Abstract
The aim of this study was to evaluate the clinical relevance and usefulness of the Onodera's prognostic nutritional index (OPNI) as a prognostic and nutritional indicator in peritoneal dialysis (PD) patients. Patients were divided into 3 groups based on the initial OPNI score: group A (n = 186, < 40), group B (n = 150, 40-45), and group C (n = 186, > 45). Group A was associated with a higher grade according to the Davies risk index than the other groups. Serum creatinine and albumin levels, total lymphocyte count, and fat mass increased with an increase in OPNI. According to the edema index, the correlation coefficient for OPNI was -0.284 and for serum albumin was -0.322. Similarly, according to the C-reactive protein (CRP), the correlation coefficient for OPNI was -0.117 and for serum albumin was -0.169. Multivariate analysis adjusted for age, Davies risk index, CRP, and edema index revealed that the hazard ratios for low OPNI, serum albumin, and CRP were 1.672 (P = 0.003), 1.308 (P = 0.130), and 1.349 (P = 0.083), respectively. Our results demonstrate that the OPNI is a simple method that can be used for predicting the nutritional status and clinical outcome in PD patients.
Keywords: Onodera's Prognostic Nutritional Index, Peritoneal Dialysis, Nutrition, Mortality, Albumin, Edema index
INTRODUCTION
Malnutrition is a common unfavorable complication prevalent in peritoneal dialysis (PD) patients and is associated with morbidity and mortality in these patients (1-5). The Kidney Disease Outcomes Quality Initiative (K/DOQI) guideline recommends various parameters for monitoring the nutritional status, such as serum albumin, subjective global assessment, anthropometry, and body composition (2). However, a standard method for the assessment of the nutritional status in PD patients does not exist.
The ideal nutritional index would provide an accurate prognosis, be associated with nutritional status, and be simple. Many nutritional indices such as the Malnutrition inflammation score, Mini nutritional assessment, Nutritional risk screening 2002, and Malnutrition universal screening tool have been established as a single predictor of mortality in malnourished patients (6, 7). These require many indicators such as serum albumin, questionnaire, weight loss, and body mass index (BMI), and are complex.
Onodera et al. (8) first reported the validity of the Onodera's prognostic nutritional index (OPNI) to predict the prognosis in 189 gastrointestinal surgical patients. The OPNI is composed of serum albumin and total lymphocyte count within the equation. A simpler tool may involve common measures and can be applied rapidly in a large number of patients. Validation of OPNI has been applied for patients with end-stage liver disease, active tuberculosis, and gastrointestinal malignancy (8-14). The aim of this study was to evaluate the clinical usefulness of the OPNI as a prognostic and nutritional indicator in PD patients.
MATERIALS AND METHODS
Selection of patients
We reviewed medical records at the Yeungnam University Hospital in Korea and identified all adults (aged > 18 yr) who received PD between April 1996 and March 2011. Among these patients, those without the information about the nutritional status evaluation or the OPNI were excluded. A total of 522 patients were included in this study. As described previously, patients were divided into 3 groups based on the initial OPNI score: group A (n = 186, < 40), group B (n = 150, 40-45), and group C (n = 186, > 45) (8, 12).
Clinical information
Clinical and laboratory data collected 1 month after the initiation of PD included age, gender, and underlying disease of end-stage renal disease, comorbidities, BMI (kg/m2), serum creatinine (mg/dL), normalized protein equivalent of nitrogen appearance (g/kg/day), C-reactive protein (CRP; mg/dL), serum albumin (g/dL), total cholesterol (mg/dL), residual renal function (RRF; mL/min/1.73 m2), edema index, PD modality, and fat mass (kg). If the patients exhibited signs of infection, laboratory findings from 2 months after the initiation of PD were substituted. Total lymphocyte counts were calculated by multiplying the percentage of lymphocytes with the total white blood cell count. Serum albumin and CRP levels were detected using an Olympus AU4500 automatic chemical analyzer; bromocresol green was used for the detection of albumin. Comorbidities were graded according to the Davies risk index and included ischemic heart disease, peripheral vascular disease, left ventricular dysfunction, diabetes mellitus, systemic collagen vascular disease, and other significant pathologies (15). Comorbidities graded by the Davies risk index were categorized as low risk (score 0), intermediate risk (score 1-2), or high risk (score ≥ 3). Furthermore, the presence of liver or spleen diseases, which are associated with changes in lymphocyte count, was identified. RRF was calculated from the collection of 24-hr urine samples as the average of the renal creatinine and urea clearances. The OPNI was calculated based on the serum albumin and total lymphocyte count, using the following equation: OPNI = 10 × serum albumin (g/dL) + 0.005 × total lymphocyte count (/mL) (8). The edema index was measured by bioimpedance analysis (Inbody 4.0 Body Composition Analyzer; Biospace, Seoul, Korea). This was defined as the ratio of the extracellular water to total body water. Fat mass was measured using a dual-energy X-ray absorptiometry (Hologic, Bedford, MA, USA).
Statistical analysis
The data were analyzed using SPSS version 19 (SPSS, Chicago, IL, USA). The distribution of continuous variables was evaluated using the Kolmogorov-Smirnov test. Normally distributed variables were expressed as mean ± standard deviation and compared using t-test or one-way analysis of variance (ANOVA). Non-parametric variables were expressed as median (range) and were compared using the Mann-Whitney or Kruskal-Wallis test. One-way ANOVA or Kruskal-Wallis test was followed by post-hoc Tukey comparison or Bonferroni correction. Categorical variables were expressed as counts and percentages. Pearson's chi-squared test or Fisher's exact test was used to analyze categorical variables. The strength of the relationship between the two variables was examined using Pearson's method of bivariate correlation. The survival estimates were calculated according to the Kaplan-Meier and Cox-regression analyses. P < 0.05 were considered statistically significant.
Ethics statement
The protocol was approved by the institutional review board of the Yeungnam University Hospital (YUH-12-0376-O41). The board waived the need for informed consent.
RESULTS
Clinical characteristics at the time of PD initiation
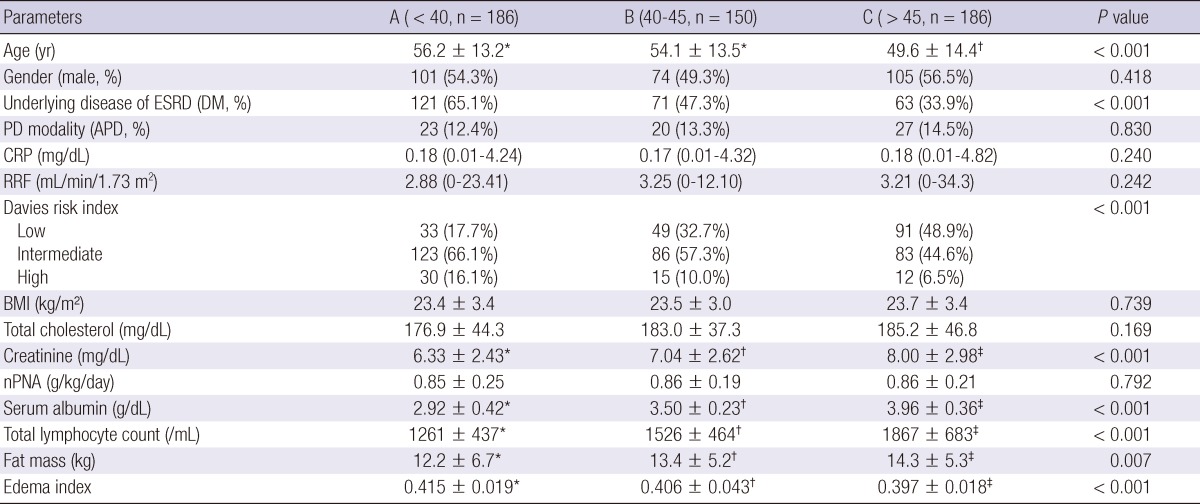
The mean age of patients was 56.2 ± 13.2 yr in group A, 54.1 ± 13.5 yr in group B, and 49.6 ± 14.4 yr in group C (P < 0.001) (Table 1). The median follow-up duration was 34.2 (1-173) months in group A, 41.9 (1-181) months in group B, and 37.6 (1-174) months in group C. Twenty-seven patients (5.2%) had liver or spleen diseases. Group A was associated with a higher incidence of diabetes, old age, and higher grade according to the Davies risk index than the other groups. Serum creatinine and albumin levels, total lymphocyte count, and fat mass increased with an increase in OPNI score. Edema index was 0.415 ± 0.019 in group A, 0.406 ± 0.043 in group B, 0.397 ± 0.018 in group C (P < 0.001). Edema index increased with a decrease in OPNI score. There were no significant differences in PD modality, CRP, RRF, BMI, and total cholesterol between 3 groups.
Table 1.
Comparison of clinical characteristics according to Onodera's prognostic nutritional index score at the time of peritoneal dialysis initiation
Data are expressed as numbers (percentages) for categorical variables and median (range) or mean ± standard deviation for continuous variables. Statistical significance was tested using one-way analysis of variance or Kruskal-Wallis test for continuous variables and Pearson's chi-squared test or Fisher's exact test for the categorical variables. Superscripts (*, †, ‡) indicate significant differences based on post-hoc Tukey comparison or Bonferroni correction. PD, peritoneal dialysis; ESRD, end-stage renal disease; DM, diabetes mellitus; APD, automated peritoneal dialysis; CRP, C-reactive protein; RRF, residual renal function; BMI, body mass index; nPNA, normalized protein equivalent of nitrogen appearance.
The OPNI and serum albumin were negatively correlated with CRP and the edema index (Table 2). According to the edema index, the correlation coefficient for OPNI was -0.284 and for serum albumin was -0.322. Similarly, according to CRP, the correlation coefficient for OPNI was -0.117 and for serum albumin was -0.169. The correlation coefficients for both variables were lower than those for serum albumin.
Table 2.
Correlation between variable markers and edema index or C-reactive protein
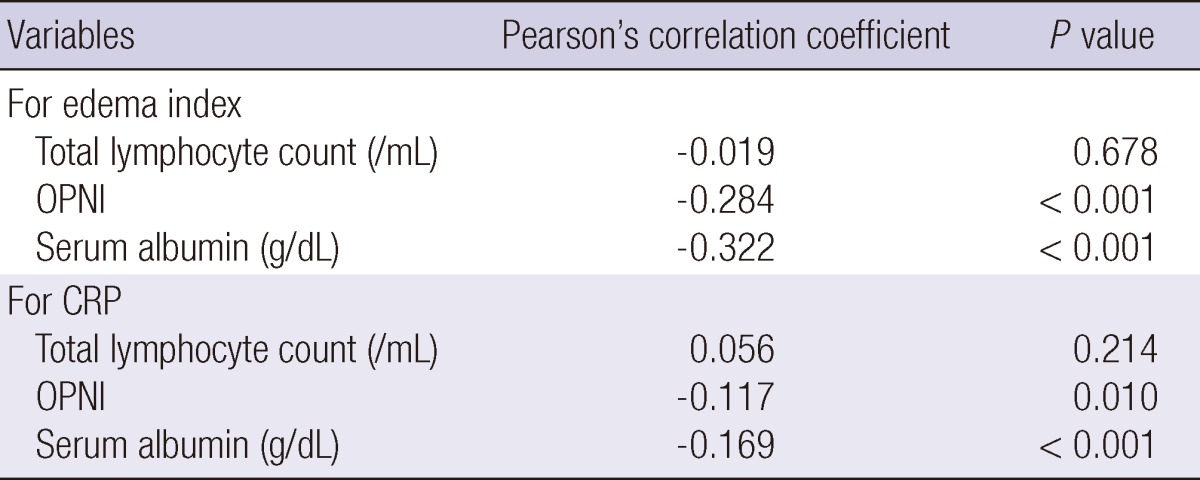
CRP, C-reactive protein; OPNI, Onodera's prognostic nutritional index.
Survival analysis
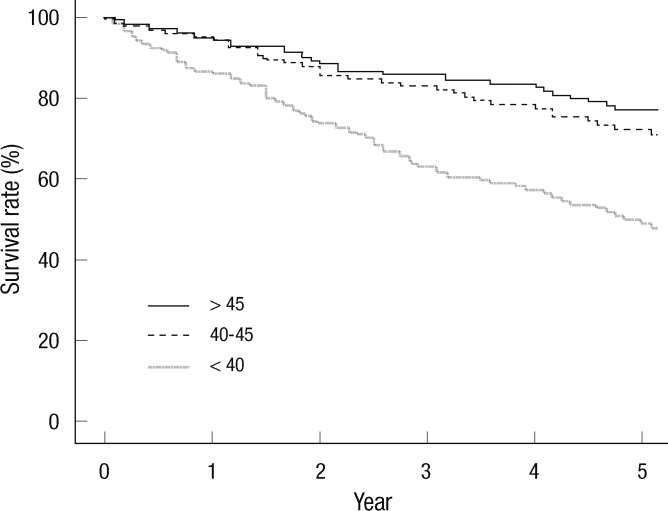
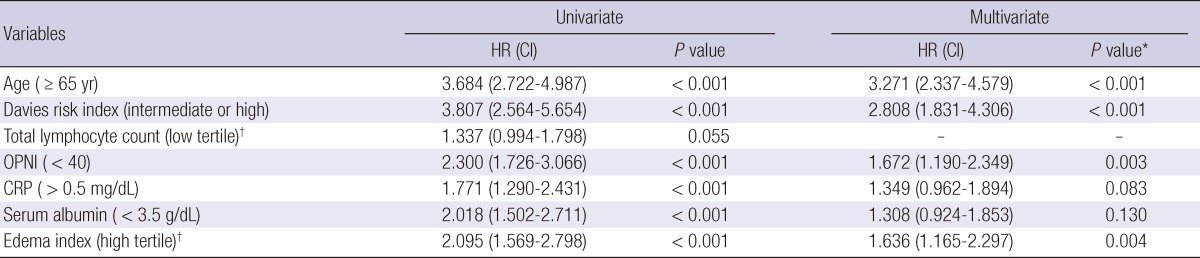
The cumulative 5-yr survival rate was 48.8%, 72.2%, and 77.1% in group A, group B, and group C, respectively (Fig. 1) (P < 0.001). Initial OPNI was associated with mortality in PD patients. Univariate analysis showed that old age (≥ 65 yr), high grade according to the Davies risk index, low OPNI (group A), hypoalbuminemia (< 3.5 g/dL), high CRP (> 0.5 mg/dL), and high edema index tertile (> 0.410) were associated with mortality in PD patients (Table 3). Multivariate analysis adjusted for age, Davies risk index, CRP, and edema index revealed that the hazard ratio for low OPNI was 1.672 (P = 0.003). Hypoalbuminemia was not associated with mortality in PD patients (hazard ratio, 1.308; P = 0.130).
Fig. 1.
Kaplan-Meier survival curve according to Onodera's prognostic nutritional index group (5-yr survival rate, 48.8% for < 40, 72.2% for 40-45, 77.1% for > 45; P < 0.001).
Table 3.
Univariate and multivariate Cox's proportional hazard analysis of variables for mortality in peritoneal dialysis patients
*Covariates for serum albumin included age, Davies risk index, CRP, and edema index. Covariates for all other variables included age, Davies risk index, OPNI, CRP, and edema index; †High edema index tertile was defined as an extracellular water/total body water > 0.410 and low total lymphocyte tertile was defined as < 1,275/mL. HR, hazard ratio; CI, confidence interval; OPNI, Onodera's prognostic nutritional index; CRP, C-reactive protein.
DISCUSSION
This study demonstrates that the OPNI is associated with the nutrition status and comorbidities. This index was less correlated to inflammation or the edema index than the serum albumin. Univariate and multivariate analyses showed that the initial low OPNI was associated with mortality in PD patients.
This study shows that the edema index and CRP are negatively correlated with serum albumin. Serum albumin has been well documented as a risk factor for mortality in dialysis patients (1, 2, 4). Canada-USA Peritoneal Dialysis Study Group (CANUSA) results showed that 1 g/L increase in serum albumin is associated with 6% decrease in mortality (16). However, serum albumin in patients with comorbidities may be more associated with inflammation or hydration than the nutritional status. While hypoalbuminemia from malnutrition alone is uncommon, malnutrition combined with hydration status or inflammation play a role in causing hypoalbuminemia in dialysis patients (17). Therefore, serum albumin in patients during the early dialysis period may be less effective as a nutritional marker than that in stable dialysis patients. Multivariate analysis adjusted for the edema index shows that serum albumin is not associated with mortality in PD patients. We found that that the correlation coefficient of OPNI for the CRP and edema index was lower than that in serum albumin. Additionally, OPNI was associated with mortality in PD patients after multivariate adjustment for the edema index and CRP. OPNI may be an independent prognostic factor, regardless of inflammation and hydration status.
Total lymphocyte count has been proposed as a prognostic factor (18). Increased total white blood cell count has been found to correlate with mortality in elderly men or after myocardial infarction (19, 20). Increased neutrophil counts have been implicated as a biomarker of atherosclerosis and/or inflammation (21). Malnutrition can induce a decrease in total lymphocyte count and suppression of cellular immunity including delayed hypersensitivity reaction (22). Immunologic changes occur early in the course of nutritional depletion (18). Reddan et al. (23) showed that the lymphocyte count was associated with the nutritional status and mortality in dialysis patients. This study shows that total lymphocyte count is an independent marker for the edema index or CRP. Although there was no statistical significance, low total lymphocyte tertile results in a hazard ratio of 1.337 for mortality. In our study, a total of 27 patients had liver or spleen diseases, which were associated with changes in the total lymphocyte count. The number of patients with 2 conditions was low, and the mortality risk of OPNI after adjusting the comorbidity index, including liver disease, was significant. These show that the clinical significance for OPNI may be valuable after correcting for these effects.
This study shows that a low OPNI score is associated with mortality in PD patients. This variable is less correlated to the edema index and CRP than serum albumin. This may be associated with addition of total lymphocyte count as an independent factor for edema index and CRP within the OPNI equation. The association between the OPNI, serum albumin, and total lymphocyte count may be due to inclusion of two variables within the OPNI equation. However, low OPNI was also associated with old age, high grade of the Davies risk index, low serum creatinine, and fat mass.
An ideal nutritional marker should be simple, have association with the nutritional status, and predict mortality. Many nutritional indices have been introduced in dialysis patients (6, 7). While these methods have been validated, using the OPNI is a simpler and easier method than previously reported indices. This index only includes complete blood count with differential count and serum albumin. This does not require additional laboratory findings or subjective questionnaires. This method has been validated in patients with malignancy or postoperative patients, who are expected to have the malnutrition in combination with inflammation, edema, or comorbidities (8-14). This study shows that the OPNI can be clinically relevant in PD patients, who are expected to have the combination of inflammation or edema.
Validation studies for OPNI were limited to Japanese subjects. There have been 4 reports examining the prognosis in patients with gastrointestinal malignancy, active tuberculosis, or end-stage liver disease (8, 10, 13, 14). There has been a report regarding the association with nutritional status alone and two reports regarding postoperative complications in patients with gastrointestinal malignancy or active tuberculosis (9, 11, 12). OPNI validation has not been widely studied in chronic kidney disease patients. This may be due to several limitations such as the application of widely known serum albumin levels and total lymphocyte count as variables for OPNI and changes in OPNI based on variable conditions. Moreover, it is not known if the results can be applicable to other populations.
There are a few limitations to this study. This was a retrospective, single-center study. Although measurements using OPNI calculation were measured 1 or 2 months after the initiating PD and patients had no definite signs of infection, OPNI was calculated using a single measurement of the total lymphocyte count. A time-averaged value may be a more precise method for predicting mortality than a single value alone. Additionally, we were unable to evaluate the lymphocyte subset. Malnutrition is associated with a decreased CD4:CD8 ratio as well as the appearance of peripheral immature T cells (24, 25). Evaluating the lymphocyte subset will help to differentiate the independent effect of malnutrition from variable conditions associated with lymphopenia, such as sepsis, malignancy, and steroid treatment (18). Decreased lymphocyte type is typically associated with a specific nutrient, e.g., the known association between β-carotene and CD19 (26). Understanding these relationships may be valuable for identifying the insufficient nutrient. Further investigation involving a more accurate lymphocyte count or subset is necessary.
In summary, our results demonstrate that the OPNI is less related to inflammation and edema than serum albumin per se. An initial low OPNI is associated with poor nutritional status and high mortality in PD patients. The OPNI is a simple method that can be used for predicting the nutritional status and clinical outcome in PD patients.
References
- 1.Avram MM, Goldwasser P, Erroa M, Fein PA. Predictors of survival in continuous ambulatory peritoneal dialysis patients: the importance of prealbumin and other nutritional and metabolic parameters. Am J Kidney Dis. 1994;23:91–98. doi: 10.1016/s0272-6386(12)80817-1. [DOI] [PubMed] [Google Scholar]
- 2.National Kidney Foundation. K/DOQI Clinical practice guidelines for nutrition in chornic renal failure. Am J Kidney Dis. 2000;35:S1–S140. doi: 10.1053/ajkd.2000.v35.aajkd03517. [DOI] [PubMed] [Google Scholar]
- 3.Stenvinkel P, Heimbürger O, Lindholm B, Kaysen GA, Bergström J. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome) Nephrol Dial Transplant. 2000;15:953–960. doi: 10.1093/ndt/15.7.953. [DOI] [PubMed] [Google Scholar]
- 4.Combe C, McCullough KP, Asano Y, Ginsberg N, Maroni BJ, Pifer TB. Kidney Disease Outcomes Quality Initiative (K/DOQI) and the Dialysis Outcomes and Practice Patterns Study (DOPPS): nutrition guidelines, indicators, and practices. Am J Kidney Dis. 2004;44:39–46. doi: 10.1053/j.ajkd.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Park JS, Jung HH, Yang WS, Kim HH, Kim SB, Park SK, Hong CD. Protein intake and the nutritional status in patients with pre-dialysis chronic renal failure on unrestricted diet. Korean J Intern Med. 1997;12:115–121. doi: 10.3904/kjim.1997.12.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kondrup J, Allison SP, Elia M, Vellas B, Plauth M Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN) ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22:415–421. doi: 10.1016/s0261-5614(03)00098-0. [DOI] [PubMed] [Google Scholar]
- 7.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2001;38:1251–1263. doi: 10.1053/ajkd.2001.29222. [DOI] [PubMed] [Google Scholar]
- 8.Onodera T, Goseki N, Nosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85:1001–1005. [PubMed] [Google Scholar]
- 9.Goseki N, Okamoto A, Onodera T. Postoperative nutritional assessment in gastric and colorectal cancer. Nihon Geka Gakkai Zasshi. 1986;87:853–858. [PubMed] [Google Scholar]
- 10.Yagi T, Yamagishi F, Sasaki Y, Mizutani F, Wada A, Kuroda F. Clinical review of pneumothorax cases complicated with active pulmonary tuberculosis. Kekkaku. 2002;77:395–399. [PubMed] [Google Scholar]
- 11.Yagi T, Yamagishi F, Sasaki Y, Itakura M, Fujikawa A, Kuga M, Ishimaru G. A study on cases developed pulmonary tuberculosis after receiving gastrectomy. Kekkaku. 2004;79:355–359. [PubMed] [Google Scholar]
- 12.Sagawa M, Katsube T, Konno S, Murayama M, Yamaguchi K, Isohata N, Yoshimatsu K, Shiozawa S, Shimakawa T, Naritaka Y, et al. The significance of Onodera's prognostic nutritional index for the treatment of gastrointestinal cancer. Gan To Kagaku Ryoho. 2008;35:2253–2255. [PubMed] [Google Scholar]
- 13.Fukushima K, Ueno Y, Kawagishi N, Kondo Y, Inoue J, Kakazu E, Ninomiya M, Wakui Y, Saito N, Satomi S, et al. The nutritional index 'COUNT' is useful for predicting long-term prognosis of patients with end-stage liver disease. Tohoku J Exp Med. 2011;224:215–219. doi: 10.1620/tjem.224.215. [DOI] [PubMed] [Google Scholar]
- 14.Okada I, Shirahata A, Soda H, Saitou M, Kigawa G, Nemoto H, Sanada Y, Hibi K. Significance of Onodera's prognostic nutritional index for treating unresectable or recurrent colorectal cancer with chemotherapy. Gan To Kagaku Ryoho. 2012;39:231–235. [PubMed] [Google Scholar]
- 15.Davies SJ, Phillips L, Naish PF, Russell GI. Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol Dial Transplant. 2002;17:1085–1092. doi: 10.1093/ndt/17.6.1085. [DOI] [PubMed] [Google Scholar]
- 16.CANADA-USA (CANUSA) Peritoneal Dialysis Study Group. Adequacy of dialysis and nutrition in continuous peritoneal dialysis: association with clinical outcomes. J Am Soc Nephrol. 1996;7:198–207. doi: 10.1681/ASN.V72198. [DOI] [PubMed] [Google Scholar]
- 17.Kaysen GA. Biological basis of hypoalbuminemia in ESRD. J Am Soc Nephrol. 1998;9:2368–2376. doi: 10.1681/ASN.V9122368. [DOI] [PubMed] [Google Scholar]
- 18.Omran ML, Morley JE. Assessment of protein energy malnutrition in older persons, Part II: Laboratory evaluation. Nutrition. 2000;16:131–140. doi: 10.1016/s0899-9007(99)00251-8. [DOI] [PubMed] [Google Scholar]
- 19.Weijenberg MP, Feskens EJ, Kromhout D. White blood cell count and the risk of coronary heart disease and all-cause mortality in elderly men. Arterioscler Thromb Vasc Biol. 1996;16:499–503. doi: 10.1161/01.atv.16.4.499. [DOI] [PubMed] [Google Scholar]
- 20.Furman MI, Becker RC, Yarzebski J, Savegeau J, Gore JM, Goldberg RJ. Effect of elevated leukocyte count on in-hospital mortality following acute myocardial infarction. Am J Cardiol. 1996;78:945–948. doi: 10.1016/s0002-9149(96)00473-0. [DOI] [PubMed] [Google Scholar]
- 21.Ernst E, Hammerschmidt DE, Bagge U, Matrai A, Dormandy JA. Leukocytes and the risk of ischemic diseases. JAMA. 1987;257:2318–2324. [PubMed] [Google Scholar]
- 22.Bistrian BR, Blackburn GL, Scrimshaw NS, Flatt JP. Cellular immunity in semistarved states in hospitalized adults. Am J Clin Nutr. 1975;28:1148–1155. doi: 10.1093/ajcn/28.10.1148. [DOI] [PubMed] [Google Scholar]
- 23.Reddan DN, Klassen PS, Szczech LA, Coladonato JA, O'Shea S, Owen WF, Jr, Lowrie EG. White blood cells as a novel mortality predictor in haemodialysis patients. Nephrol Dial Transplant. 2003;18:1167–1173. doi: 10.1093/ndt/gfg066. [DOI] [PubMed] [Google Scholar]
- 24.Kaiser FE, Morley JE. Idiopathic CD4+ T lymphopenia in older persons. J Am Geriatr Soc. 1994;42:1291–1295. doi: 10.1111/j.1532-5415.1994.tb06514.x. [DOI] [PubMed] [Google Scholar]
- 25.Schaible UE, Kaufmann SH. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med. 2007;4:e115. doi: 10.1371/journal.pmed.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grzegorzewska AE, Leander M. Total lymphocyte count and subpopulation lymphocyte counts in relation to dietary intake and nutritional status of peritoneal dialysis. Adv Perit Dial. 2005;21:35–40. [PubMed] [Google Scholar]