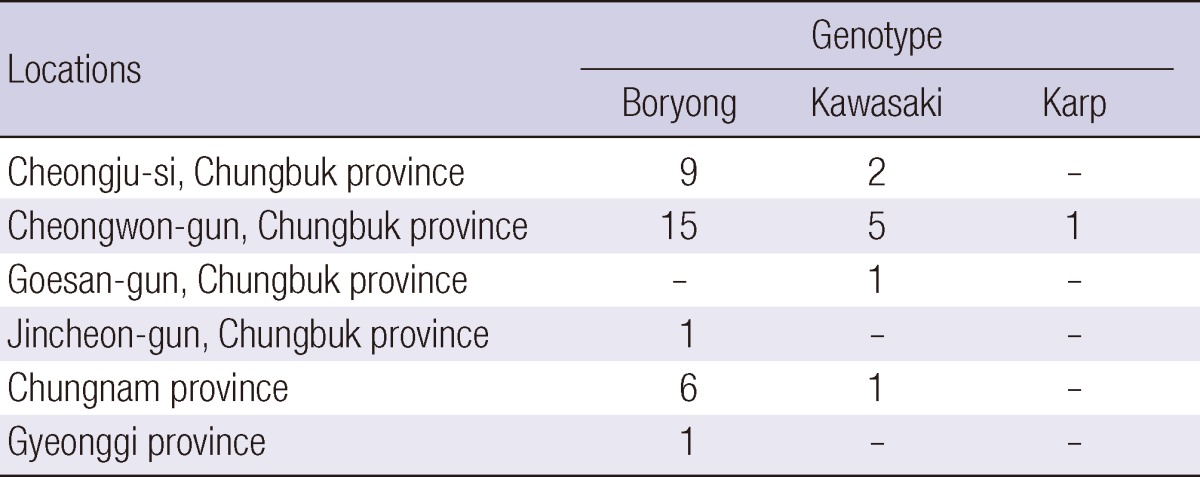
The demographic and clinical characteristics of the patients are shown in Table 3. The mean age of the patients was 62.8 yr. There were no significant differences in the time to defervescence between those with the Boryong genotype and those with the Kawasaki genotype (P = 0.282). One patient infected with the Boryong genotype presented with hypotension. Pneumonia was the only condition involving the major organs. The rates of pneumonia were not significantly different between patients infected with the Boryong and Kawasaki genotypes (4/32, 12.5% vs 1/11, 9.1%; P = 1.000). The areas in which the chigger bite was presumed to have occurred were mainly Cheongju-si and Cheongwon-gun (Table 4); although some patients were from other parts of Chungbuk province, or from Chungnam or Gyeonggi provinces. No notable differences in geographic distribution between each genotype were identified.
Table 3.
Demographic and clinical characteristics of patients and the genotypes O. tsutsugamushi based on the 56-kDa TSA nucleotide sequences
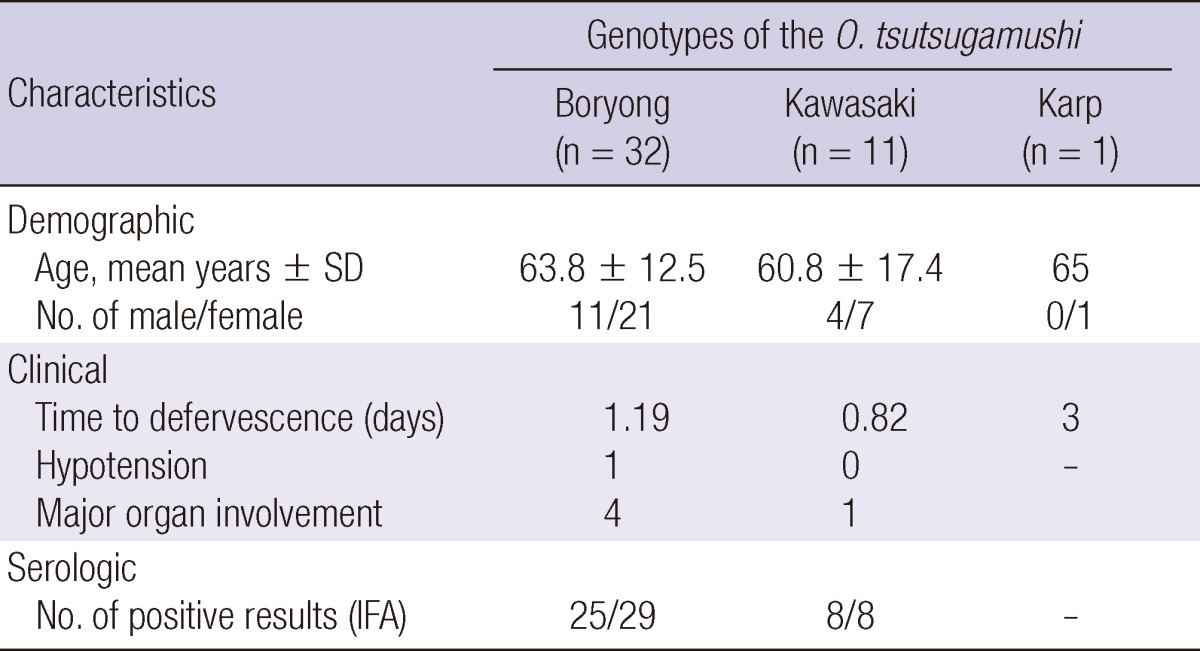
Table 4.
Genotypic distribution of O. tsutsugamushi obtained from patients with scrub typhus who visited Chungbuk National University Hospital between September 1, 2009 and December 31, 2010
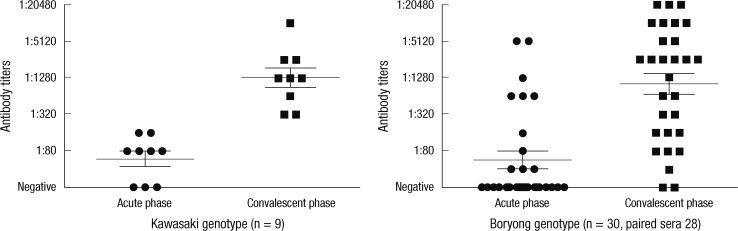
Paired sera were obtained in 37 patients. Serological tests were performed on the 37 patients using an IFA based upon the 56-kDa type-specific antigen from the Gilliam, Karp and Boryong serotypes. IFA titers of the patients infected with the Boryong and Kawasaki genotypes are shown separately in Fig. 3. The mean intervals between the first sampling and the second sampling were 8 days in both Kawasaki and Boryong genotypes. All patients infected with the Kawasaki genotype produced positive results (a ≥4-fold change in the titer in paired sera, or a titer ≥1:80 in a single serum sample) in the serological assay and 25 patients infected with the Boryong genotype produced positive results. Four patients infected with the Boryong genotype showed negative results. The sensitivity of the serological test was not different between the Boryong and the Kawasaki genotypes.
Fig. 3.
IFA titers of the patients infected with the Kawasaki and Boryong genotypes. Paired sera were obtained from each patient and IFA was done based upon the 56-kDa type-specific antigen from the Gilliam, Karp and Boryong genotype. The sensitivity of the IFA in Kawasaki genotype was not different to that in Boryong genotype suggesting cross-reactivity among genotypes.
DISCUSSION
Establishing the serotype of O. tsutsugamushi present in endemic areas is important for basic research, definitive serologic diagnosis of scrub typhus, and for the development of vaccines. Incorporating the antigens present in the prevalent genotypes of O. tsutsugamushi into geographically-specific diagnostic tests would improve the sensitivity of the serologic diagnosis. The antigenic diversity of O. tsutsugamushi in Korea has been reported in previous studies, and the Boryong serotype consistently predominates (9, 12, 17, 18).
The results of the present study showed that the Boryong genotype is most common in Chungbuk province in central Korea. This result is consistent with the findings of other studies, which include relatively small sample numbers from Chungbuk province (1, 12, 15, 19). We also found that the Kawasaki genotype of O. tsutsugamushi, which was not identified in Korea until 2010, made up a relatively large portion of the human infections in our study group (11/44 isolates; 25%) (15, 16). We believe that this is because not enough samples from Chungbuk province were included in previous studies or that there has been some change in the strains of O. tsutsugamushi present in central Korea. Recent changes in climate and environment may have affected the rodents or chiggers living in Korea. Future studies, with more nationwide sampling, are necessary to determine the contemporary distribution of O. tsutsugamushi genotypes in Korea.
The clinical symptoms and complication rates of patients infected with the two major genotypes (Boryong and Kawasaki) did not show difference. This result suggests that the pathogenicity and disease severity of the Boryong and Kawasaki genotypes are similar. More investigations into the genotypes of O. tsutsugamushi and the clinical features of infected patients are needed to know whether there are any difference of clinical courses in each genotype.
In Korea, most of the serological diagnostic tests incorporate the 56-kDa type-specific antigen from the Gilliam, Karp and Boryong strains. Though the 56-kDa type-specific antigen of the Kawasaki strain was not included in diagnostic test panel in this study, the diagnostic serological test still identified the Kawasaki strain (sensitivity 100%). This may be due to cross-reactivity between the strains. Interestingly, the sensitivity of the serological test was lower for the Boryong strain than for the Kawasaki strain (86 vs 100%). In other studies using the passive hemagglutination assay (PHA) with 56-kDa antigen from the Gilliam, Karp and Boryong strains, the sensitivity of the diagnostic test was significantly lower for the Kawasaki strain than for the Boryong strain (16). Therefore, further study is necessary to establish the sensitivity and specificity of the current serological diagnostic assay used in Korea, and to identify which strains should be included in the IFA to guarantee the best test performance.
The 56-kDa type-specific antigen is the primary immunogen that elicits neutralizing antibodies in the hosts (20-22). Some reports suggest that this protein has an important role in cellular invasion by O. tsutsugamushi (23). The protein contains four variable domains (variable domains I to IV) that differ between strains (24). Previous Korean studies analyzed partial sequences from some of the variable domains of the 56-kDa type-specific antigen to determine the genotype of O. tsutsugamushi (12, 15, 16); however, to our knowledge, the present study is the first to perform a phylogenetic analysis of the entire ORF of the 56-kDa TSA of O. tsutsugamushi collected in Korea. Also, it is the first report showing that the Kawasaki genotype is quite prevalent in Korea.
References
- 1.Ree HI, Cho MK, Lee IY, Jeon SH. Comparative epidemiological studies on vector/reservoir animals of tsutsugamushi disease between high and low endemic areas in Korea. Korean J Parasitol. 1995;33:27–36. doi: 10.3347/kjp.1995.33.1.27. [DOI] [PubMed] [Google Scholar]
- 2.Tamura A, Takahashi K, Tsuruhara T, Urakami H, Miyamura S, Sekikawa H, Kenmotsu M, Shibata M, Abe S, Nezu H. Isolation of Rickettsia tsutsugamushi antigenically different from Kato, Karp, and Gilliam strains from patients. Microbiol Immunol. 1984;28:873–882. doi: 10.1111/j.1348-0421.1984.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 3.Murata M, Yoshida Y, Osono M, Ohashi N, Oyanagi M, Urakami H, Tamura A, Nogami S, Tanaka H, Kawamura A., Jr Production and characterization of monoclonal strain-specific antibodies against prototype strains of Rickettsia tsutsugamushi. Microbiol Immunol. 1986;30:599–610. doi: 10.1111/j.1348-0421.1986.tb02987.x. [DOI] [PubMed] [Google Scholar]
- 4.Yamashita T, Kasuya S, Noda S, Nagano I, Ohtsuka S, Ohtomo H. Newly isolated strains of Rickettsia tsutsugamushi in Japan identified by using monoclonal antibodies to Karp, Gilliam, and Kato strains. J Clin Microbiol. 1988;26:1859–1860. doi: 10.1128/jcm.26.9.1859-1860.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elisberg BL, Campbell JM, Bozeman FM. Antigenic diversity of rickettsia tsutsugamushi: epidemiologic and ecologic significance. J Hyg Epidemiol Microbiol Immunol. 1968;12:18–25. [PubMed] [Google Scholar]
- 6.Yamamoto S, Kawabata N, Tamura A, Urakami H, Ohashi N, Murata M, Yoshida Y, Kawamura A., Jr Immunological properties of Rickettsia tsutsugamushi, Kawasaki strain, isolated from a patient in Kyushu. Microbiol Immunol. 1986;30:611–620. doi: 10.1111/j.1348-0421.1986.tb02988.x. [DOI] [PubMed] [Google Scholar]
- 7.Manosroi J, Chutipongvivate S, Auwanit W, Manosroi A. Determination and geographic distribution of Orientia tsutsugamushi serotypes in Thailand by nested polymerase chain reaction. Diagn Microbiol Infect Dis. 2006;55:185–190. doi: 10.1016/j.diagmicrobio.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Fournier PE, Siritantikorn S, Rolain JM, Suputtamongkol Y, Hoontrakul S, Charoenwat S, Losuwanaluk K, Parola P. Detection of new genotypes of Orientia tsutsugamushi infecting humans in Thailand. Clin Microbiol Infect. 2008;14:168–173. doi: 10.1111/j.1469-0691.2007.01889.x. [DOI] [PubMed] [Google Scholar]
- 9.Chang WH. Tsutsugamushi disease in Korea. Seoul: Seohung Press; 1994. pp. 56–57. [Google Scholar]
- 10.Kim DM, Yun NR, Neupane GP, Shin SH, Ryu SY, Yoon HJ, Wie SH, Kim WJ, Lee CY, Choi JS, et al. Differences in clinical features according to Boryoung and Karp genotypes of Orientia tsutsugamushi. PLoS One. 2011;6:e22731. doi: 10.1371/journal.pone.0022731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu H, Park SH, Kim EJ, Hwang KJ, Shim SK, Park S, Park MY. Phylogenetic clustering of 4 prevalent virulence genes in Orientia tsutsugamushi isolates from human patients. J Microbiol. 2010;48:124–128. doi: 10.1007/s12275-009-0267-7. [DOI] [PubMed] [Google Scholar]
- 12.Ree HI, Kim TE, Lee IY, Jeon SH, Hwang UW, Chang WH. Determination and geographical distribution of Orientia tsutsugamushi serotypes in Korea by nested polymerase chain reaction. Am J Trop Med Hyg. 2001;65:528–534. doi: 10.4269/ajtmh.2001.65.528. [DOI] [PubMed] [Google Scholar]
- 13.Higgins DG, Bleasby AJ, Fuchs R. CLUSTAL V: improved software for multiple sequence alignment. Comput Appl Biosci. 1992;8:189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- 14.Perriere G, Gouy M. WWW-query: an on-line retrieval system for biological sequence banks. Biochimie. 1996;78:364–369. doi: 10.1016/0300-9084(96)84768-7. [DOI] [PubMed] [Google Scholar]
- 15.Lee YM, Kim DM, Lee SH, Jang MS, Neupane GP. Phylogenetic analysis of the 56 kDa protein genes of Orientia tsutsugamushi in Southwest Area of Korea. Am J Trop Med Hyg. 2011;84:250–254. doi: 10.4269/ajtmh.2011.09-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park SW, Lee CK, Kwak YG, Moon C, Kim BN, Kim ES, Kang JM, Lee CS. Antigenic drift of Orientia tsutsugamushi in South Korea as identified by the sequence analysis of a 56-kDa protein-encoding gene. Am J Trop Med Hyg. 2010;83:930–935. doi: 10.4269/ajtmh.2010.09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korea Centers for Disease Control and Prevention. Current trends of scrub typhus. Commun Dis Monthly Rep. 2004;15:245–252. [Google Scholar]
- 18.Shim SK, Choi EN, Yu KO, Park HJ, Kim CM, Kee KH, Park JK, Park PH, Yoon MH, Park SH, et al. Characterisation of Orientia tsutsugamushi genotypes from wild rodents and chigger mites in Korea. Clin Microbiol Infect. 2009;15(Suppl 2):311–312. doi: 10.1111/j.1469-0691.2008.02254.x. [DOI] [PubMed] [Google Scholar]
- 19.Chang WH. Current status of tsutsugamushi disease in Korea. J Korean Med Sci. 1995;10:227–238. doi: 10.3346/jkms.1995.10.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seong SY, Kim HR, Huh MS, Park SG, Kang JS, Han TH, Choi MS, Chang WH, Kim IS. Induction of neutralizing antibody in mice by immunization with recombinant 56 kDa protein of Orientia tsutsugamushi. Vaccine. 1997;15:1741–1747. doi: 10.1016/s0264-410x(97)00112-6. [DOI] [PubMed] [Google Scholar]
- 21.Seong SY, Park SG, Huh MS, Jang WJ, Kim HR, Han TH, Choi MS, Chang WH, Kim IS. Mapping of antigenic determinant regions of the Bor56 protein of Orientia tsutsugamushi. Infect Immun. 1997;65:5250–5256. doi: 10.1128/iai.65.12.5250-5256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi MS, Seong SY, Kang JS, Kim YW, Huh MS, Kim IS. Homotypic and heterotypic antibody responses to a 56-kilodalton protein of Orientia tsutsugamushi. Infect Immun. 1999;67:6194–6197. doi: 10.1128/iai.67.11.6194-6197.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JH, Cho NH, Kim SY, Bang SY, Chu H, Choi MS, Kim IS. Fibronectin facilitates the invasion of Orientia tsutsugamushi into host cells through interaction with a 56-kDa type-specific antigen. J Infect Dis. 2008;198:250–257. doi: 10.1086/589284. [DOI] [PubMed] [Google Scholar]
- 24.Ohashi N, Nashimoto H, Ikeda H, Tamura A. Diversity of immunodominant 56-kDa type-specific antigen (TSA) of Rickettsia tsutsugamushi. Sequence and comparative analyses of the genes encoding TSA homologues from four antigenic variants. J Biol Chem. 1992;267:12728–12735. [PubMed] [Google Scholar]