Abstract
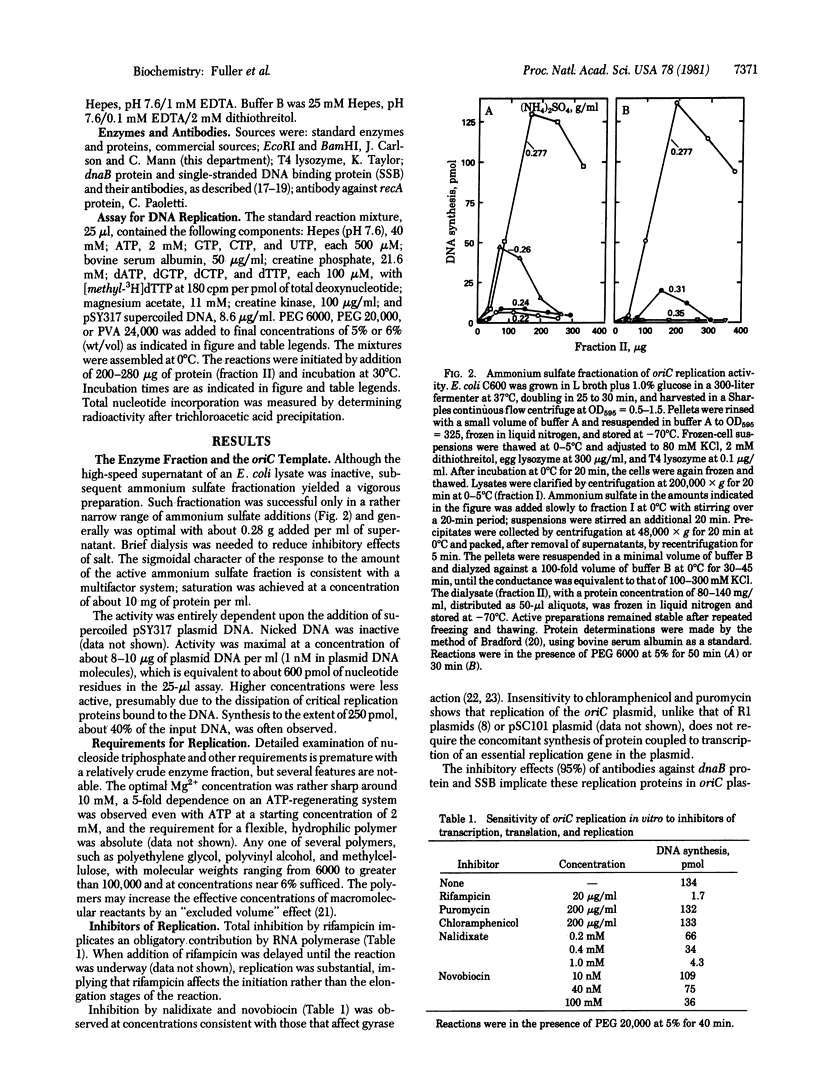
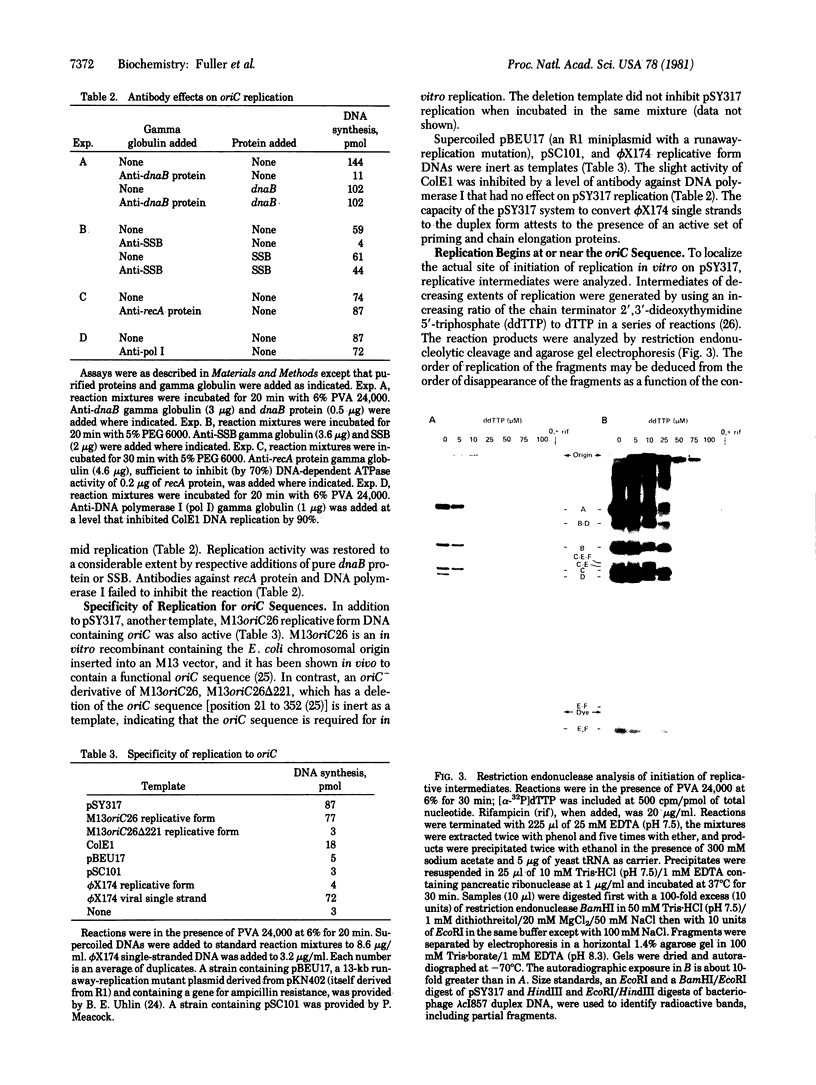
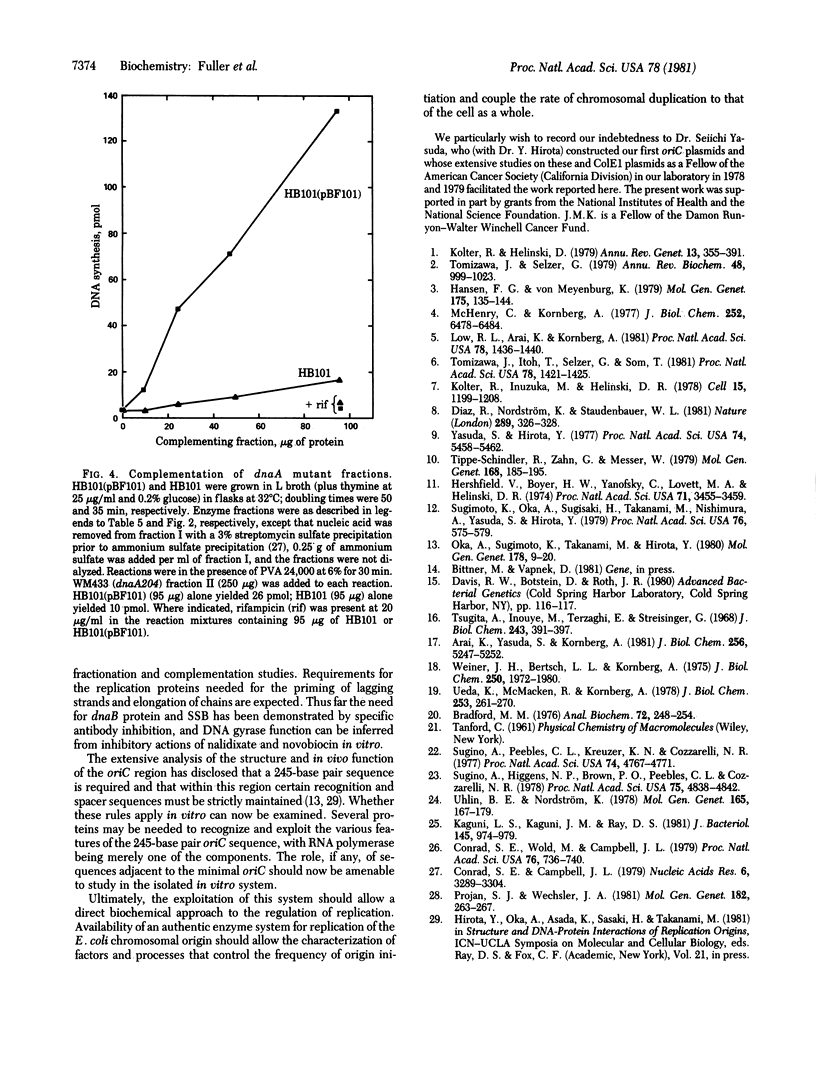
An enzyme system that replicates plasmids bearing the origin of the Escherichia coli chromosomes (oriC) has the following physiologically relevant features. The system (i) depends completely on low levels of exogenously furnished supercoiled oriC plasmids, (ii) uses only those plasmids that contain the intact oriC region of about 245 base pairs, (iii) initiates replication within or near the oriC sequence and proceeds bidirectionally, (iv) proceeds linearly, after a 5-min lag, for 30-40 min to produce as much as a 40% increase over the input DNA, (v) depends on RNA polymerase and gyrase as indicated by total inhibition by rifampicin and nalidixate, (vi) depends on replication proteins (e.g., dnaB protein and single-stranded DNA binding protein) as judged by specific antibody inhibitions, (vii) operates independently from protein synthesis, and (viii) depends on dnaA activity, as suggested by the inactivity of enzyme fraction from each of two dnaA temperature-sensitive mutant strains, and complementation (with a 15-fold overproduction of complementing activity) by a fraction from a strain containing the dnaA gene cloned in a multicopy plasmid. Resolution and analysis of factors that control the initiation of a chromosome cycle should become accessible through its enzyme system.
Full text
PDF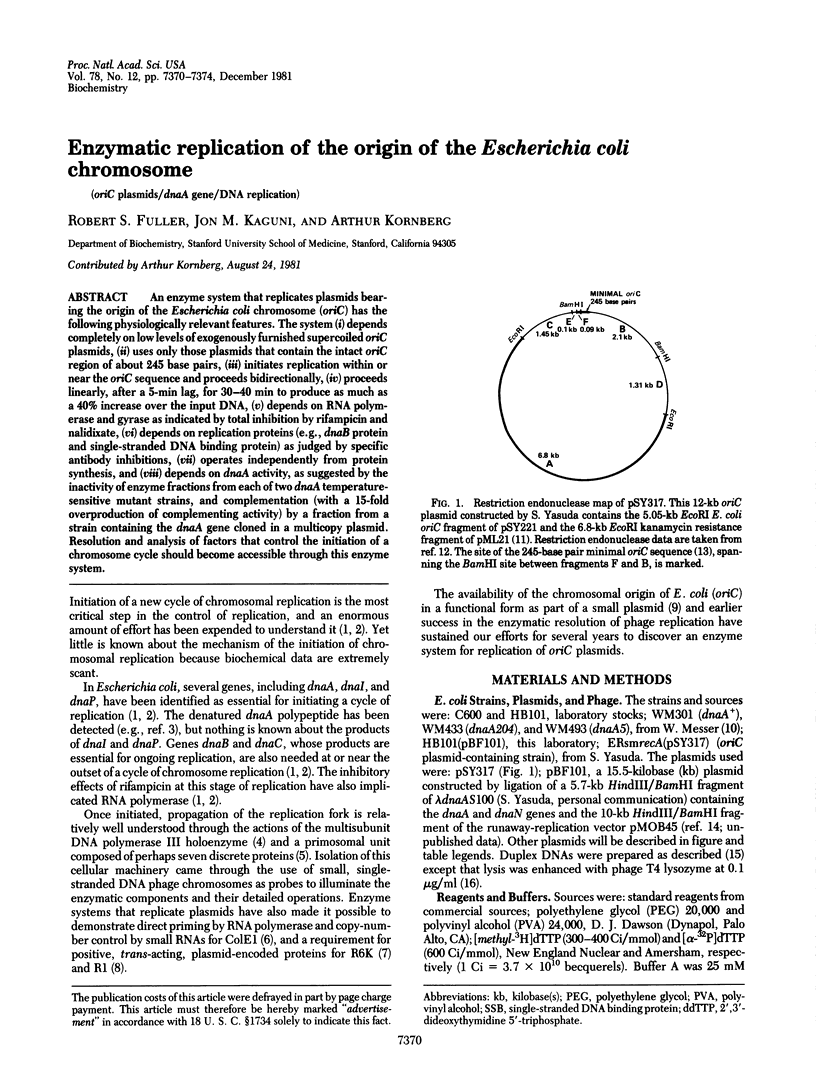

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K., Yasuda S., Kornberg A. Mechanism of dnaB protein action. I. Crystallization and properties of dnaB protein, an essential replication protein in Escherichia coli. J Biol Chem. 1981 May 25;256(10):5247–5252. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Conrad S. E., Campbell J. L. Characterization of an improved in vitro DNA replication system for Escherichia coli plasmids. Nucleic Acids Res. 1979 Jul 25;6(10):3289–3304. doi: 10.1093/nar/6.10.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad S. E., Wold M., Campbell J. L. Origin and direction of DNA replication of plasmid RSF1030. Proc Natl Acad Sci U S A. 1979 Feb;76(2):736–740. doi: 10.1073/pnas.76.2.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R., Nordström K., Staudenbauer W. L. Plasmid R1 DNA replication dependent on protein synthesis in cell-free extracts of E. coli. Nature. 1981 Jan 22;289(5795):326–328. doi: 10.1038/289326a0. [DOI] [PubMed] [Google Scholar]
- Hansen F. G., von Meyenburg K. Characterization of the dnaA, gyrB and other genes in the dnaA region of the Escherichia coli chromosome on specialized transducing phages lambda tna. Mol Gen Genet. 1979 Sep;175(2):135–144. doi: 10.1007/BF00425529. [DOI] [PubMed] [Google Scholar]
- Hershfield V., Boyer H. W., Yanofsky C., Lovett M. A., Helinski D. R. Plasmid ColEl as a molecular vehicle for cloning and amplification of DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3455–3459. doi: 10.1073/pnas.71.9.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaguni L. S., Kaguni J. M., Ray D. S. Replication of M13 oriC bacteriophages in Escherichia coli rep mutant is dependent on the cloned Escherichia coli replication origin. J Bacteriol. 1981 Feb;145(2):974–979. doi: 10.1128/jb.145.2.974-979.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter R., Helinski D. R. Regulation of initiation of DNA replication. Annu Rev Genet. 1979;13:355–391. doi: 10.1146/annurev.ge.13.120179.002035. [DOI] [PubMed] [Google Scholar]
- Kolter R., Inuzuka M., Helinski D. R. Trans-complementation-dependent replication of a low molecular weight origin fragment from plasmid R6K. Cell. 1978 Dec;15(4):1199–1208. doi: 10.1016/0092-8674(78)90046-6. [DOI] [PubMed] [Google Scholar]
- Low R. L., Arai K., Kornberg A. Conservation of the primosome in successive stages of phi X174 DNA replication. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1436–1440. doi: 10.1073/pnas.78.3.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry C., Kornberg A. DNA polymerase III holoenzyme of Escherichia coli. Purification and resolution into subunits. J Biol Chem. 1977 Sep 25;252(18):6478–6484. [PubMed] [Google Scholar]
- Oka A., Sugimoto K., Takanami M., Hirota Y. Replication origin of the Escherichia coli K-12 chromosome: the size and structure of the minimum DNA segment carrying the information for autonomous replication. Mol Gen Genet. 1980 Apr;178(1):9–20. doi: 10.1007/BF00267207. [DOI] [PubMed] [Google Scholar]
- Projan S. J., Wechsler J. A. Initiation of chromosomal DNA synthesis in vitro. Mol Gen Genet. 1981;182(2):263–267. doi: 10.1007/BF00269668. [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Oka A., Sugisaki H., Takanami M., Nishimura A., Yasuda Y., Hirota Y. Nucleotide sequence of Escherichia coli K-12 replication origin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):575–579. doi: 10.1073/pnas.76.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Higgins N. P., Brown P. O., Peebles C. L., Cozzarelli N. R. Energy coupling in DNA gyrase and the mechanism of action of novobiocin. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4838–4842. doi: 10.1073/pnas.75.10.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tippe-Schindler R., Zahn G., Messer W. Control of the initiation of DNA replication in Escherichia coli. I. Negative control of initiation. Mol Gen Genet. 1979 Jan 10;168(2):185–195. doi: 10.1007/BF00431444. [DOI] [PubMed] [Google Scholar]
- Tomizawa J., Itoh T., Selzer G., Som T. Inhibition of ColE1 RNA primer formation by a plasmid-specified small RNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1421–1425. doi: 10.1073/pnas.78.3.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J., Selzer G. Initiation of DNA synthesis in Escherichia coli. Annu Rev Biochem. 1979;48:999–1034. doi: 10.1146/annurev.bi.48.070179.005031. [DOI] [PubMed] [Google Scholar]
- Tsugita A., Inouye M. Purification of bacteriophage T4 lysozyme. J Biol Chem. 1968 Jan 25;243(2):391–397. [PubMed] [Google Scholar]
- Ueda K., McMacken R., Kornberg A. dnaB protein of Escherichia coli. Purification and role in the replication of phiX174 DNA. J Biol Chem. 1978 Jan 10;253(1):261–269. [PubMed] [Google Scholar]
- Uhlin B. E., Nordström K. A runaway-replication mutant of plasmid R1drd-19: temperature-dependent loss of copy number control. Mol Gen Genet. 1978 Oct 4;165(2):167–179. doi: 10.1007/BF00269904. [DOI] [PubMed] [Google Scholar]
- Weiner J. H., Bertsch L. L., Kornberg A. The deoxyribonucleic acid unwinding protein of Escherichia coli. Properties and functions in replication. J Biol Chem. 1975 Mar 25;250(6):1972–1980. [PubMed] [Google Scholar]
- Yasuda S., Hirota Y. Cloning and mapping of the replication origin of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5458–5462. doi: 10.1073/pnas.74.12.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]




