Abstract
Cells can migrate individually or collectively. Collective movement is common during normal development and is also a characteristic of some cancers. This review discusses recent insights into features that are unique to collective cell migration, as well as properties that emerge from these features. The first feature is that cells of the collective affect each other through adhesion, force-dependent and signalling interactions. The second feature is that cells of the collective differ from one another: leaders from followers, tip from stalk and front from back. These are dynamic differences that are important for directional movement. Last, an unexpected property is discussed: epithelial cells can rotate persistently in constrained spaces.
Keywords: collective cell movement, dynamics, mechanics, guidance, rotation
See the Glossary for abbreviations used in this article.
Glossary.
- DDR1
discoidin domain receptor 1
- ECM
extracellular matrix
- Fz
Frizzled
- Fat2
FAT tumour suppressor 2
- FGF
fibroblast growth factor
- Par3/6
partitioning defective 3/6
- RhoGAP
GTPase activating protein for Rho
- VEGF
vascular endothelial growth factor
Introduction
Collective migration refers to cells migrating together in sheets, clusters, streams, sprouts or other multicellular arrangements. It is observed frequently in animals in vivo, both as part of the normal morphogenetic programmes during development [1,2] and in pathological situations such as dissemination of carcinoma cells [3,4] or neovascularization of tumours. Cells of epithelial or endothelial origin also display collective migration in vitro: two-dimensionally in standard tissue culture dishes, or three-dimensionally in more complex matrixes [5,6,7]. Several in-depth reviews describe different types of collective cell migration [1,2,3,4] and discuss specific examples, characteristic features and model systems used in detail, including those referred to in this review.
Collective cell migration has many features in common with individual cell migration, but also has unique features. Some features might be unique to one type of migration, others more widespread. Analysis of the features shared by many types of collective cell migration might inform us about common constraints and common strategies of collective motile behaviour. In this update, I discuss studies that have improved our understanding of key features of collective cell migration and reveal some possible underlying common themes in these complex behaviours. The first is the interaction between collectively migrating cells and how the topology of this interaction can affect individual cell behaviours, as well as overall movement. The second is the differences between the migrating cells within a collective and the significance of these differences. Finally, some unexpected large-scale movements, such as rotation, can result from collective behaviours and are discussed.
Defining a collective
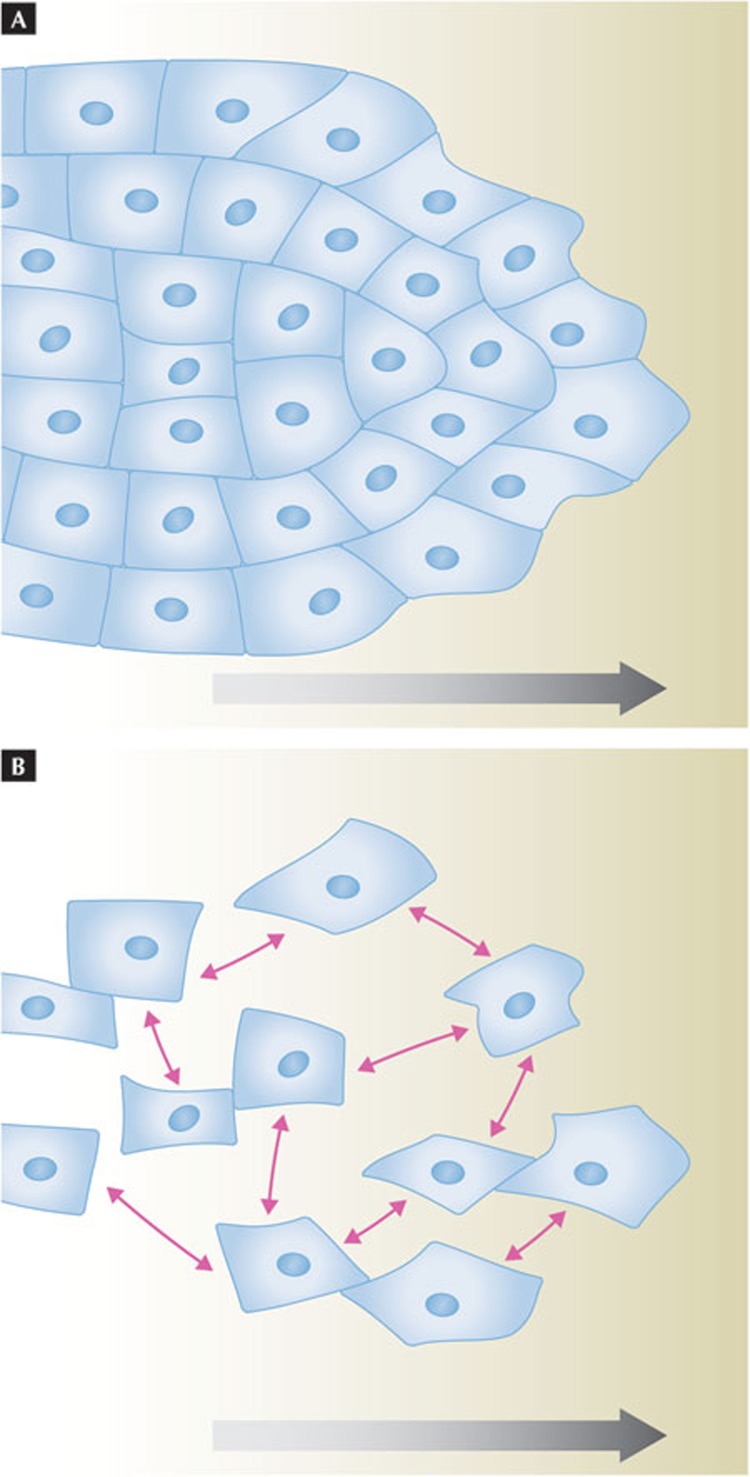
At the outset, it is worth clarifying which cell and tissue behaviours are considered to be collective cell migration (Fig 1). A strict definition describes collective migration as “tightly connected cells that migrate as cohesive structures” (Fig 1A). I favour a more inclusive definition: “collections of cells moving together and affecting one another while doing so”. This definition allows for a wider range of physical and signalling interactions between the migrating cells, and would include loosely associated streams with dynamic interactions (Fig 1B) such as neural crest cells [8] or neurons of the rostral migratory stream [9,10]. Even for closely apposed and apparently tightly connected cells, the physical and mechanical interactions can differ substantially depending on the cell type and characteristics. An open definition of collective migration emphasizes that the cell–cell connection parameter is highly variable and that this parameter should be crucially considered in each case. A recent review of cancer cell invasion classifies multicellular migration into “multicellular streaming” and “collective cell migration” [4], which would correspond to my loose and cohesive types of collective cell migration, respectively.
Figure 1.
Different interaction properties of collectively migrating cells. (A) A cohort of attached cells, in which each migrating cell is attached to its neighbours. The attachment might be relatively static or more dynamic, but gives some mechanical coupling. (B) A group of loosely interacting cells moving together. In the case of Xenopus neural crest cells, cells will locally inhibit one another on physical contact, but simultaneously attract one another (pink arrows). Large grey arrows indicate direction of movement.
Regardless of which definition is followed, two categories of interaction should be considered for cells that migrate collectively. The first consists of interactions with the environment. These are similar in principle to the interactions that an individually migrating cell has with its environment, but can show some differences in practice when executed by a group of cells. Such interactions include adhering to the substrate and getting traction on it, as well as avoiding or degrading obstacles [5,11]. The substratum can be the ECM, but it can also be other cells. Reading guidance cues is another important interaction with the environment, and one in which individual cells and collectives might differ [12,13]. The second category of interactions consists of those between the cells that are migrating collectively. These interactions can be physical and mechanical in nature, but communication by signals that do not depend on cell–cell contact can also be involved as discussed below (pink arrows in Fig 1B). This second category of interactions is used to define collective migration; the presence and consequences of these cell–cell interactions sets it apart from individual cell migration.
Interaction between cells of a tightly associated cohort is probably mediated by robust cell–cell adhesion, but what about the loose interactions? Analysis of neural crest cells might shed light on this. Neural crest cells perform long-distance migration in the vertebrate embryo and have diverse roles at their destinations. Neural crest cells can migrate as individual cells but, at least in Xenopus, movement is more directional if the cells migrate collectively [14]. N-cadherin was found to mediate a cell–cell interaction that produces contact-dependent cell polarity, which, in turn, improves collective directionality [14]. In other species, the associations between neural crest cells may be less frequent and more dynamic [8,15]. Together with the observation that neural crest cells undergo an epithelial-to-mesenchymal transition (EMT) when they delaminate from the neural tube [16], this loose association has supported the idea that neural crest cells migrate as individual mesenchymal cells. However, N-cadherin has a positive effect on net movement even for the more loosely associated cells [17], so the collective aspects of neural crest cell migration might be pervasive. Xenopus neural crest cells attract one another by secreting the complement fragment C3a, whilst also expressing the cognate receptor C3aR [18]. The resulting mutual chemoattraction keeps neural crest cells together as a loose cohort during migration. This finding helps to explain how cells that react to each other with “contact-dependent inhibition of locomotion” [19], and thereby repel one another on contact, nevertheless move together as a cohort [18] or stream [8]. The process can be described as a community effect on the basis of global attraction and local inhibition.
Information at cell contacts
The way that collectively migrating cells interact with one another usually involves some direct cell–cell contact. In neural crest cells, contact-dependent RhoA activation leads to contact-mediated repulsion [19], probably due to the ability of RhoA to stimulate contractility through Rho-kinase and myosin activation [20]. This effect is counterbalanced by the soluble attractant. For more cohesive collective movements, such as those shown by epithelial and endothelial cells, one would expect cell–cell contacts not to be repulsive. Findings suggest that the differing outcomes of cell–cell contact for these types of migrating cell—transiently touching in contrast with cohesive collective movement—is at least in part due to regulatory differences that impinge directly on RhoA [21,22]. Many cancer cells migrate and invade tissue or a three-dimensional matrix as tightly associated cohorts [3,4]. An in-depth investigation of this phenomenon using the squamous cell carcinoma cell line A431 showed that genetic manipulations that increase cortical contractility decrease cell cohesion and, interestingly, decrease tissue invasion, although the individual cells are still migratory [21]. The transmembrane protein DDR1 is necessary for cohesive movement. DDR1 reduces myosin-dependent contractility at cell–cell junctions through a set of molecular links including two polarity proteins Par3 and Par6 as well as p190ARhoGAP [21]. Local recruitment or activation of the RhoGAP is expected to reduce RhoA activation locally at cell contacts. Similarly, a screen for genes affecting collective movement of human bronchial epithelial cells identified the RhoGAP as an essential contributor that prevents cell scattering and allows collective and cohesive movement [22]. This RhoGAP, myosin-IXA, is unusual in that it also has a myosin motor domain, mediating interaction with actin filaments. Myosin-IXA is recruited to the cell cortex at new adhesions and stabilizes these adhesions. In both of these examples, local RhoA inhibition on cell–cell contact prevents contraction and cell scattering allowing for continued adhesion.
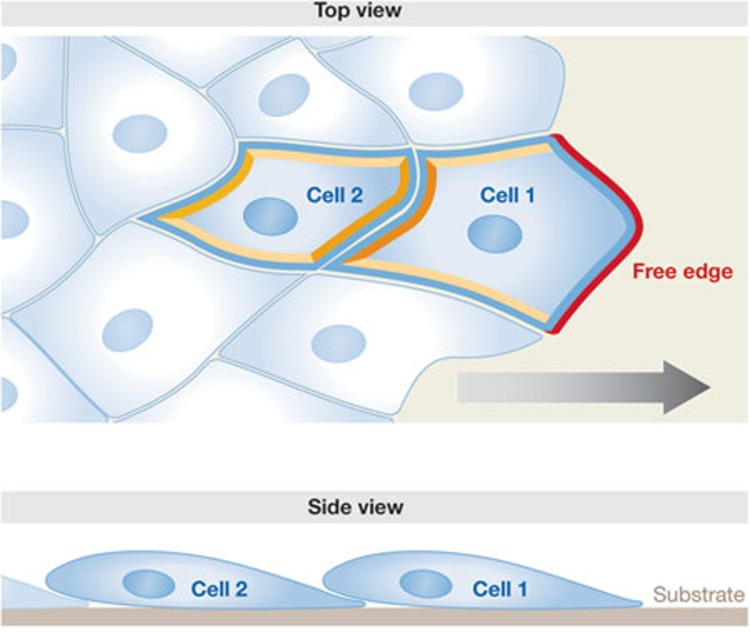
The topology of cell–cell interactions might serve as a source of spatial and directive information. This is evident when cells are in an anisotropic arrangement, for example, when only one side of a cohort is free (Fig 2). The effect of a free edge can be observed when a scratch wound is generated in a dense sheet of cells. The cells migrate with biased directionality, and often collectively, to fill the gap [7,23,24]. Interestingly, experiments have shown that when a small group of front cells in the tip of a slender moving cohort is separated from the rest by laser cutting, their highly directional movement is perturbed [25]. Slightly counter-intuitively, their ability to move forward is enhanced by being in contact with the other cells behind them. This indicates that the polarized topology of a cohort is important for effective movement. For an individual cell, the topology of its interaction with the other migrating cells can be a source of polarity and therefore of directionality. This is most clear when considering a cell at the free surface of a simple two-dimensional cohort (Fig 2, cell 1), which has obviously anisotropic interactions (red compared with yellow/orange edges). Front cells often become polarized at the subcellular level with a polarized cytoskeleton and with their protrusions preferentially oriented outwards, into the free space. In many cell types the polarity is also manifest as centrosome orientation relative to the nucleus [26,27], but this might be a consequence of migratory polarity rather than being instructive [28]. Non-front cells also become polarized [25,26,27]. In three-dimensional morphogenesis such as sprouting, one can speculate that equivalent topological instructions occur and bias the primary protruding area of a front cell to be opposite from where the cell–cell interactions occur. Coupled with substrate geometry in a tissue, such instruction can provide significant patterning in morphogenesis.
Figure 2.
Topological interactions between front cells and followers. The upper panel represents a top-view and the lower panel a side-view of the same region—the front part of a migrating cohort. As the substrate (ECM) is at the bottom, it is accessible to all migrating cells in the case of a simple monolayer. Cell 1 has one free edge (red) and three bound edges (orange and yellow) giving inherent anisotropy. The bound edges might also be under contact-dependent tension. Cell 2 has only bound edges, but they might experience different amounts and types of stress, for example due to pull from cell 1, and traction and resistance in other cells. The yellow edges might experience polarized shear stress. ECM, extracellular matrix.
How do front cells become internally polarized? The precise answer might differ depending on the cell type—epithelial, endodermal or neural—and on the geometries of cell–cell interactions between the collective. Some general ideas can, however, be explored by reconsidering the simple two-dimensional sheet type of arrangement (Fig 2). Interaction with the substrate at the free edge (Fig 2, highlighted in red) might direct front cell polarization, as ECM anisotropy can produce intracellular polarization [29]. Note that the extent of substrate interactions might initially be similar for a cell at the free surface and an internal cell of a two-dimensional monolayer (Fig 2, bottom, see side-view of cell 1 and cell 2). The difference is in available, unconstrained space. More recently, the cell–cell interaction surfaces (Fig 2, marked yellow and orange), which are also clearly anisotropic for the front cell, have attracted interest. Anisotropic cadherin-mediated cell–cell adhesion was shown to polarize multiple cell types [30,31], and thereby to direct movement [31]. Thus, by either or both mechanisms, a front cell can be directly instructed for forward movement.
It is less obvious how cells of a collective that have no free surface (Fig 2, cell 2) are oriented in behaviour. Direct observations show that such cells are polarized and contribute actively to collective migration [25,32]. As to how polarization occurs, analyses of the mechanics and forces involved seem to give some insight: physical pulling forces have been shown to alter the size of cadherin-mediated cell–cell contacts [33], just as pulling forces were previously shown to change focal adhesions to the ECM [34]. In an elegant set-up, cadherin-mediated force application, but not simply cadherin engagement, was shown to promote cell protrusions at the opposite end of mesendoderm cells [35]. Interestingly, localization of the intermediate filament keratin was strongly influenced by tension on the cell–cell contacts, apparently through recruitment of plakoglobin to cadherin under tension. Under physiological conditions, this system was found to be most important for non-front cells (Fig 2, cell 2; [35]). Although the front cell has multiple levels of polarity information to direct protrusions forward, the following cells might be more dependent on anisotropic forces to orient their protrusive activity. Force differences in collectively migrating cohorts probably have significant effects on both collective movement and individual cell behaviour [36]. The molecular link from cortical tension to intermediate filaments found in mesendoderm cells might represent a common mechanism. Intermediate filaments have been shown to transmit mechanical stress in endothelial cells [37]. Specific keratins are also required for proper wound repair in the skin of mice [38] and in collective sheet migration of epithelial cells in culture [39].
Different cell states
From the previous discussion of cell topologies, it is evident that migrating cells within a collective should sometimes be considered as two types: the front cells that have free edges or more extensive substrate interactions, and the other cells. This distinction of two cell types, two states or two behaviours seems to be a widespread phenomenon for collective cell migration. In the extreme case, there are types of collective migration in which distinct cell types, such as fibroblasts and tumour cells, co-operate [40]. Most migrating collectives, however, are homotypic—originating from one cell type. Despite this, detailed analysis of cell behaviours often identifies two types of cell, or two cell states. As this has been observed in many contexts and with varying methodology, the naming of such cells differs. In some studies, the front cells in two-dimensional sheets, in particular the most active and dominant of them, are called ‘leader cells’ with the remaining cells being called ‘followers’. For consistency, I relate to this terminology, ‘leaders’ and ‘followers’, henceforth. The presence of leader cells and distinction of two cell states is unlikely to be applicable to all types of collective movement. Certain types of branching morphogenesis, for example [41,42], might rely primarily on other cell behaviours. The same is true for rotation, as discussed in the last section.
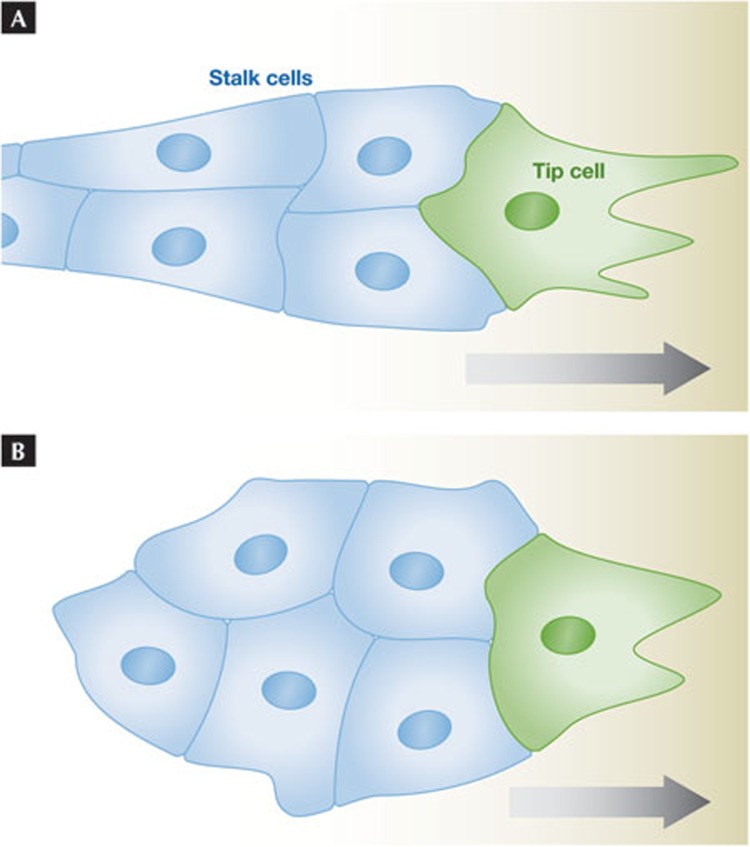
The exact topology, number and degree of specialization of leader cells varies depending on the system analysed. In sprouting morphogenesis, the leaders are the tip cells and the followers are the stalk cells (Fig 3A). These are usually described as fates and have distinct induction requirements. Tip cells are induced by VEGF in angiogenesis [43] and by FGF in tracheal development [44]. They look and behave differently from stalk cells. Tip cells have many filopodia and the ability to respond to external guidance information [45]. They also express different genes from stalk cells [46,47]. In collectively migrating and invading cancer cells, leader cells also express different genes compared with internal and follower cells [48,49,50]. Finally, data on neural crest cell migration in chicks indicate that leading and following (‘trailing’) neural crest cells not only behave differently, but also have different gene expression profiles [51]. Thus, during many types of collective cell migration, some cell specialization occurs. That this also occurs in neural crest cells supports the idea that tightly cohesive and loose, dynamic types of collective cell migration have more in common than is immediately apparent.
Figure 3.
Different cell states within a migrating collective. (A) A typical small sprout with a leading tip cell (green), followed by stalk cells (blue). The structure will move to the right. The stalk cells might be pulled by the tip cell or contribute directly to movement as seen for leaders and follower cells in a flat moving cohort. (B) A free migratory group; contrary to the situation in (A) there is no intrinsic polarity in the group, and thus no obvious leader. If we assume the green cell is the dominant ‘leader’ cell at this moment and the blue ones ‘followers’, the cluster will move to the right. If the leader state is assigned by extrinsic information such as an ‘attractant’ gradient, this might be sufficient to constitute ‘collective guidance’ [13].
Leaders in a collectively migrating sheet are functionally distinct from the other cells; this notion was reinforced in a systematic study of endothelial cells [7]. A distinct set of genes was found to be important for front cell behaviours, and was different from the genes required for general motility. Distinct roles can also be inferred from cell ablation or inactivation studies. In chains of migrating glia cells, photo-ablation studies have shown that the leader (‘pioneer’) cells are required for successful migration of the follower cells along their axonal path [52]. In the large migrating lateral line primordium in zebrafish, only the leader cells need to express the chemokine receptor Cxcr4b [53]. In developmental sheet migration surrounding a hole, the leading edge cells express unique markers and together construct a ‘purse-string’ actin cable; they also extend sensing filopodia for accurate hole closing [54]. Finally, when cohorts of epithelial cells are observed in two- or three-dimensional cultures, elongated substructures can emerge that, similarly to sprouts, migrate efficiently and directionally. Cells within them tend to be forward polarized and leader cells are obvious [24]. Ablation of the main lamellipodium in the front cell perturbs the organization and transiently impedes overall movement, showing the instructive role of leader cells on a short timescale [25]. As mentioned above, detaching the few front cells from the followers also perturbs collective directionality until the followers reattach. Thus, both leader and follower cells migrate actively and both seem to be crucial for collective directionality. However, there seems to be a clear division of labour between them in many examples of collective cell migration.
Leader and follower cells should be considered as different cell states and not different cell types. This becomes clear when collective migration is dynamically observed by live imaging. Leaders and followers are found to interconvert and change roles. It might not be surprising that this can occur in experimentally generated cell sheets or cohorts of initially equivalent cells. More surprising were the findings that in sprouting angiogenesis the leader (tip) cells exchange position, and function, with follower (stalk) cells [55,56]. A few years ago, it was shown that specification of tip cells over stalk cells is a dynamic and competitive process involving an inductive signal (VEGF) and an inhibitory signal, a type of lateral inhibition, through Notch and its ligand Delta [57,58]. This process is recapitulated in tumour angiogenesis [59,60]. A similar but more stereotypic process happens in invertebrate tracheal sprouting [61]. Live imaging has revealed that during the process of angiogenic sprouting, leader and follower cells dynamically exchange places, in the time frame of minutes to hours [55,56]. Stalk cells are observed to ‘race ahead’ and overtake the lead, displaying unanticipated dynamic migratory abilities. The stalk cells thereby become tip cells and vice versa. This indicates that tip cell behaviour is a function of position in the chain, a situation analogous to leader and follower behaviours described in vitro. This seems to be the normal process in vertebrate sprouting angiogenesis. It also helps to explain how tip cells can be quickly replaced if ablated, even in invertebrate systems that normally display limited interconversion and plasticity [62].
The appreciation that leader cell assignment is dynamic is in line with new ideas about guidance in collective cell migration. In the structures considered so far, the migrating collectives are inherently polarized (Figs 2,3A) with an attached rear. If the migrating group is unattached (Fig 3B), there is no inherent information as to the location of the front cell. Each cell with a free edge can be outward polarized and in principle behave as a leader cell. All else being equal, this would create a tug of war with no overall directionality. The migrating border cell cluster in Drosophila is an example of an unattached group, but one that migrates directionally towards its target, the giant oocyte. The border cells are derived from an epithelium and seem to be cohesive, but with frequent exchange of the front cell during migration [63,64]. Dominant leader cell behaviour can be induced in non-front cells by instant manipulation of Rac activity [65], reinforcing the idea of dynamic leader cell assignment. Interestingly, this cluster uses receptor tyrosine kinase-driven signalling for guidance, as does the vasculature or tracheal tree for both guidance and tip cell selection [1,43,44,45]. On the basis of analysis in this system, I previously proposed the idea of collective guidance [13,64], in which external guidance information directs group movement by inducing different levels of signalling in the front cell compared with the other cells (Fig 3B, higher levels in the green cell than in the blue cells). This selects one cell to be in a different state from the others, to be a transient leader. This state difference, combined with the inherent polarization of each cell, provides directionality of movement for the group. We tested this model rigorously by imposing different signalling states on different cells and observed that such externally controlled induction of leader over follower states, can indeed direct movement of a cell group [66]. Thus sprouting angiogenesis and cluster movement share both signaling pathways for guidance and dynamic leader cell assignment.
Cells and tissues rotate
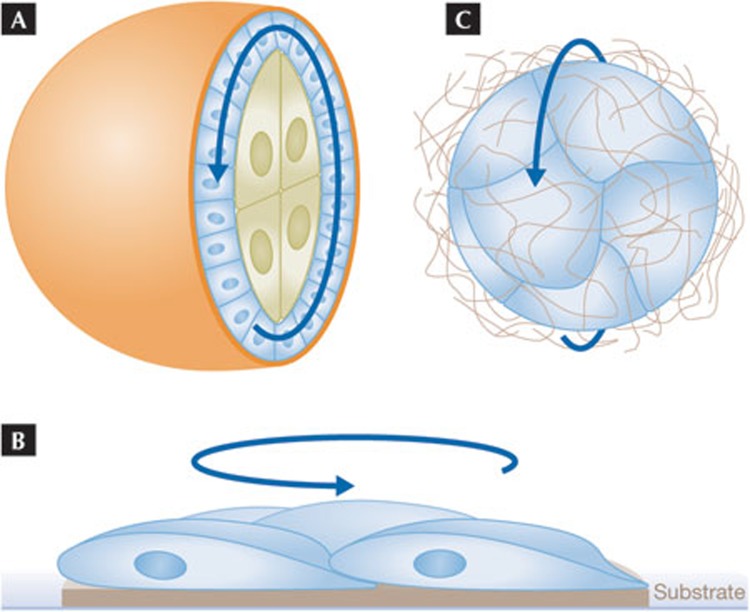
Live imaging completely changed our view of how cells in a collective differ from one another in the studies discussed above. Other surprises have come from live analysis, for example the finding that mammary epithelial cells thought to be sessile are mobile [42,67]. Also unexpected were the observations that intact epithelia and groups of epithelial cells rotate three-dimensionally [68,69]. A marked example of this is the rotation of the follicular epithelium during Drosophila oogenesis [68]. Follicle cells are somatic cells that form a monolayer epithelium enclosing central germline cells including the oocyte (Fig 4A). At a particular stage of development the whole epithelium starts to rotate, either clockwise or anti-clockwise, relative to the overlaying ECM—the basement membrane. The rotation occurs in an integrin-dependent manner and seems to continue for several complete revolutions, causing the whole structure—and the resulting egg—to become elongated. The rotating cells deposit circular tracks of ECM material such as collagen, which in turn seem to mechanically constrain tissue elongation [68]. This interesting type of morphogenesis can be viewed as a self-organized collective movement of a coherent cell sheet, but one with no free edge and no leader cells. So how do all the cells manage to move in the same direction? And is this a completely unique case or representative of a wider phenomenon? To answer these questions, it is worth considering other types of rotating movement reported for epithelial cells.
Figure 4.
Epithelial rotation. (A) A cross-section showing the internal rotating movement of the follicular epithelium (blue cells) on the ECM–basement membrane (orange layer). The internal germline cells (large green cells) follow the follicle cells. The epithelium is continuous with no free edge in the intact structure. (B) Side-view of a small group of epithelial or endothelial cells plated on an island of substrate. The cells will rotate together on the substrate for prolonged periods. (C) A small cluster of epithelial cells in a three-dimensional matrix. An apical lumen might have formed internally; the basal sides of the cells are facing outwards. Such clusters will rotate coherently in one direction. ECM, extracellular matrix.
It is well established that epithelial and endothelial cells can perform collective movements when placed in two-dimensional culture dishes, with analogy often made to wound healing. But perhaps surprisingly, when such cells are placed in a small micro-patterned area of a dish, and are thus confined with respect to substrate interaction, they tend to rotate around one another (Fig 4B; [70,71]). Apparently, mesenchymal cells do not display this behaviour [71]. When cells such as Madin–Darbey canine kidney cells or breast epithelial cells are grown under more physiological three-dimensional culture conditions, they grow in clusters, sometimes mimicking normal differentiation and forming a central, apical lumen [72,73]. These clusters rotate in a coherent fashion (Fig 4C; [69,74]). As for the follicular epithelium, the basal sides of these cells move on, and relative to, the external ECM, which is only minimally deformed by the movement [74]. Interestingly, breast cancer cells that are highly transformed and considered to have undergone EMT do not show coherent, rotating behaviour, but move erratically [69]. Thus, rotating movement seems to be a feature of normal epithelial cells when cultured under spatially confined conditions. In vivo, breast or kidney epithelial cells are part of interconnected ductal structures that would be expected to constrain rotating tendencies. Rotating movement where epithelial cells move as a group, but relative to other cells, has been described for other specialized cells in vivo. A small group of photoreceptor progenitors in the developing fly eye rotate slowly at about 5–10° per hour [75]. The migratory border cell cluster described above also rotates relative to surrounding cells, in particular when it does not undergo directional movement [64,76].
These rotational movements raise some immediate questions: how common is it? How do the cells generate traction on the substrate and move with no front? Finally, how do the cells co-ordinate their activity so that they move in the same direction?
Starting from the last question, the in vitro findings suggest that the tendency to rotate in a co-ordinated manner is an intrinsic characteristic of epithelial cells. It could primarily be cohesiveness: if cells cannot move relative to one another, any movement they make relative to a substrate must be coherent. However, in the case of the follicular epithelium, hundreds of cells are involved and movement is usually aborted if many cells are unable to interact properly with the substrate [68]. In the in vitro situations, cells are not fully differentiated and cell–cell interactions are expected to be dynamic. Therefore, co-ordinated cell participation seems to be the most probable underlying scenario for co-ordinated directional movement. One mediating principle to co-ordinate directionality could be an adhesion and force-dependent polarizing effect, as discussed in the ‘information at cell contacts’ section, which was found to be crucial for follower cells. To achieve co-ordination, differential force or tugging at a cell cortex would have to be propagated within the plane of the epithelium in a planar polarized fashion. This might be feasible by a mechanical stress effect through the cytoskeleton. Planar polarized signalling by the non-canonical Wnt–Fz pathway [77,78] might also help to organize a rotating epithelium. However, the classical planar polarity signalling pathway is not required in the follicular epithelium, whereas the atypical cadherin Fat2 is required [79]. Therefore, rotational movement might use different planar polarity signalling molecules or might primarily use mechanical effects to orient and co-ordinate movement.
The rotating movement of epithelial cells on the ECM might be analogous to sheet migration, with all cells behaving as follower cells. Follower cells can have forward protrusions along the basal substrate [32], which might allow for local traction and directed behaviour of the cell. In the context of rotation there is no inherent ‘forward’ direction, but co-ordinated protrusions could give coherent movement. It is also worth considering similarities to other epithelial movements that involve planar polarized information, but do not depend on obvious cellular protrusions. One such movement is epithelial cell intercalation resulting in tissue elongation in the fly embryo [80]. Intercalation involves significant exchange of neighbours, requiring dynamic cell–cell adhesion. Whether this also involves movement relative to an ECM, requiring dynamic cell–substrate interactions, is not clear. Vertebrate convergence extension movements also involve cell intercalation [78]; here ECM interactions seem to contribute significantly [81]. The cytoskeletal mechanisms underlying cell intercalation within an epithelium have been the focus of attention. Initial models were based on unequal myosin-based cortical tension at cell edges forcing junctions together [80,82]. More detailed imaging revealed a pulsing and anisotropic actomyosin network that affects the whole apical surface and might drive cell movements [83,84]. It is possible that forces from such dynamic cellular contractions also drive the movement of epithelial cells relative to the external ECM.
Further studies of tissues by well-resolved live imaging are required to determine whether rotational movements are actually common. A general impression is that epithelial cells do not move much in vivo. Some tissues, such as the gut, show slow and consistent epithelial cell movement due to organized turnover in the monolayer [85,86]. Other differentiated tissues might be even more static. Rigid attachment to a fixed ECM and strong cell–cell adhesion attaching cells to large, elaborate structures might prevent cell movement in such tissues. The cell culture experiments remind us, however, that motility is an intrinsic quality of epithelial and endothelial cells. Cell movement will manifest itself when the context allows. When this happens, the migration will generally be collective, as these are highly social cells. In closing, it is worth noting that our understanding of collective cell migration has advanced through studies in many different systems, and that many interesting questions remain (see Sidebar A).
Sidebar A | In need of answers.
How does the mechanical and molecular local signalling between cells of the collective change the migratory behaviour of the cells, for example their interaction with the substrate?
In a migrating collective, cells often adopt different states. What are the advantages in terms of tissue invasion, directionality, robustness or other features, of this specialization?
The leader and follower cells exchange positions and states over time. Is there a general rationale and mechanism for this?
What type of planar polarization directs coherent epithelial rotation? How widespread is this phenomenon?
To what extent does collective cell migration, compared with epithelial-to-mesenchymal transition-driven individual cell migration, contribute to cancer dissemination and metastasis?

Pernille Rørth
Footnotes
The author declares that she has no conflict of interest.
References
- Rørth P (2009) Collective cell migration. Annu Rev Cell Dev Biol 25: 407–429 [DOI] [PubMed] [Google Scholar]
- Weijer CJ (2009) Collective cell migration in development. J Cell Sci 122: 3215–3223 [DOI] [PubMed] [Google Scholar]
- Friedl P, Gilmour D (2009) Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol 10: 445–457 [DOI] [PubMed] [Google Scholar]
- Friedl P, Locker J, Sahai E, Segall JE (2012) Classifying collective cancer cell invasion. Nat Cell Biol 14: 777–783 [DOI] [PubMed] [Google Scholar]
- du Roure O, Saez A, Buguin A, Austin RH, Chavrier P, Silberzan P, Ladoux B (2005) Force mapping in epithelial cell migration. Proc Natl Acad Sci USA 102: 2390–2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga H, Irahara C, Kobayashi R, Nakagaki T, Kawabata K (2005) Collective movement of epithelial cells on a collagen gel substrate. Biophys J 88: 2250–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitorino P, Meyer T (2008) Modular control of endothelial sheet migration. Genes Dev 22: 3268–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teddy JM, Kulesa PM (2004) In vivo evidence for short- and long-range cell communication in cranial neural crest cells. Development 131: 6141–6151 [DOI] [PubMed] [Google Scholar]
- Murase S, Horwitz AF (2004) Directions in cell migration along the rostral migratory stream: the pathway for migration in the brain. Curr Top Dev Biol 61: 135–152 [DOI] [PubMed] [Google Scholar]
- Nam SC et al. (2007) Dynamic features of postnatal subventricular zone cell motility: a two-photon time-lapse study. J Comp Neurol 505: 190–208 [DOI] [PubMed] [Google Scholar]
- Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P (2007) Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol 9: 893–904 [DOI] [PubMed] [Google Scholar]
- Rørth P (2011) Whence directionality: guidance mechanisms in solitary and collective cell migration. Dev Cell 20: 9–18 [DOI] [PubMed] [Google Scholar]
- Rørth P (2007) Collective guidance of collective cell migration. Trends Cell Biol 17: 575–579 [DOI] [PubMed] [Google Scholar]
- Theveneau E, Marchant L, Kuriyama S, Gull M, Moepps B, Parsons M, Mayor R (2010) Collective chemotaxis requires contact-dependent cell polarity. Dev Cell 19: 39–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesa PM, Fraser SE (2000) In ovo time-lapse analysis of chick hindbrain neural crest cell migration shows cell interactions during migration to the branchial arches. Development 127: 1161–1172 [DOI] [PubMed] [Google Scholar]
- Duband JL, Monier F, Delannet M, Newgreen D (1995) Epithelium–mesenchyme transition during neural crest development. Acta Anat (Basel) 154: 63–78 [DOI] [PubMed] [Google Scholar]
- Kasemeier-Kulesa JC, Bradley R, Pasquale EB, Lefcort F, Kulesa PM (2006) Eph/ephrins and N-cadherin coordinate to control the pattern of sympathetic ganglia. Development 133: 4839–4847 [DOI] [PubMed] [Google Scholar]
- Carmona-Fontaine C, Theveneau E, Tzekou A, Tada M, Woods M, Page KM, Parsons M, Lambris JD, Mayor R (2011) Complement fragment C3a controls mutual cell attraction during collective cell migration. Dev Cell 21: 1026–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Fontaine C, Matthews HK, Kuriyama S, Moreno M, Dunn GA, Parsons M, Stern CD, Mayor R (2008) Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature 456: 957–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K et al. (1996) Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273: 245–248 [DOI] [PubMed] [Google Scholar]
- Hidalgo-Carcedo C, Hooper S, Chaudhry SI, Williamson P, Harrington K, Leitinger B, Sahai E (2011) Collective cell migration requires suppression of actomyosin at cell–cell contacts mediated by DDR1 and the cell polarity regulators Par3 and Par6. Nat Cell Biol 13: 49–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko T, Hall A (2012) Myosin-IXA regulates collective epithelial cell migration by targeting RhoGAP activity to cell–cell junctions. Curr Biol 22: 278–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ER, Matela AR, SundarRaj N, Iszkula ER, Klarlund JK (2004) Wounding induces motility in sheets of corneal epithelial cells through loss of spatial constraints: role of heparin-binding epidermal growth factor-like growth factor signaling. J Biol Chem 279: 24307–24312 [DOI] [PubMed] [Google Scholar]
- Poujade M, Grasland-Mongrain E, Hertzog A, Jouanneau J, Chavrier P, Ladoux B, Buguin A, Silberzan P (2007) Collective migration of an epithelial monolayer in response to a model wound. Proc Natl Acad Sci USA 104: 15988–15993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reffay M, Petitjean L, Coscoy S, Grasland-Mongrain E, Amblard F, Buguin A, Silberzan P (2011) Orientation and polarity in collectively migrating cell structures: statics and dynamics. Biophys J 100: 2566–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlieb AI, May LM, Subrahmanyan L, Kalnins VI (1981) Distribution of microtubule organizing centers in migrating sheets of endothelial cells. J Cell Biol 91: 589–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A (2001) Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell 106: 489–498 [DOI] [PubMed] [Google Scholar]
- Pouthas F, Girard P, Lecaudey V, Ly TB, Gilmour D, Boulin C, Pepperkok R, Reynaud EG (2008) In migrating cells, the Golgi complex and the position of the centrosome depend on geometrical constraints of the substratum. J Cell Sci 121: 2406–2414 [DOI] [PubMed] [Google Scholar]
- Thery M, Racine V, Piel M, Pepin A, Dimitrov A, Chen Y, Sibarita JB, Bornens M (2006) Anisotropy of cell adhesive microenvironment governs cell internal organization and orientation of polarity. Proc Natl Acad Sci USA 103: 19771–19776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupin I, Camand E, Etienne-Manneville S (2009) Classical cadherins control nucleus and centrosome position and cell polarity. J Cell Biol 185: 779–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RA, Gao L, Raghavan S, Liu WF, Chen CS (2009) Cell polarity triggered by cell–cell adhesion via E-cadherin. J Cell Sci 122: 905–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqui R, Fenteany G (2005) Multiple rows of cells behind an epithelial wound edge extend cryptic lamellipodia to collectively drive cell-sheet movement. J Cell Sci 118: 51–63 [DOI] [PubMed] [Google Scholar]
- Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, Nelson CM, Chen CS (2010) Mechanical tugging force regulates the size of cell–cell junctions. Proc Natl Acad Sci USA 107: 9944–9949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD (2001) Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol 153: 1175–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber GF, Bjerke MA, DeSimone DW (2012) A mechanoresponsive cadherin-keratin complex directs polarized protrusive behavior and collective cell migration. Dev Cell 22: 104–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepat X, Fredberg JJ (2011) Plithotaxis and emergent dynamics in collective cellular migration. Trends Cell Biol 21: 638–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmke BP, Rosen AB, Davies PF (2003) Mapping mechanical strain of an endogenous cytoskeletal network in living endothelial cells. Biophys J 84: 2691–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P, Coulombe PA (2003) Loss of keratin 6 (K6) proteins reveals a function for intermediate filaments during wound repair. J Cell Biol 163: 327–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long HA, Boczonadi V, McInroy L, Goldberg M, Määttä A (2006) Periplakin-dependent re-organisation of keratin cytoskeleton and loss of collective migration in keratin-8-downregulated epithelial sheets. J Cell Sci 119: 5147–5159 [DOI] [PubMed] [Google Scholar]
- Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E (2007) Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol 9: 1392–1400 [DOI] [PubMed] [Google Scholar]
- Larsen M, Wei C, Yamada KM (2006) Cell and fibronectin dynamics during branching morphogenesis. J Cell Sci 119: 3376–3384 [DOI] [PubMed] [Google Scholar]
- Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z (2008) Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell 14: 570–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT (2002) Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev 16: 2684–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghabrial AS, Krasnow MA (2006) Social interactions among epithelial cells during tracheal branching morphogenesis. Nature 441: 746–749 [DOI] [PubMed] [Google Scholar]
- Gerhardt H et al. (2003) VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161: 1163–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yana I et al. (2007) Crosstalk between neovessels and mural cells directs the site-specific expression of MT1-MMP to endothelial tip cells. J Cell Sci 120: 1607–1614 [DOI] [PubMed] [Google Scholar]
- del Toro R et al. (2010) Identification and functional analysis of endothelial tip cell-enriched genes. Blood 116: 4025–4033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima K, Inoue T, Shimao Y, Okada Y, Itoh Y, Seiki M, Koono M (2000) Front-cell-specific expression of membrane-type 1 matrix metalloproteinase and gelatinase A during cohort migration of colon carcinoma cells induced by hepatocyte growth factor/scatter factor. Cancer Res 60: 3364–3369 [PubMed] [Google Scholar]
- Wicki A, Lehembre F, Wick N, Hantusch B, Kerjaschki D, Christofori G (2006) Tumor invasion in the absence of epithelial–mesenchymal transition: podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell 9: 261–272 [DOI] [PubMed] [Google Scholar]
- Vignjevic D, Schoumacher M, Gavert N, Janssen KP, Jih G, Lae M, Louvard D, Ben-Ze'ev A, Robine S (2007) Fascin, a novel target of beta-catenin-TCF signaling, is expressed at the invasive front of human colon cancer. Cancer Res 67: 6844–6853 [DOI] [PubMed] [Google Scholar]
- McLennan R, Dyson L, Prather KW, Morrison JA, Baker RE, Maini PK, Kulesa PM (2012) Multiscale mechanisms of cell migration during development: theory and experiment. Development 139: 2935–2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aigouy B, Lepelletier L, Giangrande A (2008) Glial chain migration requires pioneer cells. J Neurosci 28: 11635–11641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas P, Gilmour D (2006) Chemokine signaling mediates self-organizing tissue migration in the zebrafish lateral line. Dev Cell 10: 673–680 [DOI] [PubMed] [Google Scholar]
- Jacinto A, Wood W, Balayo T, Turmaine M, Martinez-Arias A, Martin P (2000) Dynamic actin-based epithelial adhesion and cell matching during Drosophila dorsal closure. Curr Biol 10: 1420–1426 [DOI] [PubMed] [Google Scholar]
- Jakobsson L et al. (2010) Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol 12: 943–953 [DOI] [PubMed] [Google Scholar]
- Arima S et al. (2011) Angiogenic morphogenesis driven by dynamic and heterogeneous collective endothelial cell movement. Development 138: 4763–4776 [DOI] [PubMed] [Google Scholar]
- Hellstrom M et al. (2007) Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445: 776–780 [DOI] [PubMed] [Google Scholar]
- Siekmann AF, Lawson ND (2007) Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature 445: 781–784 [DOI] [PubMed] [Google Scholar]
- Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD, Thurston G (2006) Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature 444: 1032–1037 [DOI] [PubMed] [Google Scholar]
- Ridgway J et al. (2006) Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature 444: 1083–1087 [DOI] [PubMed] [Google Scholar]
- Ghabrial A, Luschnig S, Metzstein MM, Krasnow MA (2003) Branching morphogenesis of the Drosophila tracheal system. Annu Rev Cell Dev Biol 19: 623–647 [DOI] [PubMed] [Google Scholar]
- Caussinus E, Colombelli J, Affolter M (2008) Tip-cell migration controls stalk-cell intercalation during Drosophila tracheal tube elongation. Curr Biol 18: 1727–1734 [DOI] [PubMed] [Google Scholar]
- Prasad M, Montell DJ (2007) Cellular and molecular mechanisms of border cell migration analyzed using time-lapse live-cell imaging. Dev Cell 12: 997–1005 [DOI] [PubMed] [Google Scholar]
- Bianco A, Poukkula M, Cliffe A, Mathieu J, Luque CM, Fulga TA, Rørth P (2007) Two distinct modes of guidance signalling during collective migration of border cells. Nature 448: 362–365 [DOI] [PubMed] [Google Scholar]
- Wang X, He L, Wu YI, Hahn KM, Montell DJ (2010) Light-mediated activation reveals a key role for Rac in collective guidance of cell movement in vivo. Nat Cell Biol 12: 591–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaki M, Vishnu S, Cliffe A, Rørth P (2012) Effective guidance of collective migration based on differences in cell states. Proc Natl Acad Sci USA 109: 2027–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson GW, Hunter T (2007) Real-time imaging reveals that noninvasive mammary epithelial acini can contain motile cells. J Cell Biol 179: 1555–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigo SL, Bilder D (2011) Global tissue revolutions in a morphogenetic movement controlling elongation. Science 331: 1071–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner K, Mori H, Mroue R, Bruni-Cardoso A, Bissell MJ (2012) Coherent angular motion in the establishment of multicellular architecture of glandular tissues. Proc Natl Acad Sci USA 109: 1973–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne C, Huang S, Parker KK, Ingber DE, Ostuni E (2000) Symmetry breaking in cultured mammalian cells. In Vitro Cell Dev Biol Anim 36: 563–565 [DOI] [PubMed] [Google Scholar]
- Huang S, Brangwynne CP, Parker KK, Ingber DE (2005) Symmetry-breaking in mammalian cell cohort migration during tissue pattern formation: role of random-walk persistence. Cell Motil Cytoskeleton 61: 201–213 [DOI] [PubMed] [Google Scholar]
- O'Brien LE, Zegers MM, Mostov KE (2002) Building epithelial architecture: insights from three-dimensional culture models. Nat Rev Mol Cell Biol 3: 531–537 [DOI] [PubMed] [Google Scholar]
- Ferrari A, Veligodskiy A, Berge U, Lucas MS, Kroschewski R (2008) ROCK-mediated contractility, tight junctions and channels contribute to the conversion of a preapical patch into apical surface during isochoric lumen initiation. J Cell Sci 121: 3649–3663 [DOI] [PubMed] [Google Scholar]
- Marmaras A, Berge U, Ferrari A, Kurtcuoglu V, Poulikakos D, Kroschewski R (2010) A mathematical method for the 3D analysis of rotating deformable systems applied on lumen-forming MDCK cell aggregates. Cytoskeleton (Hoboken) 67: 224–240 [DOI] [PubMed] [Google Scholar]
- Escudero LM, Bischoff M, Freeman M (2007) Myosin II regulates complex cellular arrangement and epithelial architecture in Drosophila. Dev Cell 13: 717–729 [DOI] [PubMed] [Google Scholar]
- Yang N, Inaki M, Cliffe A, Rørth P (2012) Microtubules and Lis-1/NudE/dynein regulate invasive cell-on-cell migration in Drosophila. PLoS ONE 7: e40632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TJ, Mlodzik M (2005) Planar cell polarization: an emerging model points in the right direction. Annu Rev Cell Dev Biol 21: 155–176 [DOI] [PubMed] [Google Scholar]
- Roszko I, Sawada A, Solnica-Krezel L (2009) Regulation of convergence and extension movements during vertebrate gastrulation by the Wnt/PCP pathway. Semin Cell Dev Biol 20: 986–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viktorinova I, Konig T, Schlichting K, Dahmann C (2009) The cadherin Fat2 is required for planar cell polarity in the Drosophila ovary. Development 136: 4123–4132 [DOI] [PubMed] [Google Scholar]
- Bertet C, Sulak L, Lecuit T (2004) Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 429: 667–671 [DOI] [PubMed] [Google Scholar]
- Skoglund P, Keller R (2010) Integration of planar cell polarity and ECM signaling in elongation of the vertebrate body plan. Curr Opin Cell Biol 22: 589–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship JT, Backovic ST, Sanny JS, Weitz O, Zallen JA (2006) Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev Cell 11: 459–470 [DOI] [PubMed] [Google Scholar]
- Rauzi M, Lenne PF, Lecuit T (2010) Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature 468: 1110–1114 [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Zallen JA (2011) Oscillatory behaviours and hierarchical assembly of contractile structures in intercalating cells. Phys Biol 8: 045005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath JP (1996) Epithelial cell migration in the intestine. Cell Biol Int 20: 139–146 [DOI] [PubMed] [Google Scholar]
- Nelson SA et al. (2012) Tumorigenic fragments of APC cause dominant defects in directional cell migration in multiple model systems. Dis Model Mech [Epub ahead of print] doi:; DOI: 10.1242/dmm.008607 [DOI] [PMC free article] [PubMed] [Google Scholar]