Abstract
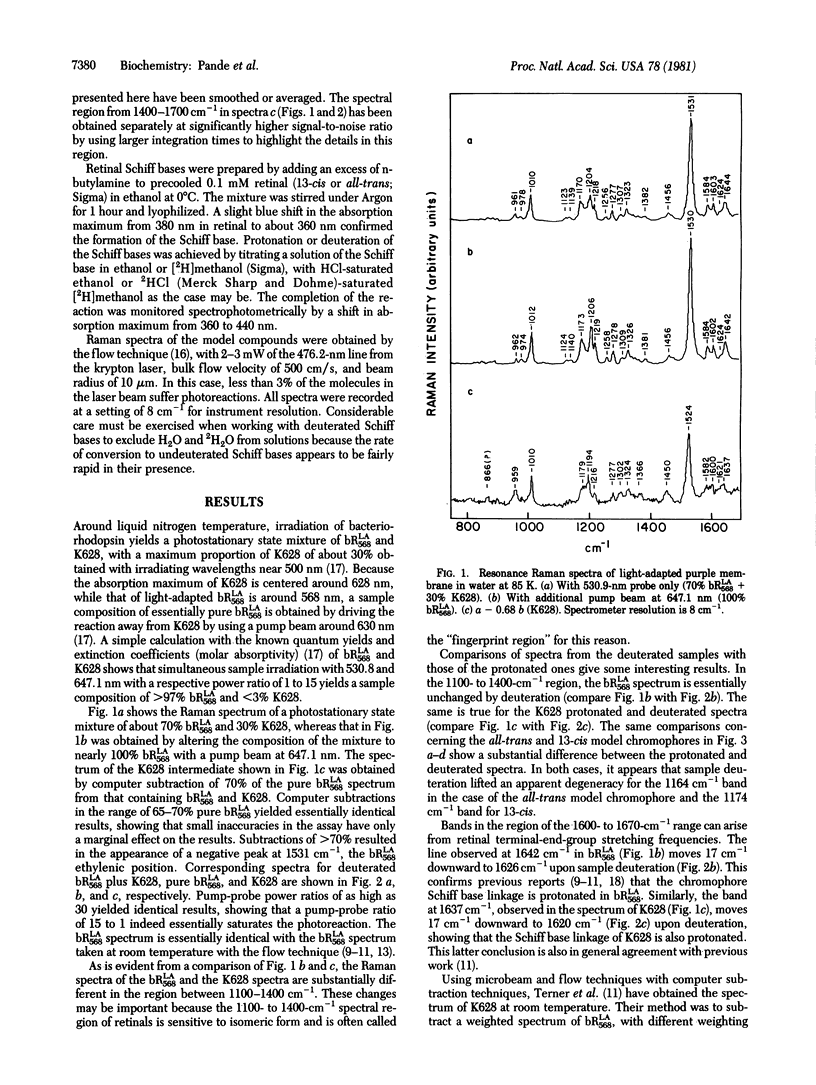
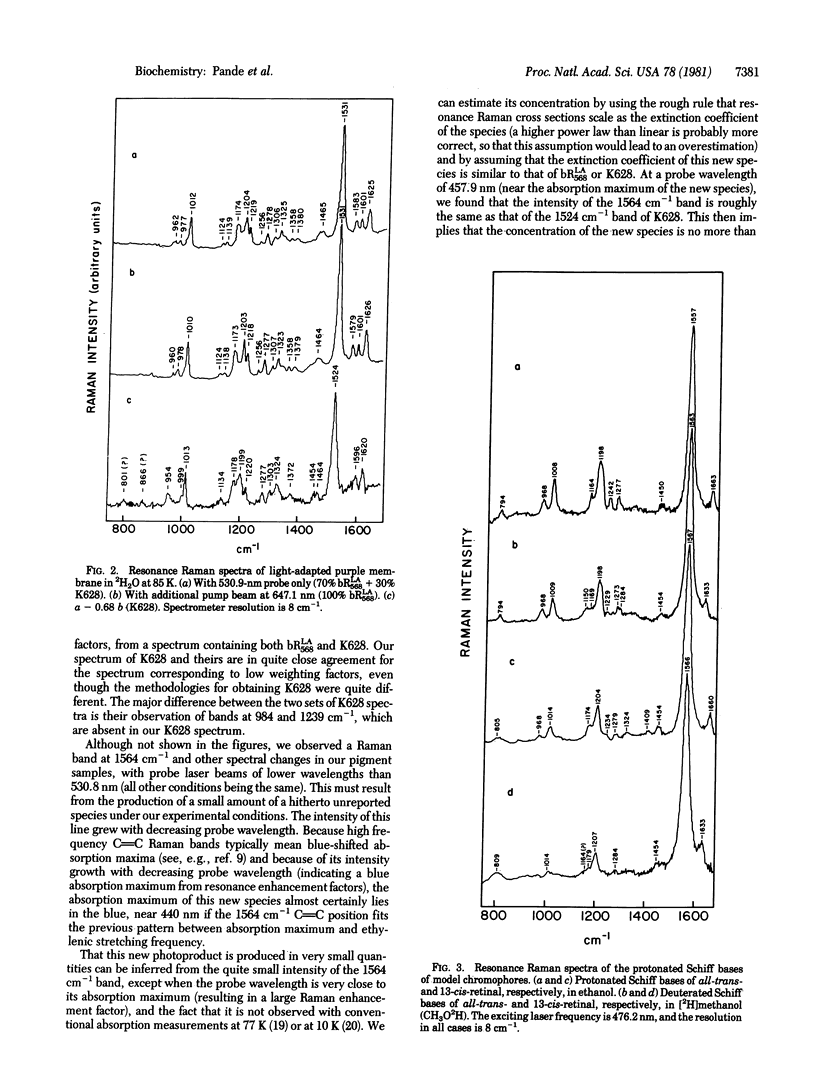
Resonance Raman multicomponent spectra of the light-adapted form of bacteriorhodopsin, bRLA568, and its first photoproduct, K628, have been obtained at liquid nitrogen temperatures. The spectra of both bRLA568 and K628 could be obtained with the known sample compositions under our irradiating conditions and computer subtraction techniques. In agreement with previous results, we find that both bRLA568 and K628 contain chromophores linked to the apoprotein by protonated Schiff bases of retinal. Neither pigment form, suspended in H2O or 2H2O, compares closely to the spectral features of all-trans and 13-cis protonated and deuterated model chromophores, respectively. The data are consistent with other results, suggesting that a chromophore isomerization takes place in the bRLA568-to-K628 phototransition. However, the exact structure of the in situ chromophore would appear not to involve simple trans-to-13-cis structures found in solution.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aton B., Doukas A. G., Callender R. H., Becher B., Ebrey T. G. Resonance Raman studies of the purple membrane. Biochemistry. 1977 Jun 28;16(13):2995–2999. doi: 10.1021/bi00632a029. [DOI] [PubMed] [Google Scholar]
- Becher B. M., Cassim J. Y. Improved isolation procedures for the purple membrane of Halobacterium halobium. Prep Biochem. 1975;5(2):161–178. doi: 10.1080/00327487508061568. [DOI] [PubMed] [Google Scholar]
- Callender R. H., Doukas A., Crouch R., Nakanishi K. Molecular flow resonance Raman effect from retinal and rhodopsin. Biochemistry. 1976 Apr 20;15(8):1621–1629. doi: 10.1021/bi00653a005. [DOI] [PubMed] [Google Scholar]
- Honig B., Ebrey T., Callender R. H., Dinur U., Ottolenghi M. Photoisomerization, energy storage, and charge separation: a model for light energy transduction in visual pigments and bacteriorhodopsin. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2503–2507. doi: 10.1073/pnas.76.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J. B., Ebrey T. G. Energy transfer in the purple membrane of Halobacterium halobium. Biophys J. 1978 Apr;22(1):49–66. doi: 10.1016/S0006-3495(78)85470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J. B., Ebrey T. G. Energy transfer in the purple membrane of Halobacterium halobium. Biophys J. 1978 Apr;22(1):49–66. doi: 10.1016/S0006-3495(78)85470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A., Spoonhower J., Bogomolni R. A., Lozier R. H., Stoeckenius W. Tunable laser resonance raman spectroscopy of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4462–4466. doi: 10.1073/pnas.71.11.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus M. A., Lewis A. Resonance Raman spectroscopy of the retinylidene chromophore in bacteriorhodopsin (bR570), bR560, M421, and other intermediates: structural conclusions based on kinetics, analogues, models, and isotopically labeled membranes. Biochemistry. 1978 Oct 31;17(22):4722–4735. doi: 10.1021/bi00615a019. [DOI] [PubMed] [Google Scholar]
- Mathies R., Freedman T. B., Stryer L. Resonance Raman studies of the conformation of retinal in rhodopsin and isorhodopsin. J Mol Biol. 1977 Jan 15;109(2):367–372. doi: 10.1016/s0022-2836(77)80040-5. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Meentzen M., Schuhmann L. Reversible dissociation of the purple complex in bacteriorhodopsin and identification of 13-cis and all-trans-retinal as its chromophores. Eur J Biochem. 1973 Dec 17;40(2):453–463. doi: 10.1111/j.1432-1033.1973.tb03214.x. [DOI] [PubMed] [Google Scholar]
- Oseroff A. R., Callender R. H. Resonance Raman spectroscopy of rhodopsin in retinal disk membranes. Biochemistry. 1974 Sep 24;13(20):4243–4248. doi: 10.1021/bi00717a027. [DOI] [PubMed] [Google Scholar]
- Pettei M. J., Yudd A. P., Nakanishi K., Henselman R., Stoeckenius W. Identification of retinal isomers isolated from bacteriorhodopsin. Biochemistry. 1977 May 3;16(9):1955–1959. doi: 10.1021/bi00628a031. [DOI] [PubMed] [Google Scholar]
- Sperling W., Carl P., Rafferty Ch, Dencher N. A. Photochemistry and dark equilibrium of retinal isomers and bacteriorhodopsin isomers. Biophys Struct Mech. 1977 Jun 29;3(2):79–94. doi: 10.1007/BF00535798. [DOI] [PubMed] [Google Scholar]
- Stockburger M., Klusmann W., Gattermann H., Massig G., Peters R. Photochemical cycle of bacteriorhodopsin studied by resonance Raman spectroscopy. Biochemistry. 1979 Oct 30;18(22):4886–4900. doi: 10.1021/bi00589a017. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]
- Terner J., Hsieh C. L., Burns A. R., El-Sayed M. A. Time-resolved resonance Raman spectroscopy of intermediates of bacteriorhodopsin: The bK(590) intermediate. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3046–3050. doi: 10.1073/pnas.76.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terner J., Hsieh C. L., El-Sayed M. A. Time-resolved resonance Raman characterization of the bL550 intermediate and the two dark-adapted bRDA/560 forms of bacteriorhodopsin. Biophys J. 1979 Jun;26(3):527–541. doi: 10.1016/S0006-3495(79)85269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M., Glaccum M., Nelson B., Ebrey T. G. Light isomerizes the chromophore of bacteriorhodopsin. Nature. 1980 Sep 25;287(5780):351–353. doi: 10.1038/287351a0. [DOI] [PubMed] [Google Scholar]



