Abstract
Adipose tissue is the largest compartment in the mammalian body for storing energy as fat, providing an important reservoir of fuel for maintaining whole body energy homeostasis. Herein, we identify the transcriptional cofactor hairless (HR) to be required for white adipogenesis. Moreover, forced expression of HR in non-adipogenic precursor cells induces adipogenic gene expression and enhances adipocyte formation under permissive conditions. HR exerts its proadipogenic effects by regulating the expression of PPARγ, one of the central adipogenic transcription factors. In conclusion, our data provide a new mechanism required for white adipogenesis.
Keywords: adipogenesis, hairless, JmjC, obesity, PPARγ
Introduction
Both excess and deficiency of adipose tissue result in severe metabolic disturbances 1]. A complex network of transcriptional events controls differentiation of adipocytes from preadipocytes of mesenchymal origin 2]. Peroxisome proliferator-activated receptor-γ (PPARγ) and the CCAAT/enhancer-binding protein (C/EBP) family of transcription factors represent core factors of the transcriptional machinery in this elaborate system. In brief, C/EBPβ and C/EBPδ mRNA and protein levels rise early in adipocyte differentiation and activate the expression of PPARγ [3]. PPARγ, considered to be the ‘master regulator’ of adipogenesis, in turn switches on transcription of C/EBPα. These two central regulators control the expression of numerous adipocyte-specific genes, as well as the transcription of each other [4, 5].
The jumonji (JmjC) domain-containing proteins have recently emerged as important factors in transcriptional regulation of cellular differentiation [6]. The JmjC domain of most members of this family has been ascribed specific histone demethylase activity, thus providing a potential common mechanism underlying modulation of transcription. Interestingly, the JmjC domain-containing histone demethylase 2A (Jhdm2a) is critically involved in energy homeostasis and obesity in mice [7]. This phenotype seemed, however, not to be caused by changes in adipogenesis. Hence, specific roles of JmjC proteins in adipogenesis are unknown thus far.
Hairless (HR) is one of the best-studied members of the JmjC protein family, but has not been linked to adipogenesis so far. Several nuclear hormone receptors have been shown to interact with HR: the thyroid hormone receptor, the vitamin D receptor and the retinoic acid receptor-related orphan receptor-α [8]. HR is crucial for several aspects of hair formation, growth and regeneration [9]. Mice lacking functional Hr show normal initial hair growth. However, after shedding at ∼3 weeks of age the hair is lost and does not regrow [10].
In this study, we identified HR as an important component of the transcriptional cascade governing white adipogenesis.
Results and discussion
Knockdown of HR impairs adipogenesis
Given the function of JmjC domain-containing proteins in cellular differentiation, we monitored mRNA expression of JmjC domain-containing genes during in vitro adipogenesis. Strikingly, hairless (Hr) was found to be the only regulated gene in differentiating 3T3-L1 preadipocytes (Fig 1A; supplementary Fig S1 online). Hr transcript levels peaked ∼24 h and dropped back to almost basal levels 48 h after differentiation induction (Fig 1A). The highest amounts of HR protein were found after 24–48 h of differentiation (Fig 1B).
Figure 1.
Hairless is required for adipocyte differentiation in vitro. (A) Relative hairless (HR) expression in 3T3-L1 cells at indicated time points of differentiation using quantitative reverse transcription–polymerase chain reaction (RT–PCR). (B) HR expression in 3T3-L1 cells at indicated time points of differentiation using western blotting. GCN5 was used as a loading control for nuclear extracts. (C) Determination of efficiency of HR knockdown in non-stimulated HR-depleted (shHr1 and shHr2) and non-silenced (ns) 3T3-L1 cells using quantitative RT–PCR. (D) Visualization of lipid incorporation by Oil Red O stains (left panels) and phase-contrast microscopy (right panels) in 3T3-L1 cells 8 days after differentiation induction. Scale bars, 50 μm. (E) Relative expression of CCAAT/enhancer-binding protein-β (C/EBPβ) and C/EBPδ in 3T3-L1 cells before (0 h) and 8 h after stimulation of adipogenesis using quantitative RT–PCR. (F) Relative expression of peroxisome proliferator-activated receptor-γ1 (PPARγ1) and PPARγ2 in 3T3-L1 cells at indicated time points of differentiation using quantitative RT–PCR. (G) Relative expression of C/EBPα, fatty acid binding protein 4 (FABP4) and adiponectin in 3T3-L1 cells before (0 h) and 8 days after stimulation of adipogenesis using quantitative RT–PCR. (H) Expression of HR, PPARγ1, PPARγ2, C/EBPα, fatty acid synthase (FASN) and FABP4 in 3T3 cells at indicated time points of differentiation using western blotting. The histone acetyl transferase GCN5 was used as a loading control for nuclear extracts. Tubulin was used as a loading control for total extracts. (I) Determination of efficiency of HR knockdown in non-stimulated HR-depleted (shHr1 and shHr2) or non-silenced (ns) primary mouse preadipocytes using quantitative RT–PCR. (J) Visualization of lipid incorporation by phase-contrast microscopy in primary mouse preadipocytes 8 days after differentiation induction. Scale bars, 50 μm. Error bars indicate standard deviations. *P<0.05; **P<0.01; ***P<0.001 (n=3 for B,C,E,F,G,I; n=4 for A, Student’s t-test). NS, nonsignificant; sh, short hairpin.
This expression pattern prompted us to investigate the requirement of HR in adipogenesis. 3T3-L1 cells infected with lentivirus containing either a non-silencing sequence or short hairpin RNA directed against HR were subjected to in vitro adipogenic differentiation. mRNA expression of Hr was reduced by ∼80% for one short hairpin (shHR1) and 60% for another short hairpin (shHR2; Fig 1C). Oil Red O staining visualized accumulation of lipids in non-silenced control cells indicating efficient differentiation into adipocytes. In contrast, cells expressing short hairpin RNA targeted against Hr showed markedly diminished Oil Red O staining and retained their fibroblastic morphology (Fig 1D). In line with silencing efficiency, depletion of HR using shHR1 resulted in a stronger inhibition of adipogenesis as compared with shHR2. Reduced adipogenesis is expected to be a result of changed expression of main transcriptional regulators. Expression of C/EBPβ and C/EBPδ, early regulators of adipocyte differentiation, were not affected in cells depleted of HR as compared with control cells (Fig 1E). However, induction of the two isoforms PPARγ1 and γ2, generated by alternative splicing and promoter usage of PPARγ, was reduced in cells depleted of HR as compared with control cells (Fig 1F). Consequently, PPARγ-dependent mRNA induction of C/EBPα, fatty acid binding protein 4 (Fabp4) and adiponectin was significantly diminished (Fig 1G). Accordingly, induction of PPARγ1 and γ2, C/EBPα, fatty acid synthase (FASN) and FABP4 proteins was markedly impaired (Fig 1H). Adipogenesis was unaffected when knockdown of HR was induced 2 or 4 days after differentiation induction (supplementary Fig S2A online), indicating that HR expression is required early during differentiation. Expression of shHr1 in cultured primary preadipocytes isolated from wild-type mice also resulted in efficient depletion of Hr mRNA (Fig 1I). These cells did not differentiate into adipocytes as compared with non-silenced control cells (Fig 1J).
3T3-L1 preadipocytes undergo approximately two rounds of mitotic clonal expansion preceding the adipogenic gene expression programme [11]. Knockdown of HR significantly, but only moderately, inhibited clonal expansion of 3T3-L1 cells as compared with control cells (supplementary Fig S2B online). HR might thus be partially required for mitotic clonal expansion, but in addition is likely to act on later transcriptional events.
The so-called ‘rhino’ mice carry a point mutation in the Hr gene, resulting in HR loss-of-function. Heterozygous animals showed no apparent skin or fur phenotype and were indistinguishable from wild-type mice. Heterozygous Hrwt/Hrrh mice were thus used as control mice in comparison to homozygous Hr mutant mice (Hrrh/Hrrh). To confirm a cell-autonomous defect, both primary preadipocytes from the stromal vascular fraction as well as primary fibroblasts were isolated from 10 weeks old mice. Almost no HR message was detected in primary preadipocytes and fibroblasts derived from Hrrh/Hrrh mice as compared with cells derived from heterozygous Hrwt/Hrrh control mice (supplementary Fig S3A online). Microscopic analysis showed that cells derived from Hrrh/Hrrh mice were unable to undergo adipogenesis to the extent of control cells. Lipid incorporation was markedly reduced in both primary Hrrh/Hrrh preadipocytes (Fig 2A) and primary Hrrh/Hrrh fibroblasts (Fig 2B) as compared with control cells. To address whether observed reduction in differentiation was due to changes in the amount of preadipocytes, we determined their numbers in the stromal vascular fraction of mice by flow cytometry. This analysis revealed no differences between Hrrh/Hrrh and control mice (supplementary Fig S3B online). Quantitative reverse transcription–polymerase chain reaction (PCR) analysis showed markedly reduced mRNA levels of PPARγ1 and γ2, C/EBPα as well as adiponectin, while expression of C/EBPβ and C/EBPδ was unchanged (Fig 2C,D). C/EBPα was highly expressed in mouse preadipocytes prior differentiation induction and was not enhanced on stimulation with adipogenic factors. Overall, our data provide strong evidence that HR is required for adipogenesis in vitro.
Figure 2.
Primary preadipocytes and fibroblasts isolated from Hr mutant mice show impaired adipogenesis. (A) Visualization of lipid incorporation by phase-contrast microscopy in differentiated primary preadipocytes isolated from heterozygous (Hrwt/Hhrh) control and homozygous (Hrrh/Hhrh) mice 8 days after differentiation induction. Scale bars, 200 μm (upper panels), 50 μm (lower panels). (B) Visualization of lipid incorporation by phase-contrast microscopy in differentiated primary fibroblasts after 8 days of differentiation. Scale bars, 200 μm (upper panels), 50 μm (lower panels). (C) Relative expression of indicated adipogenic genes in primary preadipocytes using quantitative reverse transcription–polymerase chain reaction (RT–PCR). (D) Relative expression of indicated adipogenic genes in primary fibroblasts using quantitative RT–PCR. Error bars indicate standard deviations. *P<0.05; **P<0.01 (n=4, Student’s t-test). C/EBPβ, CCAAT/enhancer-binding protein-β; HR, hairless; NS, nonsignificant; PPARγ1, peroxisome proliferator-activated receptor-γ1.
Hairless is required for adipocyte differentiation in vivo
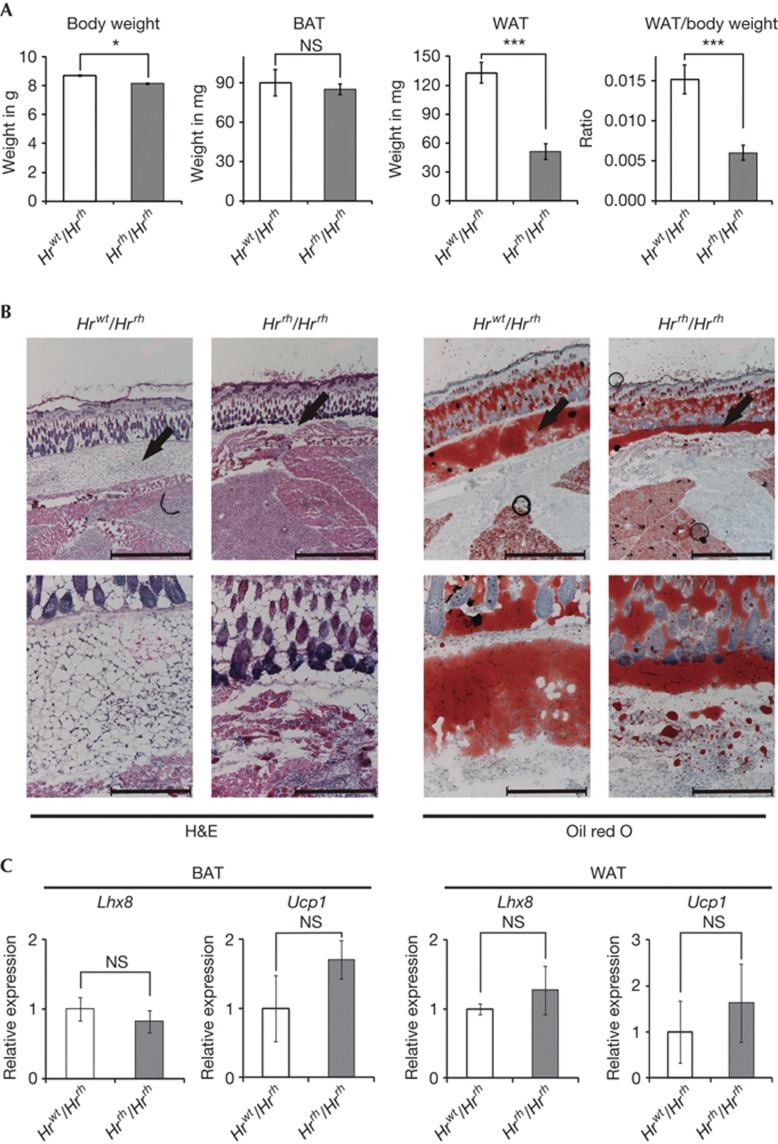
We next aimed at exploring adipose mass in HR mutant mice. They show a skin phenotype characterized by hair loss starting at ∼18 days of age. Once the hair is lost, the skin becomes gradually thickened and wrinkled [10]. At 10 weeks of age, Hrrh/Hrrh mice exhibited significantly reduced adipose mass, albeit significantly enhanced body weight, as compared with control mice (supplementary Fig S4 online). Expansion of skin is likely to account for the overall increased body weight despite reduced fat mass. To uncouple adipose tissue mass from compromised insulation due to loss of fur and altered skin, pups at 18 days of age were assessed. Subcutaneous adipose tissue in the region of the scapulae as well as brown adipose tissue (BAT) were dissected and weighted. Hrrh/Hrrh pups showed a significant but moderate reduction in body weight. While BAT weights were unchanged, a marked reduction in subcutaneous white adipose tissue (WAT) was observed in Hrrh/Hrrh pups (Fig 3A). In control mice, histology revealed a distinct subcutaneous layer of white adipose tissue. However, in Hrrh/Hrrh mice, this layer was markedly diminished (Fig 3B). mRNA expression of two BAT-specific genes, lim homeobox 8 (Lhx8) and uncoupling protein 1 (Ucp1), were not changed in BAT (Fig 3C) confirming that BAT was not affected in Hrrh/Hrrh pups. Expression of these genes in WAT was also unaltered (Fig 3C), excluding increased conversion of WAT into BAT and thus increased BAT activity. Altogether, our data indicate that HR is crucial in white adipose tissue formation in vivo independent of differences in thermoregulation.
Figure 3.
Subcutaneous white adipose tissue is reduced in Hr mutant mouse pups. (A) Body weight, weight of brown adipose tissue (BAT), weight of subcutaneous white adipose tissue (WAT) and WAT mass to body weight ratios of 18-days-old heterozygous control (Hrwt/Hrrh) and homozygous mutant (Hrrh/Hrrh) pups. (B) Transverse histological sections (haematoxylin–eosin (H&E, left) and Oil Red O (right)) at the level of the scapula from Hrwt/Hrrh and Hrrh/Hrrh pups 4 days after birth. Black arrows indicate subcutaneous adipose tissue. Scale bars, 1mm (upper panels) and 250 μm (lower panels). (C) Relative expression of LIM homeobox 8 (Lhx8) and uncoupling protein 1 (Ucp1) in BAT and WAT of 10-week-old heterozygous control (Hrwt/Hrrh) and homozygous (Hrrh/Hrrh) mutant mice using quantitative reverse transcription–polymerase chain reaction. Error bars indicate standard deviations. *P<0.05; ***P<0.001 (n=4, Student’s t-test). HR, hairless; NS, nonsignificant.
Hairless overexpression increases adipogenic potential
Very few genes were attributed enough potency to render non-adipogenic cell lines susceptible to induction of adipogenesis. 3T3-L1 preadipocytes show very high adipogenic potential in response to the standard hormonal cocktail, whereas NIH3T3 fibroblasts are largely insensitive to induction of adipocyte differentiation [12]. When comparing endogenous Hr expression levels in (undifferentiated) 3T3-L1 with those seen in NIH3T3 cells, significantly higher mRNA levels were found in 3T3-L1 cells (Fig 4A). To determine whether forced expression of Hr might induce adipogenesis in non-adipogenic cells lines, we expressed green fluorescent protein (GFP)-tagged HR and GFP alone in NIH3T3 fibroblasts (Fig 4B) and C2C12 myobloasts (Fig 4C). On HR expression, PPARγ1 and γ2, C/EBPα as well as adiponectin were induced in NIH3T3 prior induction of adipogenesis (Fig 4D), while HR had no effect on basal expression of adipogenic and myogenic genes in C2C12 cells (Fig 4E). Expression of C/EBPβ and C/EBPδ were not affected in both cell types (Fig 4D,E). Adipogenesis as well as induction of adipogenic genes were markedly enhanced in HR-expressing NIH3T3 and C2C12 cells, as compared with GFP-expressing cells under adipogenic conditions (Fig 4F–I). Myogenic markers, however, were unaltered in C2C12 cells overexpressing HR, as compared with control cells on myogenic differentiation (Fig 4J), indicating that HR is unable to change muscle fate once conditions for muscle differentiation are set in place. As shown above, the amount of preadipocytes in Hr mutant mice is not affected, challenging its requirement in adipocyte lineage commitment in vivo. Nevertheless, changes in HR expression in precursors might still be important in lineage specification as experiments in non-adipogenic cell lines hint at such a function. Importantly, the adipogenic genes were induced by ectopic expression of HR in NIH3T3 cells even under non-permissive conditions. It was shown that white and brown adipocytes do not share a direct common precursor, but that brown adipocytes are developmentally closer to myocytes [13]. Measuring expression levels of the two brown adipocyte marker genes Ucp1 and Lhx8 in C2C12 cells expressing HR showed no significantly higher induction of these factors as compared with control cells (supplementary Fig S5 online), corroborating a role of HR specifically in white adipogenesis.
Figure 4.
Forced HR expression promotes adipogenesis in NIH3T3 and C2C12 cells. (A) Relative expression of Hr in NIH3T3 and 3T3-L1 cells using quantitative reverse transcription–polymerase chain reaction (RT–PCR). (B,C) Analysis of expression of hHR–GFP and GFP alone in NIH3T3 cells (B) and C2C12 cells (C) 24 h after transfection and in non-transfected cells (control), using flow cytometry (upper panels) and fluorescence microscopy (lower panels). Scale bars, 50 μm. Histograms are representative of three flow cytometry analyses. (D,E) Relative expression of adipogenic genes in NIH3T3 cells (D) and C2C12 cells (E) ectopically expressing hHR–GFP or GFP alone prior differentiation, using quantitative RT–PCR. (F) Visualization of lipid incorporation by Oil Red O stains (upper panels) or phase-contrast microscopy (lower panels) in NIH3T3 cells 8 days after differentiation induction. Scale bars, 100 μm. (G) Relative expression of adipogenic genes in NIH3T3 cells on day 8 of differentiation using quantitative RT–PCR. (H) Visualization of lipid incorporation by Oil Red O stains (upper panels) or phase-contrast microscopy (lower panels) in C2C12 cells 8 days after differentiation induction. Scale bars, 100 μm. (I) Relative expression of adipogenic genes in C2C12 cells on day 8 of differentiation using quantitative RT–PCR. (J) Relative expression of myogenic genes in C2C12 cells on day 6 of myogenic differentiation using quantitative RT–PCR. Error bars indicate standard deviations. *P<0.05; **P<0.01; ***P<0.001 (n=3, Student’s t-test). GFP, green fluorescent protein; HR, hairless; PPARγ1, peroxisome proliferator-activated receptor-γ1.
Altogether, these results indicate that HR has a pivotal role in white adipocyte differentiation and that HR regulation might modulate adipogenic potential.
Hr promotes PPARγ transcription
To test whether the HR’s JmjC domain has an important function during HR-dependent adipogenesis, we expressed GFP alone, full-length HR–GFP and truncated HR lacking the JmjC domain in NIH3T3 cells (Fig 5A). Both cells expressing full-length HR and HR lacking the C-terminal JmjC domain showed key features of adult adipocytes on induction of differentiation (Fig 5B). This suggests that the JmjC domain within HR is not required to potentiate adipocyte differentiation.
Figure 5.
Hairless promotes PPARγ1 expression and drives adipogenesis independent of its JmjC domain. (A) Schematic representation of structural and functional domains of human HR (hHR). Repression domains (RD1, RD2, RD3); thyroid hormone receptor (TR)-interacting domains (TR-ID1, TR-ID2), RAR-related orphan receptor alpha (RORα)-interacting domains (RORα-ID1, ROR-ID2) cysteine-rich domain (Cys) and JmjC domain. (B) Visualization of lipid incorporation by phase-contrast microscopy in NIH3T3 cells ectopically expressing GFP alone, human full-length HR or a mutant form of HR lacking the JmjC domain (hHR ΔJmjC) after 8 days of differentiation. Scale bars, 50 μm. (C) Visualization of lipid incorporation by phase-contrast microscopy in HR-silenced (shHr1 and shHr2) and non-silenced (ns) 3T3-L1 cells 8 days after differentiation induction in the absence (upper panels) and presence (lower panels) of rosiglitazone. Scale bars, 100 μm. (D) Luciferase reporter assay in NIH3T3 cells coexpressing hHR–GFP or GFP alone and either an empty luciferase reporter or luciferase under the control of the PPARγ1 or PPARγ2 promoter. One day after transfection, luciferase activity was measured and plotted relative to the activity of the empty reporter. Error bars indicate standard deviations. ***P<0.001 (n=3, Student’s t-test). GFP, green fluorescent protein; HR, hairless; JmjC, jumonji; NS, nonsignificant; PPARγ1, peroxisome proliferator-activated receptor-γ1; sh, short hairpin; VDR, vitamin D receptor.
Forced expression of HR in non-adipogenic cell lines induced transcription of PPARγ1 and γ2 in NIH3T3 cells even in the absence of an adipogenic stimulus. We therefore investigated a possible regulatory mechanism of HR on PPARγ expression. Even in the presence of rosiglitazone, a potent PPARγ agonist that directly binds to its ligand-binding domain, 3T3-L1 cells depleted of HR were not able to undergo differentiation (Fig 5C). This result hints at a direct modulation of PPARγ expression by HR. To investigate this assumption, luciferase promoter assays were performed. HR stably induced reporter gene expression under the control of the PPARγ1 promoter, but failed to activate the PPARγ2 promoter (Fig 5D). Importantly however, our loss- and gain-of-function experiments mainly revealed regulation of transcription of PPARγ2 and to a lesser extend of PPARγ1. This discrepancy might be explained by a deficit in the ability of the PPARγ2 reporter to recapitulate endogenous gene expression. Overall, our data indicate that HR might control PPARγ transcription during white adipogenesis.
Concluding remarks
A tightly controlled transcriptional network regulates the process of adipocyte differentiation. Using cell culture experiments and mouse models, we showed that HR is required during both in vitro as well as in vivo white fat cell development. Furthermore, forced expression of HR in non-adipogenic cell lines increased their potential to undergo adipocyte differentiation.
HR exerts its proadipogenic function, at least partially, by promoting PPARγ transcription. The exact mechanisms of PPARγ regulation need to be addressed in the future. HR has several independent transcriptional repressor domains supporting its function as a transcriptional repressor [14]. Given positive regulation of PPARγ transcription, HR might repress the function of a negative regulator of PPARγ transcription. However, HR might also act as a transcriptional coactivator. Indeed, HR also contains motifs that have previously been shown to mediate binding of coactivators to nuclear receptors [15].
Interestingly, hair growth and cycling have recently been shown to be controlled by the subcutaneous fat tissue [16]. It will thus be interesting to investigate whether a crosstalk between subcutaneous adipocytes and hair follicle stem cells, both of which seem to be affected in Hr mutant mice, is dependent on HR. Overall, this study establishes HR as a key component in the transcriptional control of white adipocyte differentiation.
Methods
Mouse experiments. Breeding and maintenance of mice and isolation of primary cells are described in supplementary information online.
Cell culture and cell differentiation. 3T3-L1 preadipocytes, NIH3T3 fibroblasts and C2C12 myoblasts (American Type Culture Collection) were cultured in DMEM (Sigma) with 10% fetal bovine serum at 37 °C and 5% CO2. We thank A. Christiano for sharing HR overexpression constructs with us. Protocols for differentiation of cells, transfection and infection are provided in the supplementary information online.
Quantitative real-time PCR. RNA isolation, reverse transcription and PCR procedures are described in supplementary information online. Expression levels of genes in relation to the reference gene 18S were normalized to time point 0 (Figs 1A,E,F), to expression in non-silencing control cells (Fig 1C,I), to expression in heterozygous mice (Fig 3C) and to expression in GFP-expressing cells (Fig 4D,E).
Western blot analysis. Cells were collected and proteins were enriched into nuclear and cytosolic fractions as previously described [17]. Antibodies and western blotting procedures are described in the supplementary information online.
Histological analysis. Specimens were embedded in Tissue-Tek OCT Compound (Sakura) and frozen at −80 °C. Cryosections of 10 μm were then processed for haematoxylin–eosin staining and Oil Red O staining. Oil Red O staining is described in the supplementary information online.
Luciferase promoter assay. Plasmids and procedures of the assay are described in the supplementary information online. We thank J. Auwerx, ETH Lausanne, for sharing PPARγ reporter plasmids with our laboratory.
Data analysis and statistics. Statistical calculations and data handling were performed using Graph Pad Prizm 5 software (Graph Pad Prizm software). For comparison of two data sets, Student’s t-test was used. A P-value below 0.05 was accepted to deny the null hypothesis.
Supplementary information is available at EMBO reports online ( http://www.emboreports.org).
Supplementary Material
Acknowledgments
This work was supported by the Swiss National Science Foundation—SNSF (PP0033-114856), an EMBO small grant and an European Research Council (ERC) starting grant (ERC-2011-StG, 281271-STRESSMETABOL). SK has been supported by an ETH Independent Investigators' Research Awards (ETHIIRA) grant by ETH (ETH-07 09-1). M.M., A.G., A.G. and H.G. were supported by the ERC starting grant. J.M. and A.I. were supported by the Swiss National Science Foundation (SNSF). We thank L. Pouilly and O. Wendling for help with mouse histology and A. Kalousi for helping with western blot analysis.
Author contributions: S.K., M.M. and R.R. designed the research, S.K. and M.M. performed most experiments, A.G. and H.G. helped with western blotting and lentivirus infection. G.G. and C.W. performed flow cytometry experiments and helped with the isolation of preadipocytes and fibroblasts. J.S. helped with adipocyte differentiation, J.M. and A.G. helped with quantitative PCR experiments, E.G. and M.B. were involved in assessment of JmjC function in H.R., A.I. helped with the histological analysis of fat depots in mice, S.K., M.M. and R.R. wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Rosen ED, Spiegelman BM (2006) Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444: 847–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristancho AG, Lazar MA (2011) Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol 12: 722–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington GJ, Ross SE, MacDougald OA (1998) The role of C/EBP genes in adipocyte differentiation. J Biol Chem 273: 30057–30060 [DOI] [PubMed] [Google Scholar]
- Lin FT, Lane MD (1994) CCAAT/enhancer binding protein-α is sufficient to initiate the 3T3-L1 adipocyte differentiation program. Proc Natl Acad Sci USA 91: 8757–8761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Spiegelman BM (1994) Stimulation of adipogenesis in fibroblasts by PPAR γ2, a lipid-activated transcription factor. Cell 79: 1147–1156 [DOI] [PubMed] [Google Scholar]
- Cloos PA, Christensen J, Agger K, Helin K (2008) Erasing the methyl mark: histone demethylases at the center of cellular differentiation and disease. Genes Dev 22: 1115–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi K, Okada Y, Kallin EM, Zhang Y (2009) Role of Jhdm2a in regulating metabolic gene expression and obesity resistance. Nature 458: 757–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CC (2009) Hairless is a nuclear receptor corepressor essential for skin function. Nucl Recept Signal 7: e010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad W et al. (1998) Alopecia universalis associated with a mutation in the human hairless gene. Science 279: 720–724 [DOI] [PubMed] [Google Scholar]
- Mann SJ (1971) Hair loss and cyst formation in hairless and rhino mutant mice. Anat Rec 170: 485–499 [DOI] [PubMed] [Google Scholar]
- Tang QQ, Otto TC, Lane MD (2003) Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc Natl Acad Sci USA 100: 44–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Arany Z, Seale P, Mepani RJ, Ye L, Conroe HM, Roby YA, Kulaga H, Reed RR, Spiegelman BM (2010) Transcriptional control of preadipocyte determination by Zfp423. Nature 464: 619–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P et al. (2008) PRDM16 controls a brown fat/skeletal muscle switch. Nature 454: 961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter GB, Beaudoin GM 3rd, DeRenzo CL, Zarach JM, Chen SH, Thompson CC (2001) The hairless gene mutated in congenital hair loss disorders encodes a novel nuclear receptor corepressor. Genes Dev 15: 2687–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heery DM, Kalkhoven E, Hoare S, Parker MG (1997) A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 387: 733–736 [DOI] [PubMed] [Google Scholar]
- Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, Horsley V (2011) Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 146: 761–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell UR, Kvietikova I, Scheid A, Bauer C, Wenger RH, Gassmann M (2001) Induction of HIF-1α in response to hypoxia is instantaneous. FASEB J 15: 1312–1314 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.