Abstract
Quantification of quality of life (QOL) related to disease severity is important in patients with atopic dermatitis (AD), because the assessment provides additional information to the traditional objective clinical scoring systems. To document the impact of AD on QOL for both children and adults as well as to quantify the relationship with disease severity, QOL assessments were performed over a 6-month period on 415 patients with AD. A questionnaire derived from the Infants' Dermatitis Quality of Life Index (IDQOL), the Children's Dermatology Life Quality Index (CDLQI) and the Dermatology Life Quality Index (DLQI) was used to determine the QOL for 71 infants, 197 children and 147 adults, respectively. To measure AD severity, both the Rajka & Langeland scoring system and the Scoring of Atopic Dermatitis (SCORAD) index were used. The mean scores were as follows: 7.7 ± 5.5 for IDQOL, 6.6 ± 6.3 for CDLQI, and 10.7 ± 7.9 for DLQI. In conclusion, these QOL scores are correlated with AD severity scores as estimated by the Rajka & Langeland severity score and the SCORAD. The outcome of the QOL instruments in this study demonstrates that atopic dermatitis of both children and adults affects their QOL.
Keywords: Atopic Dermatitis, Disease Severity, Quality of Life
INTRODUCTION
Atopic dermatitis (AD) is a pruritic, chronic, relapsing, inflammatory skin condition that affects between 2% and 7% of adults worldwide (1), with the overall prevalence of AD reported to have increased over the last 3 decades. In Korea, the total prevalence of AD in Korean children and adults was 2.2%-2.6% (2, 3). Clinically, approximately 70% of all AD patients have personal or family histories of atopic disease, including asthma, allergic rhinitis, allergic conjunctivitis, and AD (4). Often severe, pruritus is a universal finding in AD and may result in sleep disruption, irritability and generalized stress for both the affected patients as well as family members. Other reported sequelae include difficulty falling asleep, diminished total sleep, increased sleep-related awakenings, daytime tiredness, and irritability (5, 6).
Quality of life (QOL) is a very broad concept and generally concerns whether a disease or functional impairment limits an individual's ability to complete daily tasks, it can also be used to assess the burden of illness and the outcomes of related medical treatments (7, 8). Recently, interest in measuring QOL has markedly increased, it is useful for physicians to understand patients' own perceptions of illness and its effects on day-to-day life (9). Despite the widespread prevalence of AD, little is known about how the QOL of AD patients correlates with disease severity or if it substantially differs from the QOL of the general public (7).
The Dermatology Life Quality Index (DLQI), the Children's Dermatology Life Quality Index (CDLQI) and the Infants Dermatitis Quality of Life Index (IDQOL) are such an index that has recently become popular. These questionnaires, which have been translated into several languages were easy to use in clinical situations. When comparing situations among patients in different countries, it is of great importance to use the same QOL instruments (10). These questionnaires cover, for example, symptoms and feelings/mood, disease severity, feeding, dressing/undressing, housework, leisure, school or holidays, personal relationships, sleep, expenditure and treatment.
Establishing of the degree of QOL impairment associated with AD may help providers with treatment-related decision making. As such, the aim of the present study was to evaluate the impact of AD on the QOL of both children and adults, and to identify the area of AD patients' lives most affected by the disease. In this way, a secondary goal was to use these findings to help guide the appropriate management of AD.
MATERIALS AND METHODS
Selection of patients
QOL assessments were performed over a 6-month period (from May to October 2010) on 415 patients with AD, all of whom fulfilled the criteria defined by Hanifin and Rajka (11). All subjects were seen at the dermatology clinics of a university teaching hospital that is affiliated with the Korean Atopic Dermatitis Association. Subjects were then divided into 3 groups: infants or children less than 5 yr, children between 5 and 16 yr, and adults older than 16 yr.
Assessment tools
For all measurements of AD severity, two standardized methods were used: the Rajka & Langeland (12) scoring system and the Scoring of Atopic Dermatitis (SCORAD) index (13). Specifically, the Rajka & Langeland score assess the course of disease over the most recent year as well as the present extent of eczema and associated itching, with scores ranging from 0 to 9 (mild 3-4, moderate 4.5-7.5, severe 8-9) (12).
SCORing Atopic Dermatitis (SCORAD) measures the extent, intensity, average degree of pruritus and sleep loss related to AD in the preceding 3 days. The SCORAD is a weighted index that is less related to the extent of disease (multiplying by a factor of 0.2) and places more emphasis on the intensity (a factor of 3.5) and current degree of pruritus and sleep loss (a factor of 1). Using this index, subjects were further subdivided into mild (1-15), moderate (16-40) and severe (41-103) groups based on the SCORAD results (13).
After the scoring AD severity scores were assessed, the QOL questionnaires were distributed and completed. QOL data for AD patients over the age of 16 yr were collected using self-administered questionnaires that incorporated a validated Korean version of DLQI (14). QOL was assessed in subjects between the ages of 5 and 16 yr using a validated Korean version of CDLQI questionnaire (15, 16). QOL for subjects less than 5 yr of age was assessed using a validated Korean version of IDQOL questionnaire (17). For this index, the total score is calculated by totaling the score from 10 individual questions (maximum = 30, minimum = 0), so that the higher the score, the greater the QOL impairment.
Statistical analysis
SPSS software (version 12.0; SPSS Inc. Chicago, IL, USA) was used for all analyses. The Mann-Whitney U test was used for comparisons between independent samples, while Spearman's rank order correlation coefficient was used as a measure of association. All comparisons were two-tailed, and P < 0.05 was considered significant in all cases.
Ethics statement
Appropriate ethical approval was obtained for studies enrolling both adult and child subjects by institutional review board of Chung Ang University Hospital (IRB No. C2010034-329). Informed consent was obtained from the subjects or parental guardians prior to the enrollment.
RESULTS
Subjects
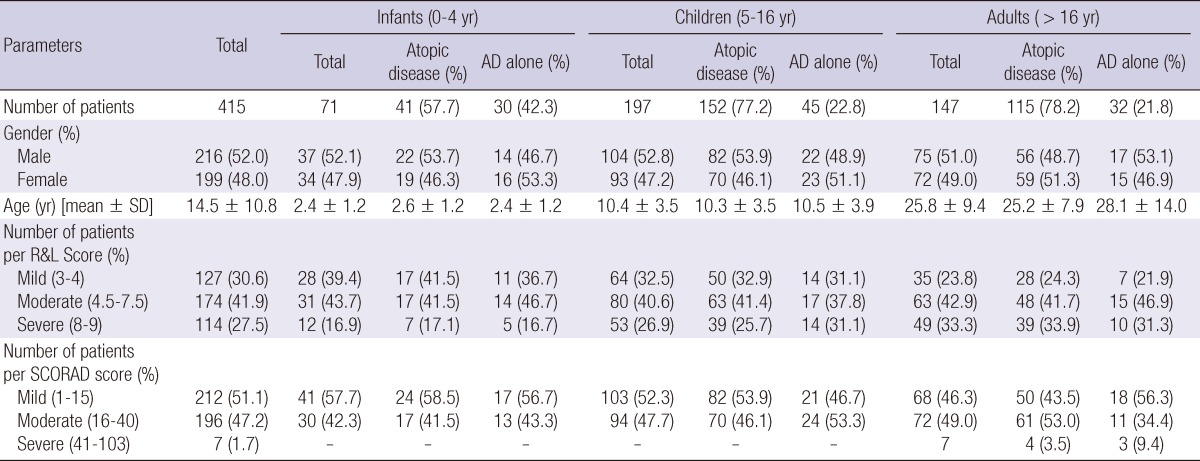
In total, 415 patients from 10 university hospitals were enrolled. The median age was 14.5 ± 10.8 yr (range: 0-85 yr), with 216 males and 199 females participating. Patients were divided into 3 groups: infants less than 5 yr (n = 71; 37 males, 34 females), children between 5 and 16 yr (n = 197; 104 males, 93 females), and adults over 16 yr (n = 147; 75 males, 72 females). The patients were divided into 2 groups: atopic disease including AD with concomitant asthma, allergic rhinitis, or allergic conjunctivitis (n = 308; 41 infants, 152 children, 115 adults), and AD alone (n = 107; 30 infants, 45 children, 32 adults). Details of the study sample are outlined in Table 1.
Table 1.
Study sample demographics and number of patients per the Rajka & Langeland (R&L) and SCORAD scores
AD, Atopic dermatitis; Atopic disease, AD with concomitant asthma, allergic rhinitis, and/or allergic conjunctivitis; R&L Score, Rajka & Langeland Score; SCORAD score, SCORing Atopic Dermatitis score.
AD severity score
The mean value ± standard deviation (SD) for the Rajka & Langeland score was 5.4 ± 1.9 in infants 0-4 yr, 5.8 ± 1.9 in children 5-16 yr, and 6.2 ± 1.9 in adults over the 16 yr. The mean SCORAD ± SD was 15.8 ± 8.4 for infants 0-4 yr of age, 16.6 ± 7.9 for children 5-16 yr, and 19.6 ± 10.0 for adults over 16 yr. The correlation coefficient between the Rajka & Langeland score and the SCORAD was 0.688 (P < 0.001).
Quality of life
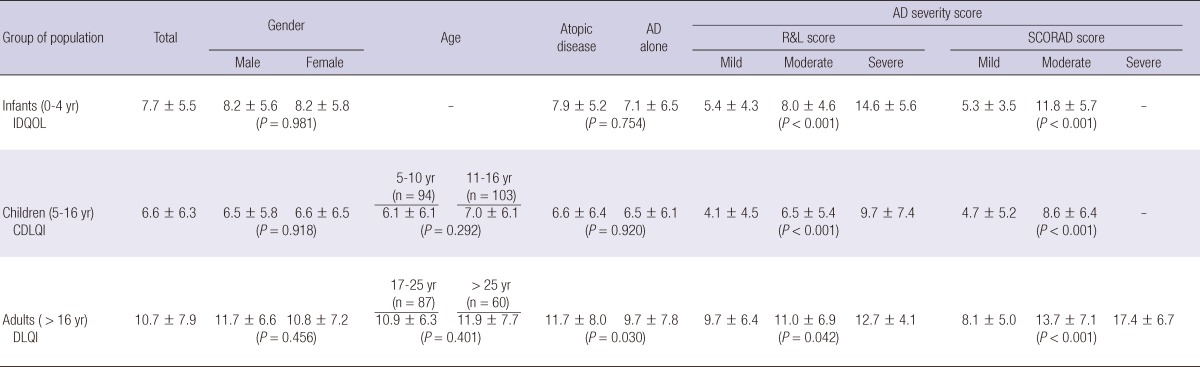
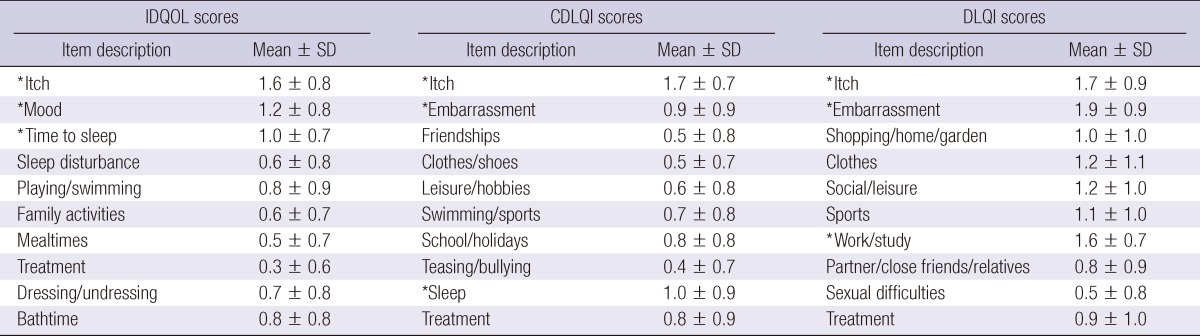
The total scores for the different QOL questionnaires are shown in Table 2. The total mean (± SD) IDQOL score was 7.7 (± 5.5). No significant differences in gender were observed (male vs female mean ± SD: 8.2 ± 5.6 vs 8.2 ± 5.8, P = 0.981). No significant differences occurring between infants with AD alone and infants with atopic disease including AD with concomitant asthma, allergic rhinitis, or allergic conjunctivitis (mean ± SD: 7.1 ± 6.5 vs 7.9 ± 5.2, P = 0.754). The 3 items in the IDQOL questionnaire with the highest scores (mean ± SD) - that is the variables that most negatively impacted the QOL - were "itching and scratching" (1.6 ± 0.8), "the child's mood" (1.2 ± 0.8) and "time to get the child to sleep" (1.0 ± 0.7), whereas the lowest-scored item was "problems caused by the treatment" (0.3 ± 0.6). The 10 detailed dimensions of the IDQOL can be seen in Table 3.
Table 2.
QOL scores (IDQOL/CDLQI/DLQI) as measured by the gender, the age, the history of atopic diseases, and the atopic dermatitis severity score
IDQOL, Infants Dermatitis Quality of Life Index; CDLQI, Children's Dermatology Life Quality Index; DLQI, Dermatology Life Quality Index; AD, Atopic dermatitis; Atopic disease, AD with concomitant asthma, allergic rhinitis, and/or allergic conjunctivitis; R&L Score, Rajka & Langeland Score; SCORAD score, SCORing Atopic Dermatitis score.
Table 3.
Mean ± standard deviation of each component of quality of life scores (IDQOL, CDLQI, and DLQI)
*The three variables in the IDQOL, CDLQI, and DLQI questionnaire with the highest scores (mean ± SD). IDQOL, Infants Dermatitis Quality of Life Index; CDLQI, Children's Dermatology Life Quality Index; DLQI, Dermatology Life Quality Index.
The total mean (± SD) CDLQI score was 6.6 (± 6.3). No significant differences in gender and age were observed (male vs female mean ± SD: 6.5 ± 5.8 vs 6.6 ± 6.5, P = 0.918; 5-10 yr vs 11-16 yr mean ± SD: 6.1 ± 6.1 vs 7.0 ± 6.1, P = 0.292). No significant differences were noticed between infants with AD alone and infants with atopic disease including AD with concomitant asthma, allergic rhinitis, or allergic conjunctivitis (mean ± SD: 6.5 ± 6.1 vs 6.6 ± 6.4, P = 0.920). The 3 items in the CDLQI questionnaire with the highest scores (mean ± SD), were "itchy, scratchy, sore or painful" (1.7 ± 0.7), "problem with sleep" (1.0 ± 0.9), and "embarrassed, self-conscious, upset or sad because of the skin" (0.9 ± 0.9), while the variable with the lowest score was "calling you names, teasing, bullying, asking questions or avoiding" (0.4 ± 0.7). The 10 detailed dimensions of the CDLQI can be seen in Table 3.
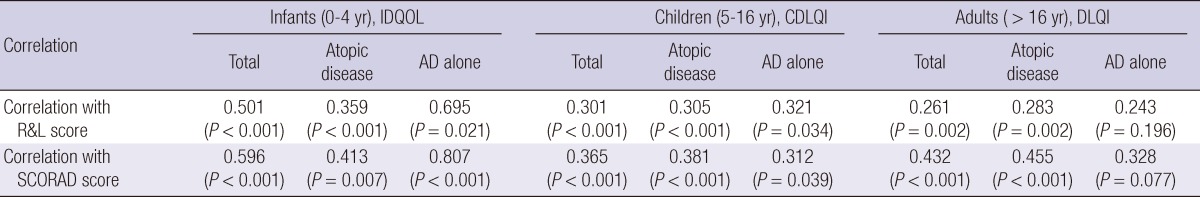
The total mean (± SD) DLQI score was 10.7 (± 7.9). No significant differences in gender and age were observed (male vs female mean ± SD: 11.7 ± 6.6 vs 10.8 ± 7.2, P = 0.456; 17-25 yr vs > 26 yr mean ± SD: 10.9 ± 6.3 vs 11.9 ± 7.7, P = 0.401). In this subset, adults with atopic disease including AD with concomitant asthma, allergic rhinitis, or allergic conjunctivitis had higher total scores than those with AD alone (mean ± SD: 11.7 ± 8.0 vs 9.7 ± 7.8, P = 0.030). The 3 variables in the DLQI questionnaire with the highest scores (mean ± SD) were "embarrassed or self conscious" (1.9 ± 0.9), "itchy, sore, painful or stinging" (1.7 ± 0.9), and "prevented from working or studying" (1.6 ± 0.7), while the item with the lowest score was "sexual difficulties" (0.5 ± 0.8). The 10 detailed dimensions of the DLQI are shown in Table 3. As shown in Table 4, both the Rajka & Langeland eczema severity score and SCORAD index correlated significantly with the all total QOL scores (IDQOL, CDLQI, and DLQI scores).
Table 4.
Correlation (Spearman's rank order) between QOL scores (IDQOL/CDLQI/DLQI) and AD severity scores (Rajka & Langeland AD severity score/SCORAD severity score)
IDQOL, Infants Dermatitis Quality of Life Index; CDLQI, Children's Dermatology Life Quality Index; DLQI, Dermatology Life Quality Index; AD, Atopic dermatitis; Atopic disease, AD with concomitant asthma, allergic rhinitis, and/or allergic conjunctivitis; R&L Score, Rajka & Langeland Score; SCORAD score, SCORing Atopic Dermatitis score.
DISCUSSION
This hospital-based cross-sectional study represented the first study in which the impact of eczema severity (assessed by a doctor) on the QOL of children and adults with AD and parents of infants with AD (assessed by themselves). In this study, we observed that AD impaired QOL among Korean children and adults did not vary by gender or age. The IDQOL, CDLQI and DLQI were correlated with the AD severity scores estimated by either the Rajka and Langeland (12) or the SCORAD scores.
The current study demonstrated and confirmed that AD impaired patients' QOL, though we were unable to detect any significant effect of gender and age on QOL scores. This finding could perhaps be explained by the similarity in life perception in the gender and age group studies. Here, the mean total IDQOL score was 7.6. These data are in agreement with prior studies by Lewis-Jones et al. (17) (mean 7.9) and Gånemo et al. (18) (men 8.6), though higher than the results described by Chinn et al. (19) (mean 5.9). However, it is important to note that in the former two studies the subjects were recruited from pediatric dermatologic clinics, while in the latter all subjects came from two general practices. As such, it seems reasonable that patients referred to a specialist would likely have more severe disease than patients treated at a general practice.
The mean total CDLQI score in the present study was 6.7. These data were in agreement with the results of a previous study by Gånemo et al. (18) (mean 7.1), though slightly lower than another comparable study (mean 7.7) (16). Lastly, the mean total DLQI score for this patient population was 10.7. These results were in agreement with another Korean study from Lee et al. (20) (mean 10.7), though higher than those described in Holm et al. (median 5) (21). However, as only a small number of patients were recruited in the latter study, the affect may be underestimated.
In the current study, a dual approach was adopted: both short- and long-term disease severity was taken into considerations during patient evaluation by combining the Rajka & Langeland severity score and SCORAD. The SCORAD comprises of objective signs (disease extent and intensity) and subjective short-term symptoms (pruritus and sleep loss) over the preceding 3 days. It is a weighted index but without a severity grading (12). The SCORAD does not provide any information on the long-term disease severity. The Rajka & Langeland severity score, on the other hand, measures the disease severity over a 12-month period (13). Although these scoring systems are effective in evaluating the severity of AD, they do not reflect the disabilities or sufferings in daily life. QOL is an important parameter in the assessment of distress experienced by children and adults with AD. Our present study further demonstrates that QOL score and the two clinical scores measure some comparable domains of the disease: the objective signs of disease correlated well with the subjective domains of QOL, as measured by the IDQOL, CDLQI and DLQI.
These results are notably similar from several prior studies which found that QOL and disease severity were correlated among AD patients (17, 20). In these reports, almost all of the subjects had mild to moderate AD, and either the Rajka & Langeland severity score or the UK diagnostic criteria for AD were used. Interestingly, in studies in which more subjects with moderate to severe AD were enrolled (22, 23), the QOL score (IDQOL, CDLQI and DLQI) is less sensitive in reflecting disease severity among AD patients with more severe disease. As such, the relationship between QOL assessments and disease severity measurements merits further study.
This study demonstrates that adults with AD concomitant other atopic diseases including asthma, allergic rhinitis, allergic conjunctivitis experienced greater QOL impairment than the patients with AD alone. We do not know how to explain the finding that only adults with atopic diseases experienced greater QOL impairment than the patients with AD alone. We suggest that adults with concomitant atopic disease have more disease complications and decreased QOL compared with children. Further research is needed to replicate and explain this finding. In fact, many have accompanying atopic dermatitis, asthma, and allergic rhinitis (23). The condition of comorbid atopic diseases may cause children and adults to be uncomfortable as a result of not only intense pruritus, sleep loss, but also coughing and/or wheezing, dyspnea, concomitant nasal congestion and/or rhinorrhea (23, 24). Those indicated important areas of concern such as withdrawal from public places, adoption of special clothing habits and concern about personal relationships (25).
This study had some limitations. First, all assessments were performed in a dermatology clinic affiliated with teaching hospitals, thus these results should not be extrapolated to a primary care setting. Second, as AD tends to improve during summer and get worse in winter, recruitment times may represent a potential confounding factor, since we did not evaluate the seasonal impact in this study. Third, the population under investigation included those with a wide range of eczema severities, with a majority of mild cases in the SCORAD index. It is unlikely that the relationship between the QOL score and disease severity will predictably remain the same in different disease severities. It may be strengthened or become weaker according to disease stage. Further research on larger numbers of AD patients in the community is currently underway.
However, part of the reason for such a study is to investigate if the QOL instrument can add some information which is useful about the disease status that cannot be determined directly by the doctor. The IDQOL, CDLQI, and DLQI proved to be useful in providing extra information about disease status as perceived by the AD patients themselves. Therefore QOL instruments should be used as extra measures of assessing outcome in clinical practice, research and clinical trials (18, 26). In conclusion, our results demonstrate that AD impairs the QOL among Korean children and adults, and does not vary by gender or age. The IDQOL, CDLQI and DLQI are correlated with the AD severity scores estimated by either the Rajka and Langeland (12) or the SCORAD scores. The outcome of the QOL instruments in this study demonstrates that atopic dermatitis of both children and adults affects their QOL.
ACKNOWLEDGMENTS
We would like to thank the AD patients and their parents who participated in this study. We thank Drs. Andrew Finlay and Sue Lewis-Jones for permission to use the IDQOL, CDLQI and DLQI.
References
- 1.Hunter JA, Herd RM. Recent advances in atopic dermatitis. Q J Med. 1994;87:323–327. [PubMed] [Google Scholar]
- 2.Yu JS, Lee CJ, Lee HS, Kim J, Han Y, Ahn K, Lee SI. Prevalence of atopic dermatitis in Korea: analysis by using national statistics. J Korean Med Sci. 2012;27:681–685. doi: 10.3346/jkms.2012.27.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim MJ, Kang TW, Cho EA, Kim HS, Min JA, Park H, Kim JW, Cha SH, Lee YB, Cho SH, et al. Prevalence of atopic dermatitis among Korean adults visiting health service center of the Catholic Medical Center in Seoul Metropolitan Area, Korea. J Korean Med Sci. 2010;25:1828–1830. doi: 10.3346/jkms.2010.25.12.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carroll CL, Balkrishman R, Feldman SR, Fleischer AB, Jr, Manuel JC. The burden of atopic dermatitis; impact on the patient, family and society. Pediatr Dermatol. 2005;22:192–199. doi: 10.1111/j.1525-1470.2005.22303.x. [DOI] [PubMed] [Google Scholar]
- 5.Chamlin SL, Frieden IJ, Williams ML, Chren MM. Effects of atopic dermatitis on young American children and their families. Pediatrics. 2004;114:607–611. doi: 10.1542/peds.2004-0374. [DOI] [PubMed] [Google Scholar]
- 6.Whalley D, Huels J, McKenna SP, Van Assche D. The benefit of pimecrolimus (Elidel. SDZ ASM981) on parents' quality of life in the treatment of pediatric atopic dermatitis. Pediatrics. 2002;110:1133–1136. doi: 10.1542/peds.110.6.1133. [DOI] [PubMed] [Google Scholar]
- 7.Kiebert G, Sorensen SV, Revicki D, Fagan SC, Doyle JJ, Cohen J, Fivenson D. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151–158. doi: 10.1046/j.1365-4362.2002.01436.x. [DOI] [PubMed] [Google Scholar]
- 8.Carr AJ, Gibson B, Robinson PG. Measuring quality of life: is quality of life determined by expectations or experience? BMJ. 2001;322:1240–1243. doi: 10.1136/bmj.322.7296.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eiser C, Morse R. The measurement of quality of life in children: past and future perspectives. J Dev Behav Pediatr. 2001;22:248–256. doi: 10.1097/00004703-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Lewis V, Finlay AY. 10 years experience of the Dermatology Life Quality Index (DLQI) J Investig Dermatol Symp Proc. 2004;9:169–180. doi: 10.1111/j.1087-0024.2004.09113.x. [DOI] [PubMed] [Google Scholar]
- 11.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh) 1980;2:44–47. [Google Scholar]
- 12.Rajka G, Langeland T. Grading of the severity of atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1989;144:13–14. doi: 10.2340/000155551441314. [DOI] [PubMed] [Google Scholar]
- 13.European Task Force on Atopic Dermatitis. Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology. 1993;186:23–31. doi: 10.1159/000247298. [DOI] [PubMed] [Google Scholar]
- 14.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): A simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 15.Waters A, Sandhu D, Beattie P, Ezughah F, Lewis-Jones S. Severity stratification of Children's Dermatology Life Quality Index (CDLQI) scores. Br J Dermatol. 2010;163(Suppl 1):121. [Google Scholar]
- 16.Lewis-Jones MS, Finlay AY. The Children's Dermatology Life Quality Index (CDLQI): Initial validation and practical use. Br J Dermatol. 1995;132:942–949. doi: 10.1111/j.1365-2133.1995.tb16953.x. [DOI] [PubMed] [Google Scholar]
- 17.Lewis-Jones MS, Finlay AY, Dykes PJ. The infants' dermatitis quality of life index. Br J Dermatol. 2001;144:104–110. doi: 10.1046/j.1365-2133.2001.03960.x. [DOI] [PubMed] [Google Scholar]
- 18.Gånemo A, Svensson A, Lindberg M, Wahlgren CF. Quality of life in Swedish children with eczema. Acta Derm Venereol. 2007;87:345–349. doi: 10.2340/00015555-0245. [DOI] [PubMed] [Google Scholar]
- 19.Chinn DJ, Poyner T, Sibley G. Randomized controlled trial of a single dermatology nurse consultation in primary care on the quality of life of children with atopic eczema. Br J Dermatol. 2002;146:432–439. doi: 10.1046/j.1365-2133.2002.04603.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee HJ, Park CO, Lee JH, Lee KH. Life quality assessment among adult patients with atopic dermatitis. Korean J Dermatol. 2007;45:159–164. [Google Scholar]
- 21.Holm EA, Wulf HC, Stegmann H, Jemec GB. Life quality assessment among patients with atopic eczema. Br J Dermatol. 2006;154:719–725. doi: 10.1111/j.1365-2133.2005.07050.x. [DOI] [PubMed] [Google Scholar]
- 22.Hon KL, Leung TF, Wong KY, Chow CM, Chuh A, Ng PC. Does age or gender influence quality of life in children with atopic dermatitis? Clin Exp Dermatol. 2008;33:705–709. doi: 10.1111/j.1365-2230.2008.02853.x. [DOI] [PubMed] [Google Scholar]
- 23.O'Connell EJ. The burden of atopy and asthma in children. Allergy. 2004;59(Suppl 78):7–11. doi: 10.1111/j.1398-9995.2004.00563.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee SM, Ahn JS, Noh CS, Lee SW. Prevalence of allergic diseases and risk factors of wheezing in Korean military personnel. J Korean Med Sci. 2011;26:201–206. doi: 10.3346/jkms.2011.26.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112(6 Suppl):S118–S127. doi: 10.1016/j.jaci.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 26.Ben Gashir MA, Seed PT, Hay RJ. Quality of life and disease severity are correlated in children with atopic dermatitis. Br J Dermatol. 2004;150:284–290. doi: 10.1111/j.1365-2133.2004.05776.x. [DOI] [PubMed] [Google Scholar]