Abstract
Dexmedetomidine, which is a selective α2-adrenoceptor agonist, was recently introduced into clinical practice for its analgesic properties. The purpose of this study was to evaluate the effects of dexmedetomidine in a vincristine-evoked neuropathic rat models. Sprague-Dawley rats were injected intraperitoneally with vincristine or saline (0.1 mg/kg/day) using a 5-day-on, 2-day-off schedule for 2 weeks. Saline and dexmedetomidine (12.5, 25, 50, and 100 µg/kg) were injected to rats developed allodynia 14 days after vincristine injection, respectively. We evaluated allodynia at before, 15, 30, 60, 90, 120, 180, and 240 min, and 24 hr after intraperitoneal drug (normal saline or dexmedetomidine) injection. Saline treatment did not show any differences for all the allodynia. Maximal paw withdrawal thresholds to mechanical stimuli were 3.0 ± 0.4, 9.1 ± 1.9, 13.0 ± 3.6, 16.6 ± 2.4, and 24.4 ± 1.6 g at saline, 12.5, 25, 50, and 100 µg/kg dexmedetomidine injection, respectively. Minimal withdrawal frequency to cold stimuli were 73.3 ± 4.2, 57.1 ± 6.8, 34.3 ± 5.7, 20.0 ± 6.2, and 14.3 ± 9.5 g at saline, 12.5, 25, 50, and 100 µg/kg dexmedetomidine injection, respectively. Dexmedetomidine shows a dose-dependent antiallodynic effect on mechanical and cold stimuli in vincristine-evoked neuropathic rat models (P < 0.05).
Keywords: Dexmedetomidine, Neuropathy, Pain, Vincristine
INTRODUCTION
Vincristine-evoked painful neuropathy is one of the major dose-limiting adverse effects by cancer chemotherapy with vincristine. Not only does it lead to termination of the treatment of cancer patients, but also worsens the quality of their lives (1). The exact mechanism underlying vincristine-induced peripheral neuropathy is still unknown (2). Until now, tricyclic antidepressants, anti-convulsants, opioids, and non-steroidal anti-inflammatory drugs are prescribed for the treatment of vincristine-induced peripheral neuropathy, but their effect and safety are limited (3).
Dexmedetomidine, which is a selective and potent α2-adrenergic receptor agonist, is well known for its sedation and analgesic effects during the perioperative period and in critical care (4). It is also reported that dexmedetomidine showed an analgesic effect in an ischemic, acute postoperative, and intractable cancer pain of human and herpetic stomatitis after living-donor lung transplantation (5-7). Moreover, dexmedetomidine exhibits antinociceptive actions on postoperative, streptozotocin-induced, complete Freund's adjuvant (CFA)-induced, formalin-induced, carrageenan-induced inflammatory, acute nociceptive, and neuropathic pain in various animal models (8, 9). However, there is not any report on the effect of dexmedetomidine on chemotherapy-evoked peripheral neuropathy including vincristine-evoked neuropathy in clinical or preclinical settings.
Therefore, we investigated the analgesic effects of intraperitoneal dexmedetomidine by assessing the withdrawal responses to mechanical and cold stimuli in vincristine-evoked peripheral neuropathic rat models in order to examine the possibility of treating vincristine-evoked peripheral neuropathy in cancer patients with dexmedetomidine.
MATERIALS AND METHODS
Animals
The animal studies were performed after receiving approval of our institutional animal care and use committee at the Catholic University of Korea (approval No. CUMC-2010-0062-05, the date of protocol approval: February 1, 2010). Rats were housed up to 3-4 per plastic cage and maintained on a 12 hr light/dark cycle. Food and water were available freely. Sprague-Dawley male rats (200-250 g) were used in this experiment. These rats were adjusted to the environment for at least 7 days before starting the experiment. In all studies, the investigator was blinded to drug treatments.
Vincristine injection
The vincristine-evoked peripheral neuropathic model was used in this study. Baseline responses to mechanical, cold, and heat stimuli of the both hindpaws were used on day 1 (baseline). After behavioral testing, rats received intraperitoneal injections of either vincristine sulfate (Sigma Aldrich Co., St. Louis, MO, USA) (0.1 mg/kg/day; n = 11) or saline (0.1 mg/kg/day; n = 5) daily for 2 weeks. The treatment schedule consisted of 5 daily injections, followed by a 2-day off which means no injections, and then followed by 5 daily injections, as described by Weng et al. (10). On day 15, rats that showed withdrawal responses to 4 g or less von Frey hairs (Stoelting Co., Wood Dale, IL, USA) were considered allodynic, and used in the study.
Drug administration
Neuropathic rats were allocated into 5 groups randomly. Each group received intraperitoneally either normal saline (n = 6) or dexmedetomidine (Precedex® Inj., Hana Pharm Co., Seoul, Korea) at 12.5, 25, 50, and 100 µg/kg (n = 7 per each group).
Behavioral tests
All behavioral testing was conducted during the daytime to avoid circadian rhythm errors by the same investigator. Rats were subjected to mechanical withdrawal threshold test, cold withdrawal frequency test, and heat noxious threshold test. All tests were performed at before (Pre), 15, 30, 60, 90, 120, 180, and 240 min, and 24 hr after the administration. The mechanical threshold was measured using the up-down method with the von Frey hairs (1.0-26.0 g). We recorded the gram of minimum von Frey hair that initiated a withdrawal response. The cut-off threshold was recorded as 26.0 g.
Cold withdrawal frequency test was measured using a percentage withdrawal frequency (the number of withdrawal responses/the number of trials × 100) after application of 100% acetone to the both hindpaws. The test was repeated 5 times. The heat thresholds for hyperalgesia were measured using an increasing-temperature hot plate (IITC Life Science, Woodland Hills, CA, USA), which was heated up from 30℃ at a rate of 12℃/min until the rat showed a withdrawal response. The final cut-off temperature was 55℃. All data were presented as the mean of two values from both hindpaws. We also evaluated the rotarod test (Ugo Basile, Comerio, VA, Italy) to observe changes in the locomotor function of the treated rats. After acclimatization for 2 days (3 training trials on the revolving drums of 10-15 rpm), we measured the rotarod time at each time point after intraperitoneal administration.
Statistical analysis
Results are presented as mean ± standard error of mean. Two ways ANOVA was performed to determine general differences, depending on the treatment group and time. This was followed by post hoc Bonferroni multiple comparisons' test. A percentage of the maximum possible effect (%MPE) was analyzed with one-way ANOVA, followed by post hoc Newman-Keuls multiple comparisons' test for dose-response curves. A %MPE was calculated as follows: (treatment threshold-baseline threshold)/(cut-off threshold-baseline threshold) × 100 for mechanical allodynia; and (baseline frequency-treatment frequency)/(baseline frequency) × 100 for cold allodynia. ED50 was calculated from nonlinear regression with variable slope using Prism 5 software. All analyses were performed with Prism 5 (GraphPad Software Inc., La Jolla, CA, USA) and a P value of < 0.05 was considered significant.
RESULTS
Effects of the vincristine injection on allodynic thresholds
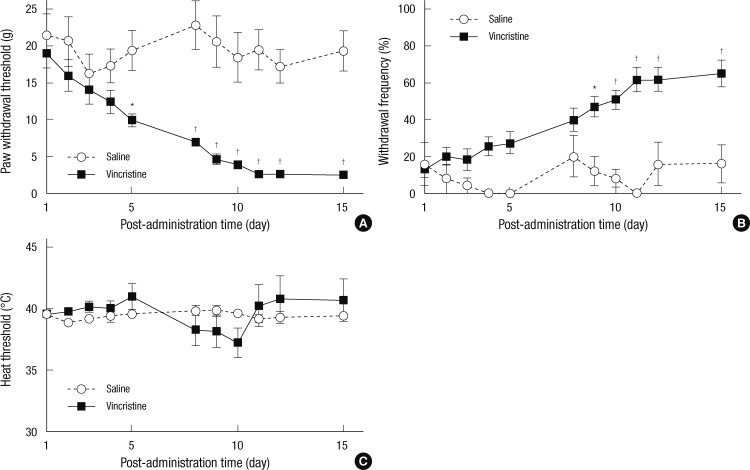
No rats injected with either vincristine or saline showed any motor abnormality or complication. The vincristine-injected rats did not gain weight during the injection schedule. However, they looked healthy. Saline did not induce any hyperalgesia or allodynia. Vincristine injection produced significant mechanical and cold allodynia after the 2-week injection schedule (P = 0.002 and P = 0.005) (Fig. 1A, B). However, heat nociception remained stable over the 2-week observation period (P = 0.710) (Fig. 1C). Mechanical threshold was significantly decreased during 5 days to 15 days after vincristine injection (Fig. 1A) and cold withdrawal frequency was increased during 9 days to 15 days after vincristine injection (Fig. 1B).
Fig. 1.
Changes of withdrawal responses during vincristine treatment. (A) Mechanical allodynia. (B) Cold allodynia. (C) Heat hyperalgesia. Results are presented as mean ± standard error of mean (n = 11 in vincristine group, n = 5 in saline group) (*P < 0.01 vs saline; †P < 0.001 vs saline).
Mechanical allodynia
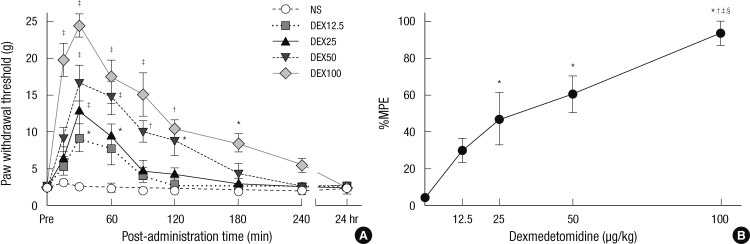
After vincristine injection, mechanical allodynia developed in 75.5% of the experimental rats after two weeks. In the vehicle group, the mechanical thresholds were 2.3 ± 0.6, 3.0 ± 0.4, 2.5 ± 0.5, 2.3 ± 0.5, 1.9 ± 0.5, 1.9 ± 0.5, 1.8 ± 0.5, 1.9 ± 0.5, and 2.3 ± 0.6 g at before (Pre), 15, 30, 60, 90, 120, 180, and 240 min, and 24 hr after saline injection, respectively. The withdrawal threshold increased at 30 min for 12.5 µg/kg, from 30 to 60 min for 25 µg/kg, from 30 to 120 min for 50 µg/kg, and from 15 to 180 min for 100 µg/kg, as compared to the vehicle group (P = 0.002) (Fig. 2A). Intraperitoneal injection of dexmedetomidine decreased mechanical allodynia dose-dependently (P < 0.001) (Fig. 2B). The ED50 was calculated to be 35.71 µg/kg (95% CI, 24.83-46.60).
Fig. 2.
Dexmedetomidine affects mechanical allodynia of vincristine treated rats. (A) The withdrawal threshold to mechanical stimuli in the vincristine-induced neuropathic rats. The withdrawal threshold was observed before (Pre) and after injection of normal saline (NS), dexmedetomidine 12.5 µg/kg (DEX12.5), dexmedetomidine 25 µg/kg (DEX25), dexmedetomidine 50 µg/kg (DEX50), dexmedetomidine 100 µg/kg (DEX100). Results are presented as mean ± standard error of mean (n = 6 in saline group, n = 7 per each dexmedetomidine group) (*P < 0.05 vs NS; †P < 0.01 vs NS; ‡P < 0.001 vs NS). (B) Dose-response curve of the percentage maximum possible effect (%MPE) on mechanical analgesia in the dexmedetomidine groups. This shows a mechanical analgesia dose-dependently. Each point is represented the mean ± standard error of mean (n = 6 in saline group, n = 7 per each dexmedetomidine group) (*P < 0.05 vs NS; †P < 0.05 vs 12.5 µg/kg; ‡P < 0.05 vs 25 µg/kg; §P < 0.05 vs 50 µg/kg of dexmedetomidine).
Cold allodynia
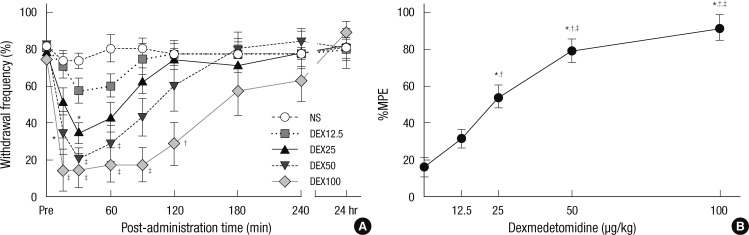
During the vincristine injection schedule, cold allodynia developed in 69.4% of the experimental rats after two weeks. In the vehicle group, the cold withdrawal frequencies were 80.0 ± 5.2, 73.3 ± 4.2, 73.3 ± 4.2, 80.0 ± 7.3, 80.0 ± 5.2, 76.7 ± 6.1, 80.0 ± 5.2, 83.3 ± 6.1, and 80.0 ± 5.2% at before (Pre), 15, 30, 60, 90, 120, 180, and 240 min, and 24 hr after saline injection, respectively. Injection of dexmedetomidine decreased the withdrawal frequency at 30 min for 25 µg/kg, from 15 to 60 min for 50 µg/kg, and from 15 to 120 min for 100 µg/kg (P = 0.004), as compared to the vehicle group (Fig. 3A). Intraperitoneal administration of dexmedetomidine also attenuated cold allodynia dose-dependently (P < 0.001) (Fig. 3B). The ED50 was calculated to be 24.78 µg/kg (95% CI, 19.71-29.86).
Fig. 3.
Dexmedetomidine reverses cold allodynia of vincristine treated rats. (A) The withdrawal frequency to cold stimuli. The frequency was measured before (Pre) and after injection of normal saline (NS), dexmedetomidine 12.5 µg/kg (DEX12.5), dexmedetomidine 25 µg/kg (DEX25), dexmedetomidine 50 µg/kg (DEX50), dexmedetomidine 100 µg/kg (DEX100). Results are presented as mean ± standard error of mean (n = 6 in saline group, n = 7 per each dexmedetomidine group) (*P < 0.05 vs NS; †P < 0.01 vs NS; ‡P < 0.001 vs NS). (B) Dose-response curve of the percentage maximum possible effect (%MPE) on cold analgesia in the dexmedetomidine groups. This shows a cold analgesia dose-dependently. Each point is represented the mean ± standard error of mean (n = 6 in saline group, n = 7 per each dexmedetomidine group) (*P < 0.05 vs NS; †P < 0.05 vs 12.5 µg/kg; ‡P < 0.05 vs 25 µg/kg of dexmedetomidine).
Rotarod test
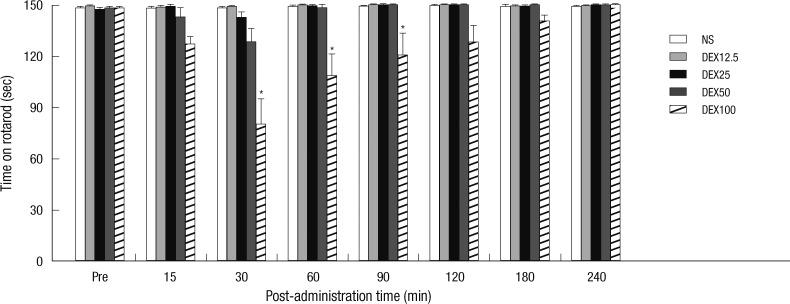
Injection of 12.5, 25, and 50 µg/kg of dexmedetomidine did not reduce the rats' rotarod time significantly, when compared to the vehicle group (P = 0.341). However, the rotarod time was reduced to 80.0 ± 15.1, 108.4 ± 12.7, and 120.6 ± 12.9 sec at 30, 60, and 90 min after 100 µg/kg dexmedetomidine administration, respectively (P < 0.001) (Fig. 4).
Fig. 4.
The rotarod time of dexmedetomidine-injected rats. Rotarod time was observed before (Pre) and after dexmedetomidine or saline injection. Rotarod time was not decreased by injection of normal saline (NS) and 12.5, 25, and 50 µg/kg of dexmedetomidine. Only 100 µg/kg of dexmedetomidine decreased rotarod time at from 30 to 90 min. Results are presented as mean ± standard error of mean (n = 6 in saline group, n = 7 per each dexmedetomidine group) (*P < 0.001 vs NS).
DISCUSSION
In this study, we validated vincristine-induced rat models showed mechanical and cold allodynia. Additionally, we found that intraperitoneally injected dexmedetomidine could reverse mechanical and cold allodynia dose-dependently in vincristine-induced neuropathic rats.
Until now, there has been no study that has described the effect of dexmedetomidine in an animal chemotherapy-induced peripheral neuropathic model. Considering that dexmedetomidine is a selective and potent α2-adrenergic receptors agonist, there is only one study that has presented analgesic effects of clonidine as one of the alpha-2 adrenoceptor agonists in chemotherapy-induced neuropathic pain model. According to the study by Lynch et al. (3), clonidine demonstrated effects of reducing mechanical allodynia in a vincristine-induced neuropathic rat model. However, in this study, the vincristine-filled mini-osmotic pump (30 µg/kg/day for 14 days, intravenously) developed by Nozaki-Taguchi et al. (11) was implanted in rats and made assessments on the effects of clonidine on mechanical allodynia only at a specific time point. Unlike the study performed by Lynch et al., we used the method of Weng et al. (10) (0.1 mg/kg/day, 5-day-on, 2-day-off schedule for 2 weeks, intraperitoneally). We also identified that dexmedetomidine had dose-dependent analgesic effects on mechanical and cold allodynia at pre-injection and 15-, 30-, 60-, 90-, 120-, 180-, and 240-min, and 24 hr after injection, by classifying the doses of dexmedetomidine as 12.5, 25, 50, and 100 µg/kg. Given that dexmedetomidine is highly selective and specific to alpha-2 adrenoceptor, 7-8 times more than clonidine, and does not trigger severe respiratory depression (12, 13), it was expected that dexmedetomidine would have stronger analgesic effects with less side effects. Therefore, it is considered more useful than clonidine for actual application in a clinical patient.
It is still unclear how dexmedetomidine manifests an antiallodynic effect on vincristine-evoked peripheral neuropathy. However, the result that intraperitoneal administration of dexmedetomidine showed a dose-dependent reversal of allodynia in the vincristine-induced neuropathic rats from this study implicates that alpha-2 adrenoceptor is related to the pain control of chemotherapy-induced neuropathy. The alpha-2 adrenoceptor is distributed in the nerve system including primary afferents and dorsal horn neurons in the spinal cord as well as the brain (14). According to Brede et al. (15), dexmedetomidine in this study might have an analgesic effect through alpha-2A and alpha-2C adrenoceptor. Dexmedetomidine demonstrated the antinociceptive effect when administered to the locus coeruleus of the brainstem in rat (16), and systemically administered medetomidine in rat produced antinociceptive responses of both medial thalamic and spinothalamic tract neurons in a dose-dependent manner (17). However, in that study, the finding that 100 µg/kg medetomidine inhibited the nociceptive response of the medial thalamic neuron more significantly than that of the spinothalamic tract neuron (17) made it possible to explain the supraspinal level mechanism of dexmedetomidine. In addition, it has been well known that dexmedetomidine has antinociceptive effects at the spinal cord level from intrathecal or epidural administrations of dexmedetomidine in various animal models (8, 9). Lastly, a study addressed that administration of a peripherally restricted alpha-2 adrenoceptor antagonist blocks the analgesic actions of dexmedetomidine in neuropathic rats (18). Another recent clinical research demonstrated that the intra-articular administration of dexmedetomidine had postoperative analgesic effects in arthroscopic knee surgery (5). The other clinical study showed that topically administered dexmedetomidine to the wound sites presented as having antihyperalgesic effect in bilateral third molar surgery (6). These three articles suggest that dexmedetomidine had peripheral analgesic effects. Therefore, it was likely that the analgesic effect from intraperitoneal administration of dexmedetomidine in this study was accomplished through mechanisms at the supraspinal, spinal and peripheral levels.
Meanwhile, the intraperitoneal administration of dexmedetomidine in a spinal cord injury model of rat had significantly reduced the level of tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-6 which increased after spinal cord injury (19). Besides, the administration of dexmedetomidine reduced the levels of TNF-α, IL-1, and IL-6 in patients with severe septic manifestation subsequent to the ileus surgery (20). Although the mechanism of vincristine to trigger neuropathic pain has not been well understood, various hypotheses have emerged. One of them states that an increase of TNF-α was observed from the sciatic nerve of a vincristine-induced neuropathic rat model (21). TNF-α was significantly up-regulated at spinal cord of vincristine-induced allodynic mice. But, when administered a neutralizing antibody of TNF-α, vincristine-induced mechanical allodynia was significantly reduced (22). Also, when administered a drug agent that blocks IL-1β release in the vincristine-induced neuropathic rat model, tactile allodynia reduced (23). The IL-6 levels were increased in both sciatic nerve and dorsal root ganglion of vincristine-induced allodynic mice whereas a neutralizing antibody of IL-6 administered to an ambient area of the sciatic nerve significantly reduced vincristine-induced mechanical allodynia (24). Moreover, the occurrence of vincristine-induced mechanical allodynia in IL-6 knock-out mice was lower than in wild type mice (24). To this end, it was suggested that induction of proinflammatory cytokine such as TNF-α, IL-1, and IL-6 could be the cause of vincristine-induced neuropathy (25). Thus, it is considered that anti-inflammatory action of dexmedetomidine could have effects on analgesia of vincristine-induced neuropathy.
Secondly, the intrathecal administration of dexmedetomidine in the postoperative pain model and formalin model of rats reduced Fos activation of the spinal dorsal horn (9, 26). Fos expression was observed as significantly activated not only at superficial and deep layers but also at intermediate layers of spinal cord in the vincristine-induced neuropathy rat model (12). Interestingly, Fos protein was known as being distributed widely in the dorsal horn of a vincristine-evoked neuropathy rat model than in that of a nerve injury rat model (27). Therefore, dexmedetomidine-induced reduction of Fos activation at the spinal cord level can be considered to be another anti-allodynia-presenting mechanism in vincristine-induced neuropathy.
Thirdly, the intrathecal administration of dexmedetomidine in anaesthetized rats had dose-dependently reduced nociceptive C fiber-evoked responses by transcutaneous electrical stimulation (28). C-fiber nociceptors had shown significant hyperresponsiveness against suprathreshold mechanical and heat stimuli in the vincristine-induced neuropathy rat model (29). To this end, it would be possible to presume that the effects of dexmedetomidine on reduction of nociceptive C fiber-evoked responses would be the third mechanism that showed anti-allodynia in vincristine-induced neuropathy.
Finally, both intraperitoneal and intrathecal administrations of dexmedetomidine in a monoarthritic rat model significantly inhibited the glial activation of astrocytes and microglia in the spinal cord (30, 31). The intraperitoneal administration of propentofyllinne in vincristine-induced neuropathic rats, which is a glial modulating agent, reduced the mechanical allodynia as well as the spinal astrocytes and microglia activation (32). This made it possible to infer the inhibition effects of dexmedetomidine on spinal glial cell activation as the last mechanism that showed anti-allodynia in vincristine-induced neuropathy.
In summary, the beneficial effect of dexmedetomidine on vincristine-induced peripheral neuropathy in this study may be due to a combination of anti-inflammatory effects, like as Fos expression-reductive effect, nociceptive C fiber-evoked responses-reductive effect, and spinal glial cell activation-inhibitive effect.
The most common side effect of dexmedetomidine is sedation (33). The examination of this side effect is clinically important because the side effect limits the use of higher dose dexmedetomidine to continuously treat cancer patients. Additionally, if rats in this experiment were sedated by dexmedetomidine, the analgesic effect of dexmedetomidine would have been exaggerated. But, according to rotarod testing, only the 100 µg/kg of systemic dexmedetomidine decreased the rotarod performance. So, it is thought that the anti-allodynic effect of dexmedetomidine at 12.5, 25, and 50 µg/kg is not exaggerated by the sedative effect of dexmedetomidine.
In the present study, identifying how dexmedetomidine have analgesic effects on vincristine-induced neuropathy was something lacking. It is considered that future studies may be conducted to identify the exact action mechanism of dexmedetomidine on vincristine-induced neuropathy and reduce the side effects of dexmedetomidine. Of course, clinical studies should be implemented pre-emptively before applying to patients in actual practice.
In conclusion, this study shows that vincristine induces peripheral neuropathy in rats and dexmedetomidine attenuates hyperalgesia by mechanical and cold stimuli in these neuropathic rats, but sedation appears at the dosage of 100 µg/kg.
ACKNOWLEDGMENTS
We thank Yuri Choi of Anesthesiology Laboratory of Seoul St. Mary's Hospital for her technical assistance.
References
- 1.Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer. 2008;44:1507–1515. doi: 10.1016/j.ejca.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 2.Park BY, Park SH, Kim WM, Yoon MH, Lee HG. Antinociceptive effect of memantine and morphine on vincristine-induced peripheral neuropathy in rats. Korean J Pain. 2010;23:179–185. doi: 10.3344/kjp.2010.23.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynch JJ, 3rd, Wade CL, Zhong CM, Mikusa JP, Honore P. Attenuation of mechanical allodynia by clinically utilized drugs in a rat chemotherapy-induced neuropathic pain model. Pain. 2004;110:56–63. doi: 10.1016/j.pain.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Gerlach AT, Murphy CV, Dasta JF. An updated focused review of dexmedetomidine in adults. Ann Pharmacother. 2009;43:2064–2074. doi: 10.1345/aph.1M310. [DOI] [PubMed] [Google Scholar]
- 5.Al-Metwalli RR, Mowafi HA, Ismail SA, Siddiqui AK, Al-Ghamdi AM, Shafi MA, El-Saleh AR. Effect of intra-articular dexmedetomidine on postoperative analgesia after arthroscopic knee surgery. Br J Anaesth. 2008;101:395–399. doi: 10.1093/bja/aen184. [DOI] [PubMed] [Google Scholar]
- 6.Cheung CW, Ng KF, Choi WS, Chiu WK, Ying CL, Irwin MG. Evaluation of the analgesic efficacy of local dexmedetomidine application. Clin J Pain. 2011;27:377–382. doi: 10.1097/AJP.0b013e318208c8c5. [DOI] [PubMed] [Google Scholar]
- 7.Ohashi Y, Ohta N, Hirao O, Uchiyama A, Mashimo T, Fujino Y. Analgesic effect of dexmedetomidine in a patient with herpetic stomatitis after living-donor lung transplantation. J Anesth. 2008;22:297–299. doi: 10.1007/s00540-008-0634-2. [DOI] [PubMed] [Google Scholar]
- 8.Onttonen T, Pertovaara A. The mechanical antihyperalgesic effect of intrathecally administered MPV-2426, a novel alpha2-adrenoceptor agonist, in a rat model of postoperative pain. Anesthesiology. 2000;92:1740–1745. doi: 10.1097/00000542-200006000-00034. [DOI] [PubMed] [Google Scholar]
- 9.Nazarian A, Christianson CA, Hua XY, Yaksh TL. Dexmedetomidine and ST-91 analgesia in the formalin model is mediated by alpha2A-adrenoceptors: a mechanism of action distinct from morphine. Br J Pharmacol. 2008;155:1117–1126. doi: 10.1038/bjp.2008.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weng HR, Cordella JV, Dougherty PM. Changes in sensory processing in the spinal dorsal horn accompany vincristine-induced hyperalgesia and allodynia. Pain. 2003;103:131–138. doi: 10.1016/s0304-3959(02)00445-1. [DOI] [PubMed] [Google Scholar]
- 11.Nozaki-Taguchi N, Chaplan SR, Higuera ES, Ajakwe RC, Yaksh TL. Vincristine-induced allodynia in the rat. Pain. 2001;93:69–76. doi: 10.1016/S0304-3959(01)00294-9. [DOI] [PubMed] [Google Scholar]
- 12.Haselman MA. Dexmedetomidine: a useful adjunct to consider in some high-risk situations. AANA J. 2008;76:335–339. [PubMed] [Google Scholar]
- 13.Virtanen R, Savola JM, Saano V, Nyman L. Characterization of the selectivity, specificity and potency of medetomidine as an a2-adrenoceptor agonist. Eur J Pharmacol. 1988;150:9–14. doi: 10.1016/0014-2999(88)90744-3. [DOI] [PubMed] [Google Scholar]
- 14.Unnerstall JR, Kopajtic TA, Kuhar MJ. Distribution of alpha 2 agonist binding sites in the rat and human central nervous system: analysis of some functional, anatomic correlates of the pharmacologic effects of clonidine and related adrenergic agents. Brain Res. 1984;319:69–101. doi: 10.1016/0165-0173(84)90030-4. [DOI] [PubMed] [Google Scholar]
- 15.Brede M, Philipp M, Knaus A, Muthig V, Hein L. Alpha2-adrenergic receptor subtypes - novel functions uncovered in gene-targeted mouse models. Biol Cell. 2004;96:343–348. doi: 10.1016/j.biolcel.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Xu M, Wei H, Kontinen VK, Kalso E, Pertovaara A. The dissociation of sedative from spinal antinociceptive effects following administration of a novel alpha-2-adrenoceptor agonist, MPV-2426, in the locus coeruleus in the rat. Acta Anaesthesiol Scand. 2000;44:648–655. doi: 10.1034/j.1399-6576.2000.440604.x. [DOI] [PubMed] [Google Scholar]
- 17.Pertovaara A, Kauppila T, Jyväsjärvi E, Kalso E. Involvement of supraspinal and spinal segmental alpha-2-adrenergic mechanisms in the medetomidine-induced antinociception. Neuroscience. 1991;44:705–714. doi: 10.1016/0306-4522(91)90089-7. [DOI] [PubMed] [Google Scholar]
- 18.Poree LR, Guo TZ, Kingery WS, Maze M. The analgesic potency of dexmedetomidine is enhanced after nerve injury: a possible role for peripheral alpha2-adrenoceptors. Anesth Analg. 1998;87:941–948. doi: 10.1097/00000539-199810000-00037. [DOI] [PubMed] [Google Scholar]
- 19.Can M, Gul S, Bektas S, Hanci V, Acikgoz S. Effects of dexmedetomidine or methylprednisolone on inflammatory responses in spinal cord injury. Acta Anaesthesiol Scand. 2009;53:1068–1072. doi: 10.1111/j.1399-6576.2009.02019.x. [DOI] [PubMed] [Google Scholar]
- 20.Tasdogan M, Memis D, Sut N, Yuksel M. Results of a pilot study on the effects of propofol and dexmedetomidine on inflammatory responses and intraabdominal pressure in severe sepsis. J Clin Anesth. 2009;21:394–400. doi: 10.1016/j.jclinane.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Muthuraman A, Singh N, Jaggi AS. Protective effect of Acorus calamus L. in rat model of vincristine induced painful neuropathy: an evidence of anti-inflammatory and anti-oxidative activity. Food Chem Toxicol. 2011;49:2557–2563. doi: 10.1016/j.fct.2011.06.069. [DOI] [PubMed] [Google Scholar]
- 22.Kiguchi N, Maeda T, Kobayashi Y, Kishioka S. Up-regulation of tumor necrosis factor-alpha in spinal cord contributes to vincristine-induced mechanical allodynia in mice. Neurosci Lett. 2008;445:140–143. doi: 10.1016/j.neulet.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Honore P, Donnelly-Roberts D, Namovic MT, Hsieh G, Zhu CZ, Mikusa JP, Hernandez G, Zhong C, Gauvin DM, Chandran P, et al. A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J Pharmacol Exp Ther. 2006;319:1376–1385. doi: 10.1124/jpet.106.111559. [DOI] [PubMed] [Google Scholar]
- 24.Kiguchi N, Maeda T, Kobayashi Y, Kondo T, Ozaki M, Kishioka S. The critical role of invading peripheral macrophage-derived interleukin-6 in vincristine-induced mechanical allodynia in mice. Eur J Pharmacol. 2008;592:87–92. doi: 10.1016/j.ejphar.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Cata JP, Weng HR, Lee BN, Reuben JM, Dougherty PM. Clinical and experimental findings in humans and animals with chemotherapy-induced peripheral neuropathy. Minerva Anestesiol. 2006;72:151–169. [PubMed] [Google Scholar]
- 26.Shimode N, Fukuoka T, Tanimoto M, Tashiro C, Tokunaga A, Noguchi K. The effects of dexmedetomidine and halothane on Fos expression in the spinal dorsal horn using a rat postoperative pain model. Neurosci Lett. 2003;343:45–48. doi: 10.1016/s0304-3940(03)00309-4. [DOI] [PubMed] [Google Scholar]
- 27.Coggeshall RE. Fos, nociception and the dorsal horn. Prog Neurobiol. 2005;77:299–352. doi: 10.1016/j.pneurobio.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan AF, Kalso EA, McQuay HJ, Dickenson AH. The antinociceptive actions of dexmedetomidine on dorsal horn neuronal responses in the anaesthetized rat. Eur J Pharmacol. 1992;215:127–133. doi: 10.1016/0014-2999(92)90617-d. [DOI] [PubMed] [Google Scholar]
- 29.Tanner KD, Reichling DB, Levine JD. Nociceptor hyper-responsiveness during vincristine-induced painful peripheral neuropathy in the rat. J Neurosci. 1998;18:6480–6491. doi: 10.1523/JNEUROSCI.18-16-06480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu B, Zhang WS, Yang JL, Xu H, Deng XM, Zhang YQ. Dexmedetomidine blocks thermal hyperalgesia and spinal glial activation in rat model of monoarthritis. Acta Pharmacol Sin. 2010;31:523–530. doi: 10.1038/aps.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu B, Zhang WS, Yang JL, Lû N, Deng XM, Xu H, Zhang YQ. Evidence for suppression of spinal glial activation by dexmedetomidine in a rat model of monoarthritis. Clin Exp Pharmacol Physiol. 2010;37:e158–e166. doi: 10.1111/j.1440-1681.2010.05426.x. [DOI] [PubMed] [Google Scholar]
- 32.Sweitzer SM, Pahl JL, DeLeo JA. Propentofylline attenuates vincristine-induced peripheral neuropathy in the rat. Neurosci Lett. 2006;400:258–261. doi: 10.1016/j.neulet.2006.02.058. [DOI] [PubMed] [Google Scholar]
- 33.Chan AK, Cheung CW, Chong YK. Alpha-2 agonists in acute pain management. Expert Opin Pharmacother. 2010;11:2849–2868. doi: 10.1517/14656566.2010.511613. [DOI] [PubMed] [Google Scholar]